Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3787
Peer-review started: January 30, 2021
First decision: February 25, 2021
Revised: March 2, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: May 26, 2021
Processing time: 101 Days and 0.7 Hours
Diabetic ketoacidosis (DKA) is a serious complication of type 1 diabetes mellitus (T1DM). Very rarely does DKA lead to cerebral edema, and it is even rarer for it to result in cerebral infarction. Bilateral internal carotid artery occlusion (BICAO) is also rare and can cause fatal stroke. Moreover, case reports about acute cerebral infarction throughout both internal carotid arteries with simultaneous BICAO are very scarce. In this study, we present a patient with BICAO, T1DM, hypertension, and hyperlipidemia, who had a catastrophic bilateral cerebral infarction after a DKA episode. We briefly introduce BICAO and the mechanisms by which DKA results in cerebral infarction.
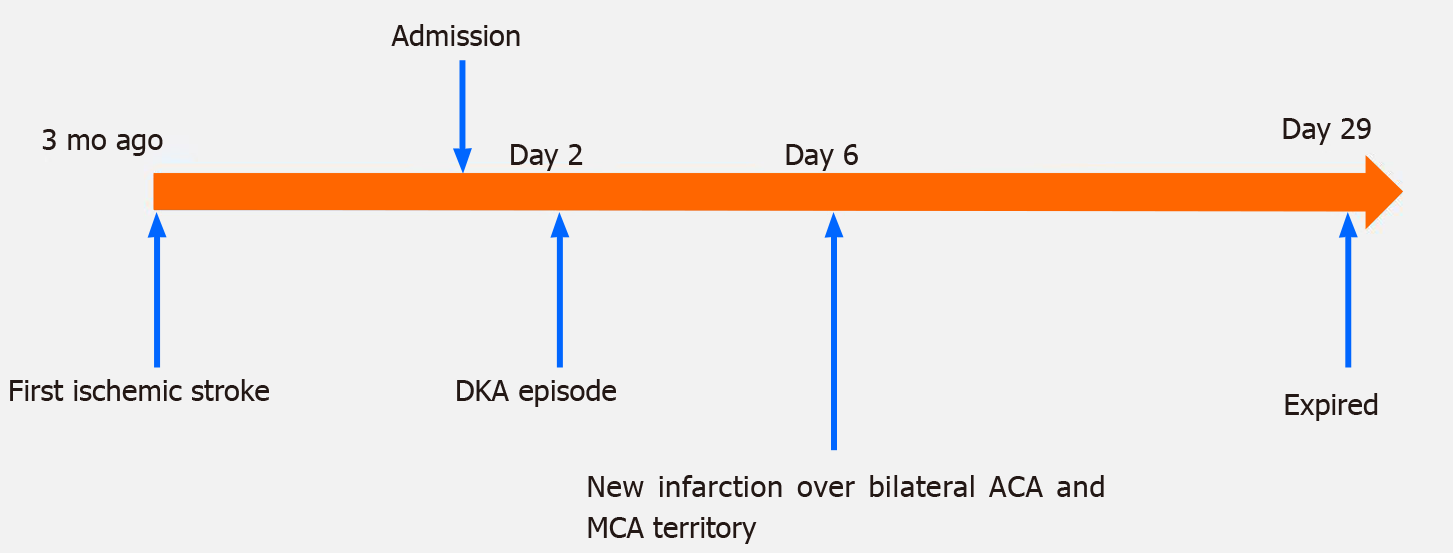
A 41-year-old woman presented with ischemic stroke that took place 3 mo prior over the left corona radiata, bilateral frontal lobe, and parietal lobe with right hemiplegia and Broca’s aphasia. She had a history of hypertension for 5 years, hyperlipidemia for 4 years, hyperthyroidism for 3 years, and T1DM for 31 years. The first brain magnetic resonance imaging not only revealed the aforementioned ischemic lesions but also bilateral internal carotid artery occlusion. She was admitted to our ward for rehabilitation due to prior stroke sequalae. DKA took place on hospital day 2. On hospital day 6, she had a new massive infarction over the bilateral anterior cerebral artery and middle cerebral artery territory. After weeks of aggressive treatment, she remained in a coma and on mechanical ventilation due to respiratory failure. After discussion with her family, compassionate extubation was performed on hospital day 29 and she died.
