Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.3943
Peer-review started: November 18, 2020
First decision: December 30, 2020
Revised: January 9, 2021
Accepted: February 10, 2021
Article in press: February 10, 2021
Published online: June 6, 2021
Processing time: 176 Days and 17.8 Hours
Transplant renal artery stenosis is a relatively frequent vascular complication after transplantation. However, extra-renal pseudo-aneurysms (EPSAs) are rare after transplantation; they can be life-threatening and usually need open surgical repair. We discuss the diagnosis and spontaneous healing of an asymptomatic renal allograft EPSA caused by renal artery anastomotic stenosis, which was diagnosed in a timely manner and managed by conservative treatments.
We present a 37-year-old male patient diagnosed with a renal allograft EPSA caused by renal artery anastomotic stenosis due to multiple atherosclerotic plaques with ultrasonographic examination 6 mo post transplantation. The stenosis rate of 90% and the EPSA were verified by computed tomography angiography. The diagnosis was further confirmed with digital subtraction angiography. Percutaneous transluminal angiography was conducted, and a metallic stent was successfully implanted at the stenosed site of the main renal artery trunk. No further intervention for the EPSA was undertaken due to the difficulty of stenting and the risk of bleeding; regular ultrasonographic follow-ups were recommended. The stenosis was significantly relieved immediately after stent implantation and the EPSA was healed spontaneously by completely filling with hypo-echoic thrombosis 8 mo after stenting.
Ultrasonography combined with a high-frequency linear probe can detect vascular complications post renal transplantation at an early stage and improve prognosis.
Core Tip: Extra-renal pseudo-aneurysms (EPSAs) are rare post-kidney transplantation, which might be life-threatening and usually need open surgical repair or even nephrectomy. EPSA combined with, or more precisely, caused by transplant renal artery stenosis is rare. There is still no consensus for the treatment of EPSAs. We report the diagnosis and spontaneous healing of an asymptomatic renal allograft EPSA caused by renal artery anastomotic stenosis, which was diagnosed in a timely manner and managed by conservative treatments. The stenosis was relieved after stent implantation, and the EPSA was cured spontaneously by completely filling with hypo-echoic thrombosis 8 mo after stenting.
- Citation: Xu RF, He EH, Yi ZX, Li L, Lin J, Qian LX. Diagnosis and spontaneous healing of asymptomatic renal allograft extra-renal pseudo-aneurysm: A case report. World J Clin Cases 2021; 9(16): 3943-3950
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/3943.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.3943
Renal transplantation is one of the effective therapies for end-stage renal disease. With the improvement in immunosuppression in renal transplantation, there has been a significant decrease in premature graft loss and acute rejection in the past two decades[1,2]. Vascular complications are common among renal transplantation patients, including thrombosis, stenosis, pseudo-aneurysm, arteriovenous fistula, and so on[3,4]. Pseudo-aneurysms can be intra-renal or extra-renal (the renal graft artery trunk or iliac artery at the anastomosis site). Extra-renal pseudo-aneurysms (EPSAs) are usually rare and still without treatment consensus, which can lead to devastating consequences and could be fatal and sometimes may require renal graft nephrectomy[5,6]. Transplant renal artery stenosis (TRAS) can happen at any time post transplantation, which usually occurs within 3 years post transplantation[7]. The prevalence of TRAS and EPSAs is less than 1% and 1%-23%, respectively. EPSA combined with, or, more precisely, caused by TRAS is rare. We manifest the diagnosis and prognosis of an asymptomatic renal allograft EPSA caused by renal artery anastomotic stenosis, which was diagnosed in time and managed under conservative treatments.
A 37-year-old male patient presented to the ultrasound department for regular ultrasonographic follow-up, without complaints of specific discomforts.
The patient underwent allogeneic kidney transplantation (donation after cardiac death) because of IgA nephropathy, chronic renal insufficiency and uremia at our hospital 6 mo ago.
The patient was diagnosed with renal hypertension 10 years ago, and his blood pressure was stable after dialysis with medication administration. He was diagnosed with renal anemia 10 years ago and was treated with hemopoietin regularly. He was diagnosed with renal osteopathy 10 years ago, and his condition was stable with oral administration of calcium tablets.
Personal and family history were unremarkable.