DKA can lead to cerebral infarction due to several mechanisms. In people with existing BICAO and several stroke risk factors such as hypertension, T1DM, hyperlipidemia, DKA has the potential to cause more serious ischemic strokes.
Core Tip: Diabetic ketoacidosis (DKA) causes vascular endothelial injury, systemic inflammation, vasoconstriction, abnormal coagulation cascade, increased platelet aggregation, and increased blood viscosity, all of which are associated with cerebral infarction. Bilateral internal carotid artery occlusion (BICAO) is a rare condition that can give rise to devastating ischemic strokes. DKA acts as an additional risk factor for ischemic stroke in a high-risk patient with BICAO, hypertension, hyperlipidemia, and type 1 diabetes mellitus.
- Citation: Chen YC, Tsai SJ. Bilateral cerebral infarction in diabetic ketoacidosis and bilateral internal carotid artery occlusion: A case report and review of literature. World J Clin Cases 2021; 9(15): 3787-3795
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3787.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3787
Hypertension[1], hyperlipidemia[2] and diabetes mellitus[3] are risk factors for ischemic stroke. People with diabetes have more than double the risk of ischemic stroke in comparison with individuals without diabetes[3]. The recurrent stroke incidence in people with prior stroke in 5 years has been reported to range from 19% to 32%[4]. Diabetic ketoacidosis (DKA) often occurs in people with type 1 diabetes mellitus (T1DM)[5] whose neurological complications include cerebral edema. However, literature about the link between ischemic stroke and DKA is not very common in adults. Bilateral internal carotid artery occlusion (BICAO) can lead to fatal stroke[6] and only accounts for 0.4% of people with cerebral vascular accident[7]. Moreover, acute cerebral infarction throughout both internal carotid arteries (ICAs) with simultaneous BICAO is also rare[8]. This article presents a 41-year-old woman with T1DM for 31 years, as well as hypertension and hyperlipidemia for several years, and who suffered from BICAO and ischemic stroke over the left corona radiata, bilateral frontal lobe, and parietal lobe 3 mo prior. She had a DKA episode and a massive infarction in the bilateral anterior cerebral artery (ACA) and middle cerebral artery (MCA) territories afterward. We will discuss the mechanisms of DKA and BICAO that may be involved in such massive brain infarction.
A 41-year-old woman was admitted to our ward for rehabilitation due to ischemic stroke 3 mo prior.
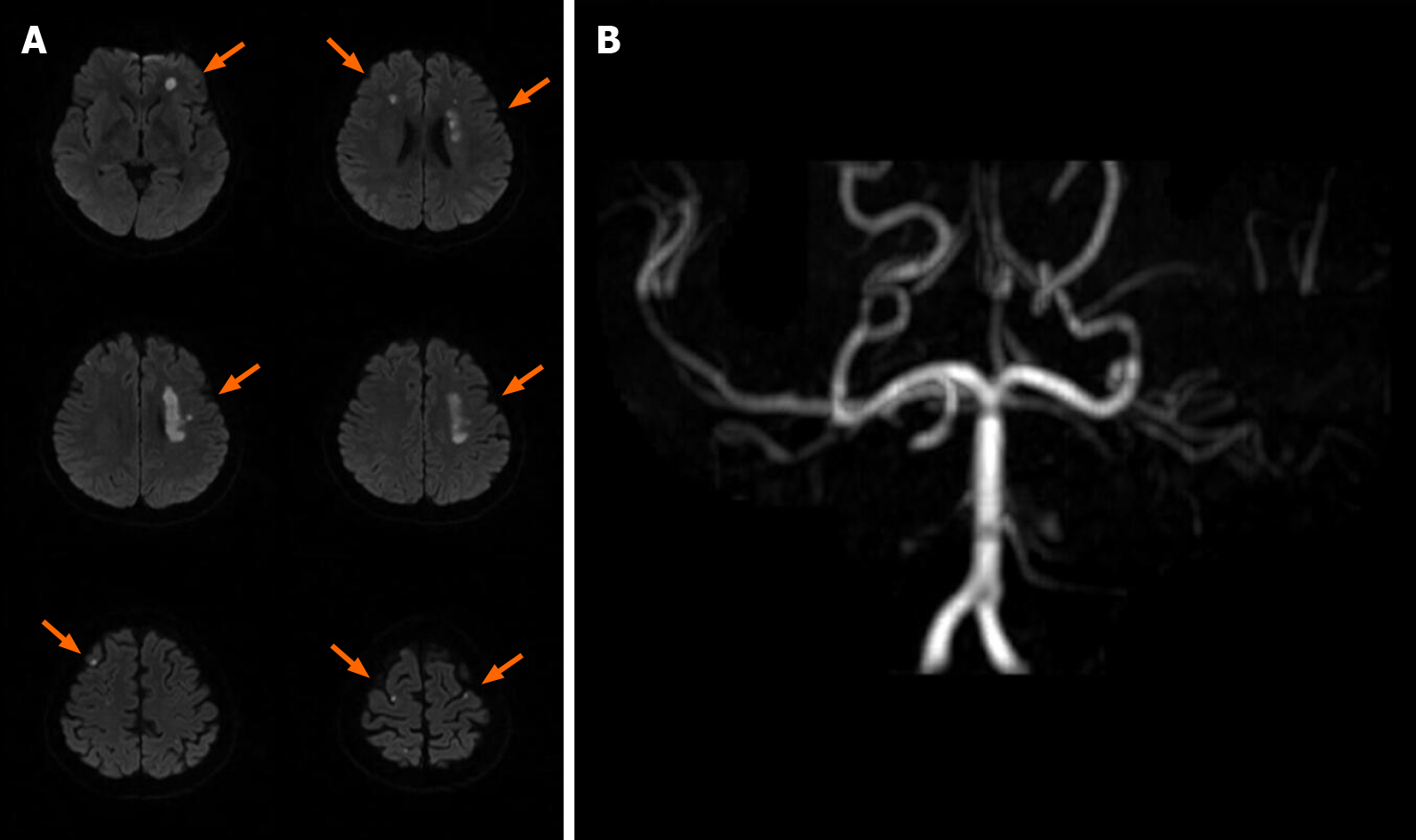
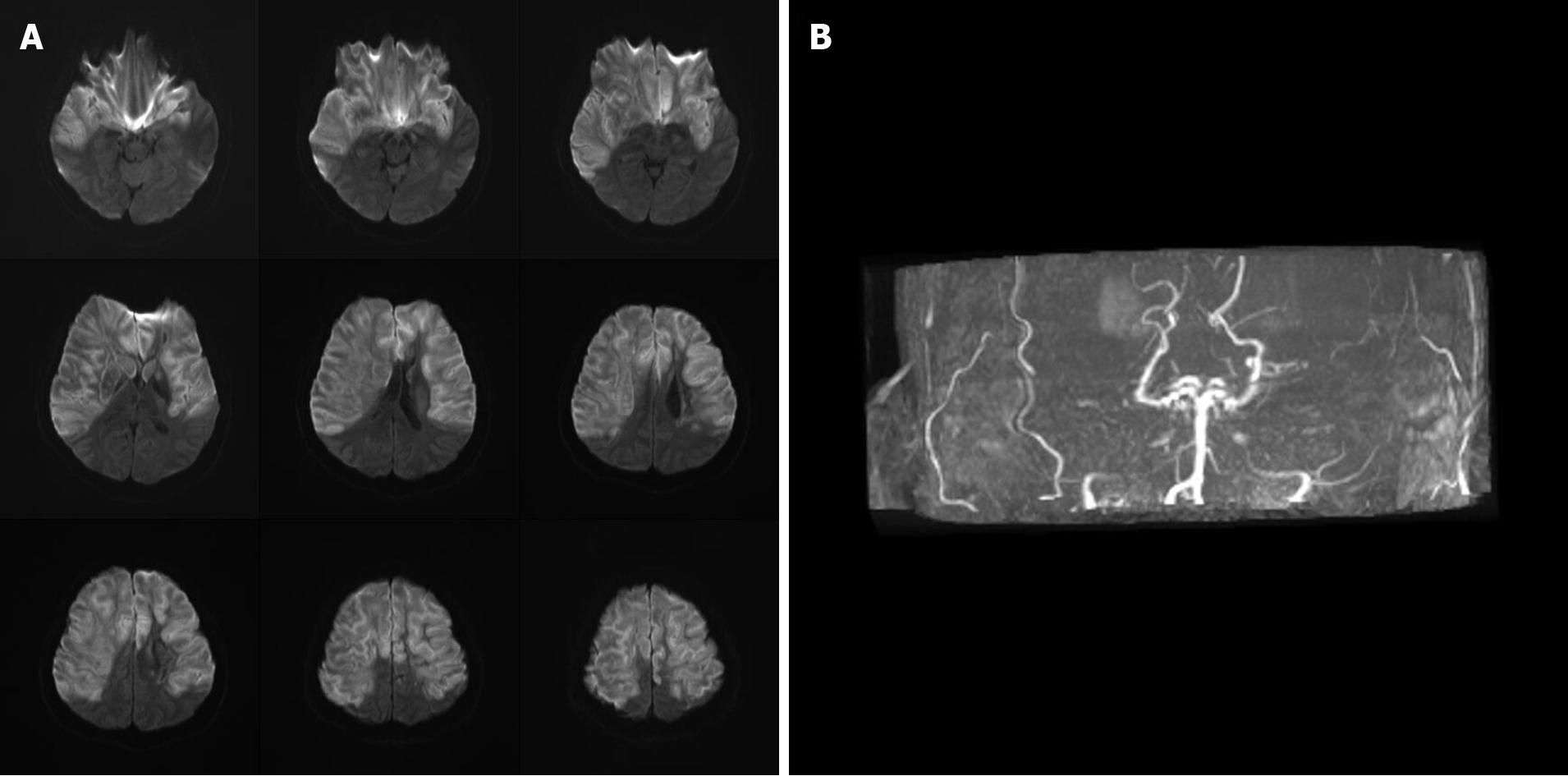
The patient had ischemic stroke over the left corona radiata, bilateral frontal lobe, and parietal lobe 3 mo prior with right hemiplegia and Broca’s aphasia (Figure 1) and received inpatient rehabilitation programs in other hospitals. There were no major accidents such as recurrent stroke, urinary tract infection, or pneumonia during the 3 mo. This time she was transferred to our ward for further rehabilitation. Upon admission, she had significant right hemiplegia but she still could ambulate with use of a quad cane for short distances. Her consciousness was alert but Broca’s aphasia was presented. Her symptoms included dizziness, vomiting, Kussmaul breathing, and lowered response to verbal commands