The patient’s body temperature was 36.3 ℃, heart rate was 90 bpm, respiratory rate was 18 breaths per minute, and blood pressure was 105/64 mmHg. One 15-cm oblique scar could be seen in the right lower abdomen. Arteriovenous fistula could be seen in the left forearm.
The post-transplant serum creatinine fluctuated around 90 μmol/L during follow-ups. However, the creatinine at this time was 165.4 μmol/L, which was higher than the normal level.
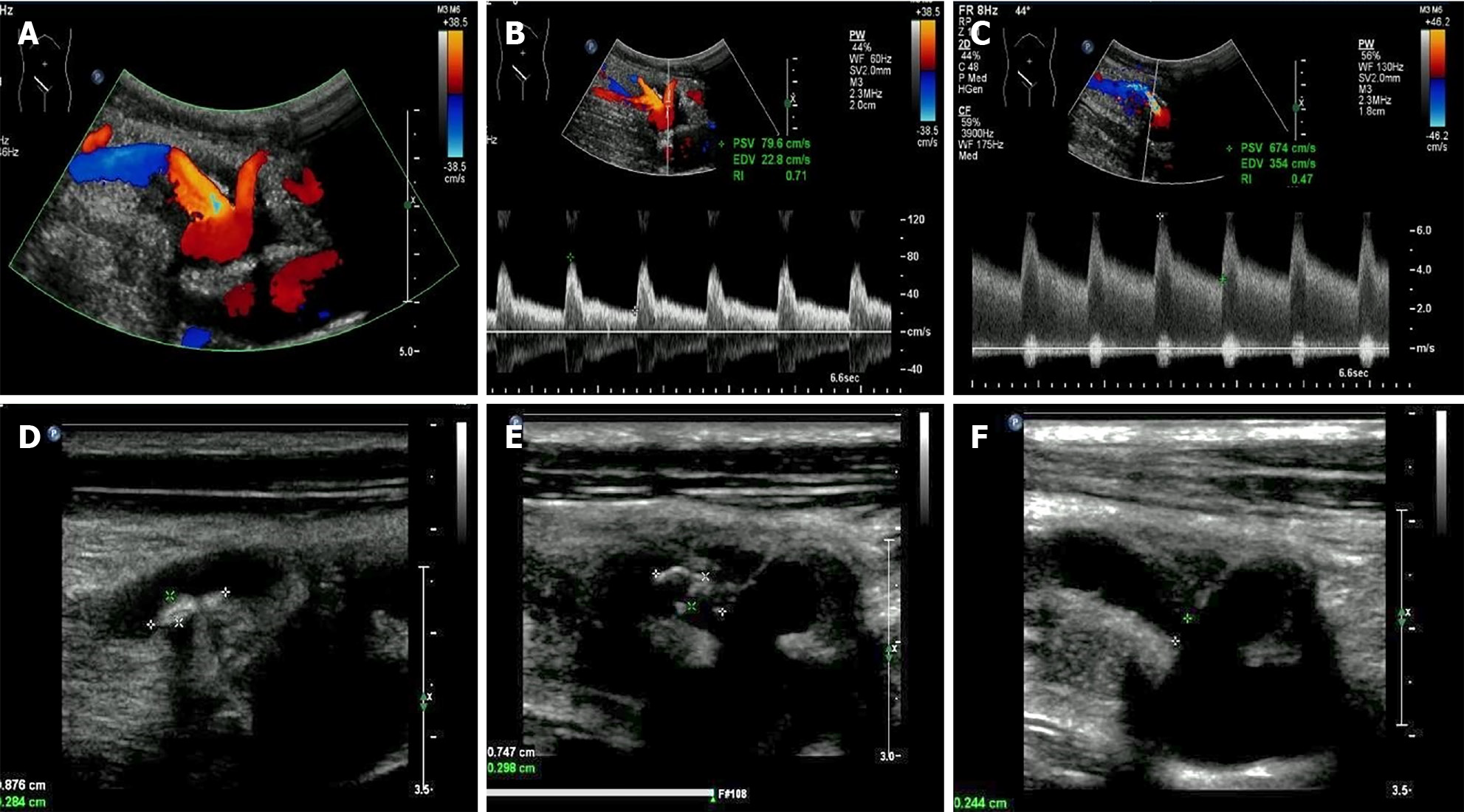
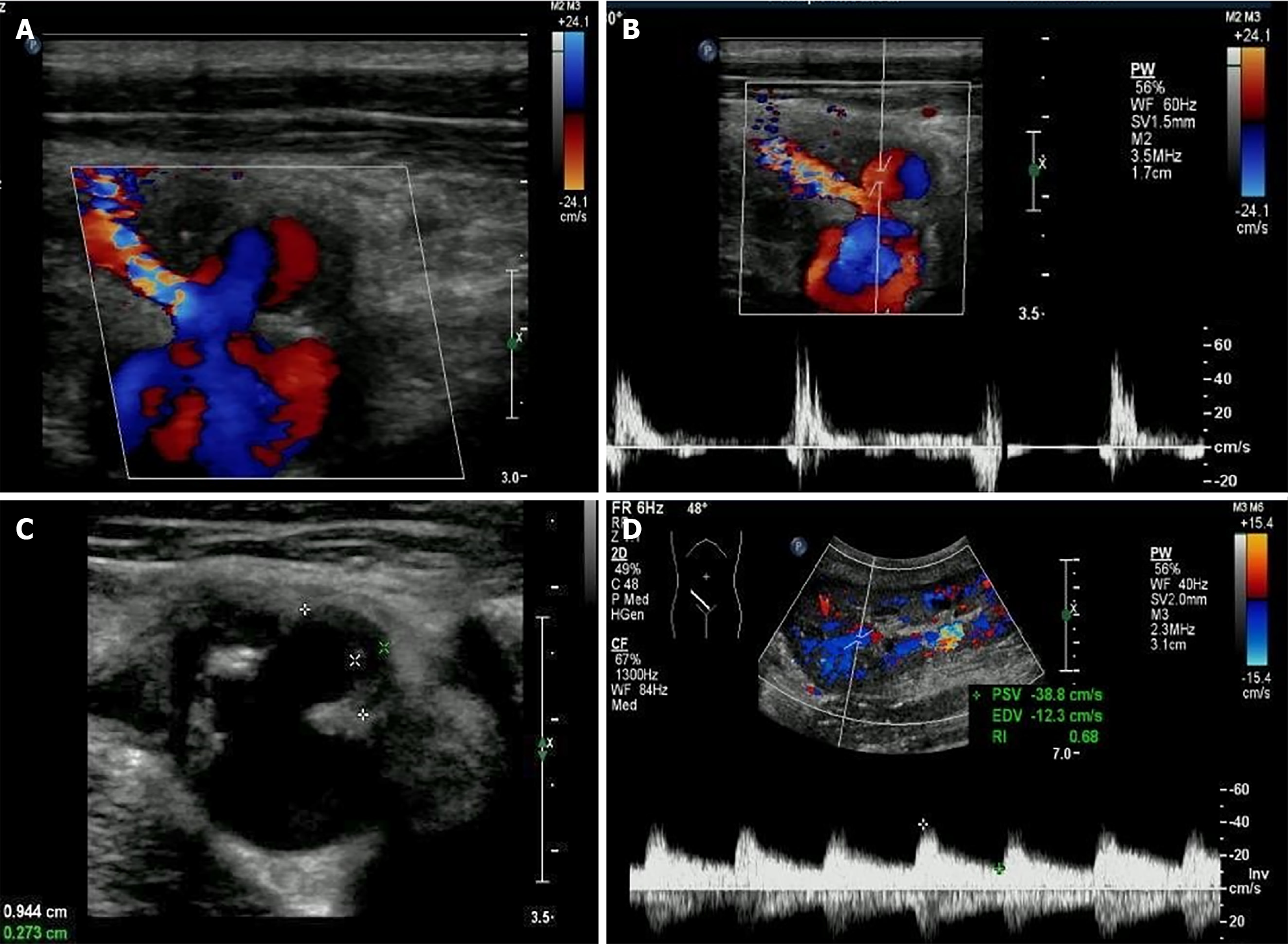
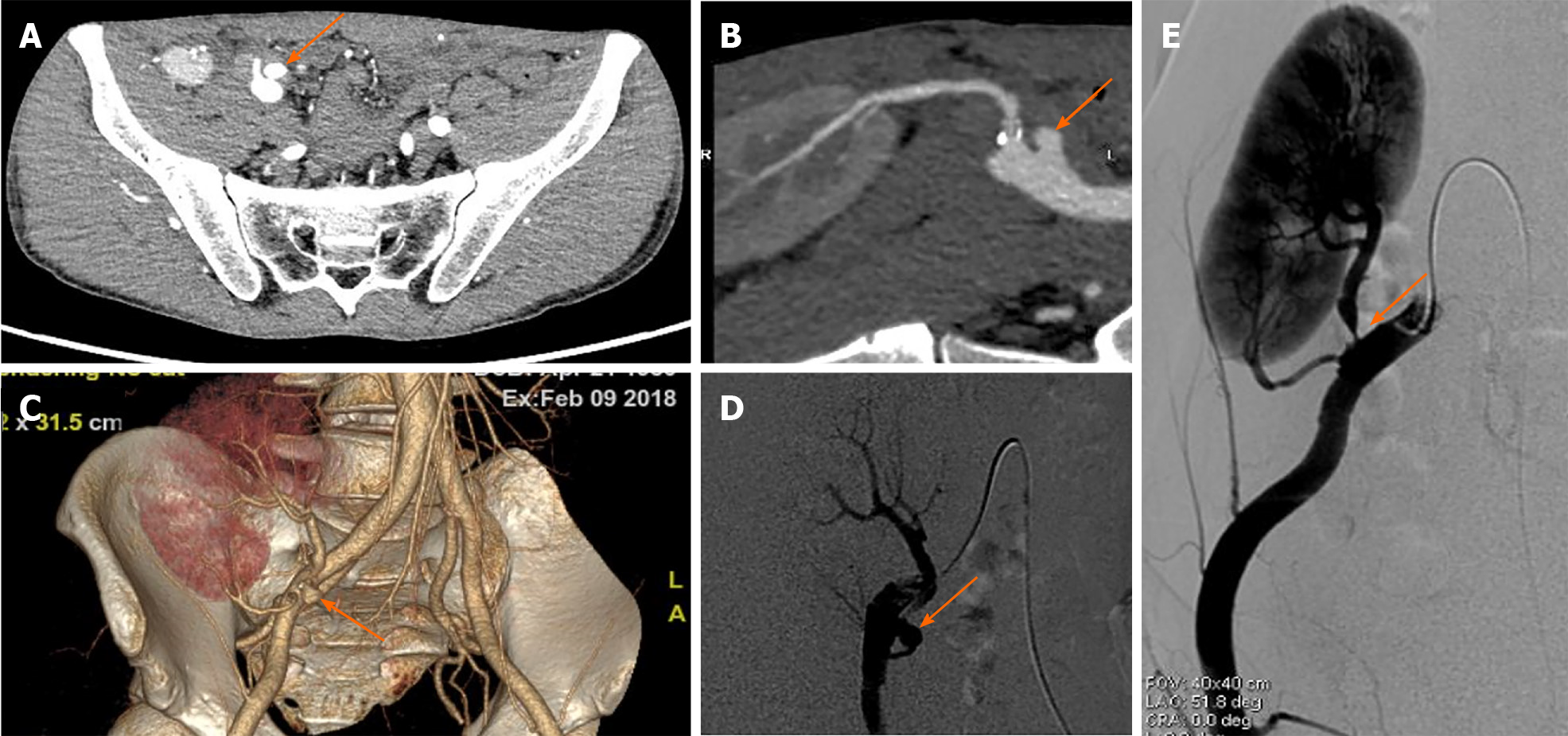
Two artery trunks of the renal graft anastomosed with the side of the external iliac artery were detected during the ultrasonographic examination (1-5 MHz convex transducer, iuElite; Philips, Bothell, WA98021, United States) (Figure 1A and B). Color aliasing indicating high-velocity turbulent flow at the proximal site of the main renal artery trunk (donor