on the morning of hospital day 2. There were no new appearances of limb weakness. Hyperglycemia persisted despite sequential subcutaneous rapid-acting insulin injections. Arterial blood gas analysis showed metabolic acidosis (pH 7.255, PCO2 15.9 mmHg, HCO3- 7.1 mmol/L, base excess -16.8 mmol/L). Urine analysis disclosed ketone 3+. DKA was suspected, and she was transferred to the intensive care unit for further care, which included aggressive hydration, insulin pump, bicarbonate infusion, and electrolyte correction. Serum ketone body on hospital day 3 was 5.5 mmol/L. After DKA, the patient was still conscious and alert, but her arterial blood gas had revealed metabolic acidosis with respiratory compensation from day 2 to day 5 (Table 1). However, on the morning of hospital day 6, she became comatose and hemodynamic instability was also noted. The brain magnetic resonance imaging (MRI) disclosed: (1) Acute infarction lesions over the bilateral MCA and bilateral ACA territory with brain swelling of infarction lesions and midline shift; and (2) Occlusion of bilateral ICA (Figure 2). Glycerol was administered for increased intracranial pressure, and endotracheal intubation was performed. Echocardiograms revealed normal left ventricle contractility and no evidence of thrombus or vegetation formation. Hemodynamic instability, respiratory failure, and severe hyperglycemia persisted. The patient was in a coma from hospital day 6 to day 29, and mechanical ventilation was needed. After discussion with her family about her poor prognosis, compassionate extubation was performed, and she expired on hospital day 29. The timeline of the patient is presented in Figure 3.