portion) was detected, with peak systolic velocity (PSV) of 674 cm/s on pulsed-wave Doppler (PWD) (Figure 1C). The renal artery to external iliac artery PSV ratio was larger than 3:1, which further confirmed stenosis at the site. Stenosis at the proximal site of the main renal artery trunk (donor portion) was considered. Multiple hyper-echo plaques attached to the wall of the main renal artery trunk were detected after meticulous scanning with the high frequency 9-3 MHz linear probe (Figure 1D and E). The largest plaque of the proximal site of the main renal artery trunk close to the anastomosis was about 0.7 cm × 0.3 cm, and the plaque size of the middle segment of the main renal artery trunk was about 0.9 cm × 0.3 cm. The residual lumen at the proximal site of the main renal artery trunk was about 0.2 cm in diameter, and one 1.2 cm × 0.8 cm external protruding cystic structure was detected at the anastomotic site between the main renal artery trunk and the external iliac artery. Blood flow signals inside the cystic structure were visible in color Doppler flow imaging, showing as swirling pattern, and to-and-fro disorganized arterial spectrum was detected with PWD (Figure 2A and B). Hypo-echoic thrombus with thickness of 0.3 cm was observed inside the cystic structure, and an EPSA at the anastomotic site caused by renal artery atherosclerotic stenosis with thrombosis formed inside was considered (Figure 2C). The spectral waveform of the perforating area inside the renal allograft was observed, showing as prolonged acceleration time longer than 70 ms (Figure 2D). Stenosis rate of 90% at the proximal site of the main renal artery trunk accompanied with a pseudo-aneurysm at the anastomosis with the external iliac artery was confirmed by further computed tomography angiography and digital subtraction angiography (Figure 3).
An EPSA at anastomotic site caused by renal artery atherosclerotic stenosis with thrombosis formed inside was considered.
After reviewing the benefits and risks of all treatment options, the physician and the patient decided to conduct percutaneous transluminal angiography. Balloon dilation was conducted at first and then a metallic stent of 6 mm × 20 mm was implanted at the proximal stenosed site of the main renal artery trunk successfully under angiography. Since the pseudo-aneurysm was at the anastomotic site, there was no adequate landing zone available for the placement of another stent due to the complexity of an anastomosis with a reconstructed main renal artery combined with the lower pole renal artery. According to our judgement, the pseudo-aneurysm might be the result of the high pressure caused by the severe stenosis and there was thrombosis formed inside the sac. Therefore, no further intervention was chosen according to the size of aneurysm and the danger of bleeding after stent implantation. We recommended close ultrasonographic follow-ups.
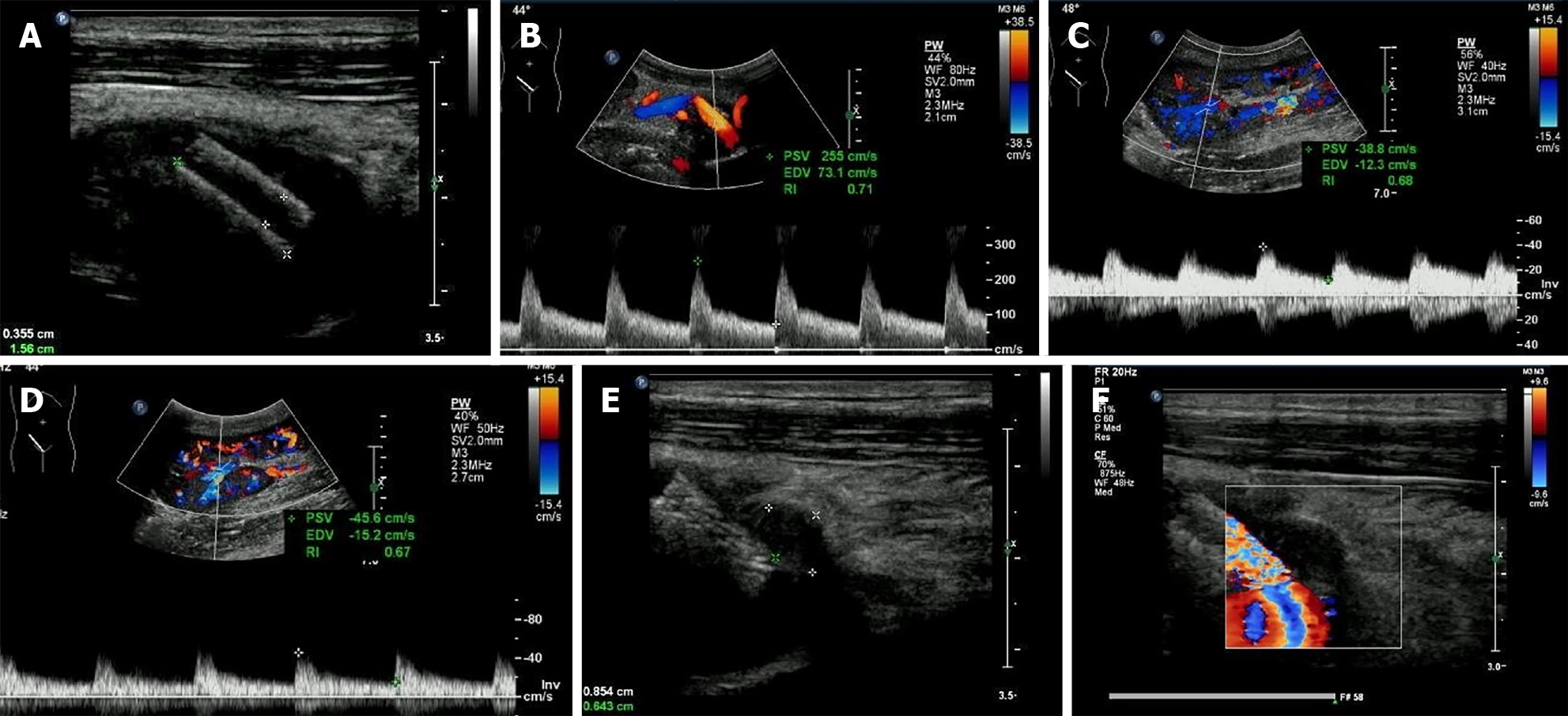
The stenosis was significantly relieved after stent implantation, manifested as the PSV of stenosed site decreasing to 255 cm/s (Figure 4A and B) and the disappearance of the prolonged acceleration time of the arterial spectral waveform inside the renal graft (Figure 4C and D). During regular ultrasonographic follow-ups, the lumen of the pseudo-aneurysm narrowed and was replaced by hypo-echoic thrombosis gradually. The pseudo-aneurysm was completely filled with hypo-echoic thrombosis 8 mo after stenting (Figure 4E and F).
TRAS is relatively common, compared with EPSAs, which occurs in less than 1% of patients[8]. These are mostly the result of technical errors or infection, and frequently happen at the arterial anastomotic site. It might lead to renal graft dysfunction and/or uncontrolled post-transplant hypertension and even allograft loss[9,10].