| Arterial blood gas | Reference range | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 8 | Day 14 | Day 17 | Day 22 |
| pH | 7.35-7.45 | 7.255 | 7.326 | 7.39 | 7.411 | 7.211 | 7.433 | 7.368 | 7.258 | 7.372 |
| PCO2 (mmHg) | 35-45 | 15.9 | 18.1 | 22.7 | 20.1 | 17.4 | 24.6 | 31 | 51 | 34.4 |
| HCO3 (mmol/L) | 22-26 | 7.1 | 9.5 | 13.9 | 12.9 | 7.0 | 16.6 | 18.0 | 23.0 | 20.2 |
| Base excess (mmol/L) | -2 to +2 | -16.8 | -13.4 | -8.6 | -9.0 | -18.1 | -5.1 | -5.8 | -3.9 | -3.9 |
Three months prior, the patient had an ischemic stroke in the left corona radiata, bilateral frontal lobe, and parietal lobe as well as BICAO (Figure 1). There was no major accident in the 3 mo before this admission. The patient also had T1DM for 31 years, hypertension for 5 years, hyperlipidemia for 4 years, and hyperthyroidism for 3 years. She had regular subcutaneous insulin injections and medical control for the above diseases. The medications being taken were methimazole 5 mg once daily, aspirin 100 mg once daily, and atorvastatin 20 mg once daily.
The patient did not drink or smoke. The patient’s family history was negative.
At admission, the patient's body temperature was 36.1 °C, pulse was 84 beats/min, and blood pressure was 125/78 mmHg. Her breathing was steady, averaging 18 breaths per minute. Neurological examinations showed clear consciousness, right central facial palsy and right hemiplegia. The Brunnstrom stage of her right upper limb and right lower limb were III/III (proximal/distal) and IV, respectively. For functional status, she could ambulate with use of a quad cane for short distances. She was able to feed herself. Broca’s aphasia was noted.
The blood test on hospital day 2 showed a glycohemoglobin level of 7.8%, hyperglycemia (344 mg/dL), and normal thyroid function. Other levels were within normal range. Urine analysis on hospital day 1 showed neither ketonuria nor pyuria. The detailed blood examinations during hospitalization are listed in Table 2. The thyroid function at admission and stroke survey after the second stroke are listed in Table 3. The arterial blood gas analyses during hospitalization are listed in Table 1.