The causes for TRAS are mostly technical, vascular clamps, vessel lesions during preservation, angulation or kinking of the artery. Stenosis can also occur due to donor or recipient atherosclerosis[11]. The incidence of multiple renal arteries is about 17%-35% reported by autopsy studies, depending on the ethnic origin[12]. The use of renal grafts with multiple renal arteries is quite common clinically, due to the donor shortage[13]. Accessory arteries are usually ligated if the vascularizing area is less than 5%-10% of the kidney. The ligation of the lower pole renal artery should be avoided since it might lead to ureteral ischemia or necrosis. In our case, the lower pole renal artery and the main renal artery trunk were anastomosed with the external iliac artery, after the arteries were reconstructed with aortic patches when the graft was still on ice.
The treatment measures for TRAS include optical medical administration alone, surgical revascularization and percutaneous transluminal angioplasty with or without stenting[14]. As a less invasive approach than surgery, percutaneous transluminal angioplasty with stenting has become the first-line option without the support of very strong evidence[15,16].
The etiology of EPSAs includes perivascular infection, degeneration, defective vascular reconstruction, iatrogenic damage, suture rupture, vessel wall ischemia or anastomotic leakage[6,17]. EPSAs exclusively happen at the anastomotic site and are usually singular, due to defective vascular reconstruction. For donors with multiple renal arteries, even though aortic patches are frequently used to avoid individual reconstruction in deceased donor grafts, the incidence of vascular complications of recipients with multiple renal arteries is still significantly higher than those with single renal artery, 10.8% vs 8.1%[17]. The reason might be the complexity of the anastomosis with a reconstructed renal artery combined with accessory arteries of small diameters. EPSAs are potentially devastating and might lead to graft loss due to rupture[18].
Patients with EPSAs post renal transplantation can be asymptomatic and be incidentally found by ultrasonography or computed tomography[19]. Some patients present with anemia, fever, pulsatile masses, hypertension, renal function impairment, and abdominal pain, and few EPSAs are life-threatening due to acute rupture [17,19-21]. Ultrasonography can be used to recognize most of the lesions, manifesting as simple or complex cysts. Color Doppler can show the intra-cystic blood flow and PWD can detect arterial waveforms[22]. The creatinine during the follow-up at this time was 165.4 μmol/L, which was a little bit higher than normal. However, our patient did not present any symptoms or signs directly caused by EPSA. The EPSA in our case was not visible with the 1-5 MHz convex transducer at first. The plaques and EPSA were not found until the high frequency 3-9 MHz transducer was used, which indicates the importance of combination use of the low and high frequency probes to make an accurate diagnosis. We propose the scanning of the renal allograft with high a frequency linear probe whenever possible.
Even though EPSAs rupture can lead to life-threatening hemorrhage that needs urgent intervention and graft nephrectomy might be needed if the graft is jeopardized. The indications and options for treatment of EPSAs are still subject to debate at present[19]. There is no identified diameter to predict the risk of EPSA rupture. Published reports[19,23] recommend indications for repair as follows: symptomatic EPSAs, presence of infection, large size (diameter larger than 2.5 cm), progressive enlargement and signs of rupture. Treatments for EPSAs include open surgical repair, endovascular approach and ultrasound-guided thrombin injection. In the last decade, the endovascular approach has become more popular for EPSAs[19,24,25], which reduces the risk of rupture by excluding blood flow into the EPSAs with stent placement.
We assume that the cause of EPSA of our case was the high pressure caused by the severe stenosis of the main renal artery and the complexity and weakness of the anastomosis, especially by the former. Due to the difficulty of stenting at the EPSA site, its size, and the cause of EPSA, conservative closely monitoring was considered for the EPSA management. The prognosis turned out that our judgment was correct. Thrombosis filled in the EPSA sac gradually after the high pressure around the anastomosis decreased after the stenosis was relieved. However, this is only a case report, which needs accumulation of more cases.
EPSAs of renal transplantation are life threatening with the risk of rupture and might need graft nephrectomy when allograft function was devastated. We present a patient with an asymptomatic EPSA caused by para-renal artery anastomotic atherosclerotic stenosis, which was spontaneously relieved by removing the cause and close conservative observation. It is still on debate how, when and whether to treat EPSAs. Diagnosis of EPSAs as early as possible is important for a better prognosis, and scanning of the renal allograft with a high frequency linear probe might change the prognosis unexpectedly.
The language was edited by professor David Ari Rubenstein.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali FE S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li X
| 1. | Bruno S, Remuzzi G, Ruggenenti P. Transplant renal artery stenosis. J Am Soc Nephrol. 2004;15:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Ghazanfar A, Tavakoli A, Augustine T, Pararajasingam R, Riad H, Chalmers N. Management of transplant renal artery stenosis and its impact on long-term allograft survival: a single-centre experience. Nephrol Dial Transplant. 2011;26:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Hagen G, Wadström J, Magnusson M, Magnusson A. Outcome after percutaneous transluminal angioplasty of arterial stenosis in renal transplant patients. Acta Radiol. 2009;50:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Ren Y, Xiong F, Kan X, Qian K, Cao Y, Chen L, Xiong B, Zhou G, Zheng C. Endovascular management of transplant renal artery stenosis: A single-center retrospective study. Catheter Cardiovasc Interv. 2020;95:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Sachdeva A, Bhatia N, Paul B, Kumar V. External iliac artery pseudoaneurysm complicating renal transplantation. J Assoc Physicians India. 2013;61:752-753. [PubMed] |