| Biochemical data | Reference range | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 14 | Day 17 | Day 22 |
| WBC (/μL) | 4000-11000 | 8600 | 16600 | 32510 | - | 19890 | 18930 | 13520 | 13870 |
| Neutrophil (%) | 40-75 | 61.2 | 89 | 87 | - | 70.8 | 75 | 81.3 | 59.2 |
| Hemoglobin (g/dL) | 12-16 | 14.3 | 13.5 | - | - | 13.8 | 11.7 | 10.3 | - |
| Platelet (/μL) | 150000-400000 | 250000 | 356000 | - | - | 181000 | - | 472000 | - |
| Sodium (mmol/L) | 136-144 | 134 | 148 | 146 | 180 | 166 | 152 | 154 | - |
| Potassium (mmol/L) | 3.6-5.1 | 4.3 | 3.1 | 3.4 | 3.0 | 3.8 | 4.3 | 3.6 | - |
| Creatinine (mg/dL) | 0.44-1.03 | 0.75 | 0.74 | - | - | 1.59 | 2.94 | 2.49 | 1.55 |
| C-reactive protein (mg/dL) | < 0.748 | 1.345 | - | - | - | - | - | - | - |
| Biochemical data | Reference range | Day 2 | Day 7 |
| Free T4 (ng/dL) | 1.04-1.27 | 1.04 | - |
| TSH (μIU/mL) | 0.38-5.33 | 2.58 | - |
| T3 (ng/dL) | 76-155 | 53.5 | - |
| Complement C3 (mg/dL) | 79-152 | - | 70.9 |
| Complement C4 (mg/dL) | 16-38 | - | 21 |
| ANA (titer) | < 1: 80X | - | Homogeneous type 1: 80X |
| Anti-dsDNA (IU/mL) | < 200 | - | 62.33 |
| ANCA (titer) | Negative (< 1: 40X) | - | Negative |
Brain MRI for the first stroke before this admission revealed left corona radiata, bilateral frontal lobe, and parietal lobe infarction as well as BICAO (Figure 1). Brain MRI for the second stroke disclosed: (1) Acute infarction lesions over the bilateral MCA and bilateral ACA territory with brain swelling of infarction lesions and midline shift; and (2) Occlusion of bilateral ICA (Figure 2).
The final diagnosis of the presented case was: (1) Bilateral MCA and bilateral ACA territory infarction with brain swelling and midline shift; (2) BICAO; and (3) T1DM with DKA.
Aggressive hydration, insulin pumping, electrolyte correction, vasopressors, glycerol, and mechanical ventilation were applied.
On hospital day 6, the patient went into a coma after bilateral MCA and bilateral ACA territory infarction, and mechanical ventilation was necessary due to respiratory failure. On hospital day 29, we discussed about her poor prognosis even with intensive treatment with her family. She expired on hospital day 29 after compassionate extubation.
Strokes occurring in people under the age of 45 are generally called young strokes[9]. Congenital heart disease, hematologic disorders, and metabolic disorders result in young stroke mainly in children and adolescents[10]. In young adults, the common reasons for young stroke are smoking, hypertension, diabetes, and hyper-cholesterolaemia[10]. The formation of advanced glycation end products plays a crucial role in diabetic vascular complications including atherosclerosis[11]. Other causes for young adult stroke include hypercoagulable states, metabolic disorders, vasculopathy, cardiac defects, drug use, and migraines[12].
Carotid stenosis is an important risk factor for ischemic stroke and is majorly attributed to atherosclerosis[13], and it is a slow and progressive process, from intima-medial thickening, carotid plaque, asymptomatic carotid stenosis to symptomatic carotid disease. However, in our patient, the brain MRI of the first stroke had disclosed BICAO. BICAO carries can lead to fatal stroke[6] and only accounts for 0.4% of people with cerebrovascular accident[7]. The clinical course of BICAO is often chronic due to collateral circulation[14]. In BICAO, the basilar artery and ophthalmic artery are the main collateral systems to support the ACA and MCA territory. Moreover, acute cerebral infarction throughout both ICAs with simultaneous bilateral carotid artery occlusion is rarer. Ota et al[8] summarized previous 9 reports regarding simultaneous BICAO with infarction. Only one survived. The speculated pathogeneses of BICAO for them include cardioembolism, atrial myxoma, atherothrombosis and unknown etiology. Treatment options for carotid stenosis include medical management, carotid stenting, and carotid endarterectomy[15]. However, carotid revascularization is not recommended for patients with chronic total occlusion of the carotid artery[16]. To date, clinical data on medical or surgical bypass management in BICAO are limited.
DKA, one of the most severe complications of diabetes, is mostly seen in patients with T1DM. The precipitating factors for DKA are infection, surgery, trauma, myocardial ischemia, pancreatitis, psychological stress, stroke and inadequate insulin treatment or noncompliance[17]. Clinical manifestations include nausea, vomiting, abdominal pain[18], neurological symptoms[19], decreased skin turgor, dry mucosa, and Kussmaul breathing. Laboratory examinations may reveal ketonemia, ketonuria, hyperglycemia, and anion gap metabolic acidosis. The complications of DKA include cerebral edema[20] and noncardiogenic pulmonary edema[20].
Stroke, a form of acute stress, is a precipitating factor for DKA[21]. However, DKA rarely causes cerebral infarction and occurs more frequently in children and young adolescents than in adults[22]. It has been demonstrated that hyperglycemia and acidosis produce oxidative stress and cause tissue ischemia[23]. An increase in oxidative stress brings about diffuse vascular injury[24]. DKA results in significant fluid loss and decreased velocity of blood flow[25]. Hyperglycemia and acidosis induce red blood cell rigidity (increased blood viscosity)[26,27]. The vascular response in hyperglycemia has generally been considered vasoconstrictive, which may be related to decreased availability of nitric oxide[28]. The probable reasons for thrombosis formation by DKA are decreased levels of protein C and protein S activity and increased von Willebrand factor[29]. DKA also elevates C-reactive protein (CRP) and cytokines (IL-6, IL-1β, TNF-α) and activates complements[30-34]. Activation of platelet and coagulation systems are also present in uncomplicated DKA[35]. In short, mechanisms of DKA that can lead to cerebral infarction include systemic inflammation, increased oxidative stress, abnormal coagulation cascade, platelet dysfunction, fluid loss, and increased blood viscosity.
The precipitating factors for DKA were mentioned previously. In our patient, there was no significant leukocytosis or elevation of CRP in the initial stage of DKA. There was also no pyuria in urinary analysis at admission. Furthermore, she had no pneumonia, trauma, myocardial infarction, and pancreatitis. We treated her with the same dosage of subcutaneous insulin as her usual. However, we noticed her hyperglycemia during hospital day 1 to day 2. Insufficient insulin dose might be the predisposing factor for this DKA. The timing of her change in consciousness was after 4 d of DKA. Before this, she was conscious and alert. Thus, we excluded the possibility that the second stroke was prior to DKA. The blood examination on hospital day 4 showed white blood cell (WBC): 16600/μL, segment: 89%, no bandemia. The arterial blood gas analysis had revealed metabolic acidosis with respiratory compensation from hospital day 2 (DKA onset) to hospital day 6 (secondary stroke onset) despite aggressive treatment. From admission to hospital day 6, she had no signs or symptoms of potential infection such as bacteremia, urinary tract infection, gastroenteritis, or pneumonia. It is noticed that patients with DKA often present with leukocytosis without infection, and may be related to ketonemia, increased catecholamine and hypercortisolemia[36]. Nonetheless, it is seldom to see that WBC > 25000 mm3 or the presence of > 10% neutrophil bands in the absence of bacterial infection[36]. Therefore, sepsis with persistent metabolic acidosis was considered to a lesser extent.
We speculate that the patient developed BICAO due to long-term vasculopathy of T1DM, hypertension, and hyperlipidemia. Computed tomography angiogram and magnetic resonance angiography showed no moyamoya disease. Thus, the diagnosis was unlikely to be congenital carotid stenosis. Because of the secondary stroke, we carefully reviewed our patient and her electrocardiogram was sinus rhythm. Echocardiography showed no vegetations. Embolic stroke was less likely. Clinical data did not demonstrate any drop in blood pressure to suggest hypoperfusion before the second stroke. Our stroke survey disclosed no significantly elevated antinuclear antibody, antineutrophil autoantibodies, or anti-dsDNA titers, therefore autoimmune-related stroke was not favored. Because of BICAO, her perfusion in the ACA and MCA territories was frail and relied on collateral circulations. However, she might survive for a long time even with BICAO because the clinical course of BICAO is often chronic due to collateral circulation[14]. We assumed that DKA for several days, with effects of diffuse vascular injury