| 6. | Shamsaeefar A, Nikeghbalian S, Kazemi K, Mansorian M, Motazedian N, Malekhosseini SA. Kidney Transplant From a Deceased Donor With Renal Artery Aneurysm: A Case Report. Exp Clin Transplant. 2019;17:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Hurst FP, Abbott KC, Neff RT, Elster EA, Falta EM, Lentine KL, Agodoa LY, Jindal RM. Incidence, predictors and outcomes of transplant renal artery stenosis after kidney transplantation: analysis of USRDS. Am J Nephrol. 2009;30:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Dimitroulis D, Bokos J, Zavos G, Nikiteas N, Karidis NP, Katsaronis P, Kostakis A. Vascular complications in renal transplantation: a single-center experience in 1367 renal transplantations and review of the literature. Transplant Proc. 2009;41:1609-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Seratnahaei A, Shah A, Bodiwala K, Mukherjee D. Management of transplant renal artery stenosis. Angiology. 2011;62:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Kolli KP, LaBerge JM. Interventional Management of Vascular Renal Transplant Complications. Tech Vasc Interv Radiol. 2016;19:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Buturović-Ponikvar J. Renal transplant artery stenosis. Nephrol Dial Transplant. 2003;18 Suppl 5:v74-v77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Taghizadeh Afshari A, Mohammadi Fallah MR, Alizadeh M, Makhdoomi K, Rahimi E, Vossoghian S. Outcome of Kidney Transplantation From Living Donors With Multiple Renal Arteries Versus Single Renal Artery. Iran J Kidney Dis. 2016;10:85-90. [PubMed] |
| 13. | Giovanardi F, Nudo F, Lai Q, Garofalo M, Consolo A, Choppin De Janvry E, Arroyo Murillo GA, Ursi P, Stabile D, Melandro F, Berloco PB, Pretagostini R, Poli L. Surgical Technique Notes of Arterial Vascular Reconstruction During Kidney Transplantation: Personal Experience and Literature Review. Transplant Proc. 2019;51:128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Bull AS, Piovesan AC, Marchini GS, Yamaçake KGR, Antonopoulos IM, Falci R, Kanashiro H, Ebaid G, Carnevale FC, Messi G, Nahas WC. Outcomes of endovascular treatment of renal arterial stenosis in transplanted kidneys. Int Braz J Urol. 2019;45:925-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Valle LGM, Cavalcante RN, Motta-Leal-Filho JM, Affonso BB, Galastri FL, Doher MP, Guimarães-Souza NK, Cavalcanti AKN, Garcia RG, Pacheco-Silva Á, Nasser F. Evaluation of the efficacy and safety of endovascular management for transplant renal artery stenosis. Clinics (Sao Paulo). 2017;72:773-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Li CM, Shang T, Tian L, Zhang HK. Short-Term Outcomes Using a Drug-Coated Balloon for Transplant Renal Artery Stenosis. Ann Transplant. 2018;23:75-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Nguan CY, Luke PP. Renal artery pseudoaneurysm of infectious etiology: a life-threatening complication after renal transplantation. Urology. 2006;68:668-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Edwards JB, Wooster MD, Tanious A, Back MR. Management of Aortoiliac Aneurysms with Atypical Renal Artery Anatomy. Ann Vasc Surg. 2019;54:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Bracale UM, Carbone F, del Guercio L, Viola D, D'Armiento FP, Maurea S, Porcellini M, Bracale G. External iliac artery pseudoaneurysm complicating renal transplantation. Interact Cardiovasc Thorac Surg. 2009;8:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Li Z, Li Y, Jing Z, Feng R. Endovascular Treatment for a True Aneurysm of the Transplant Renal Artery using Noncovered Stent-Assisted Coil Embolization. Ann Vasc Surg 2018; 51: 325.e5-325. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Kountidou CS, Stier K, Niehues SM, Lingnau A, Schostak M, Fuller TF, Lützenberg R. Successful repair of post-transplant mycotic aneurysm of iliac artery with renal graft preservation: a case report. Urology. 2012;80:1151-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Ardelean A, Mandry D, Claudon M. [Vascular complications following renal transplantation: diagnostic evaluation]. J Radiol. 2011;92:343-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Luzzio CC, Waclawik AJ, Gallagher CL, Knechtle SJ. Iliac artery pseudoaneurysm following renal transplantation presenting as lumbosacral plexopathy. Transplantation. 1999;67:1077-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Patil VV, Roytman M, Ames S, Beckerman W, Lookstein RA. Endovascular Repair of Renal Artery Anastomotic Pseudoaneurysm Following Living Donor Kidney Transplant. Cardiovasc Intervent Radiol. 2015;38:1640-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Haijie C, Fubo S, Xiaoying L, Ying Y, Zenghui P. Retrospective evaluation of the endovascular repair of the anastomotic pseudoaneurysm of the transplanted renal artery using the snorkel technique. Vascular. 2020;28:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |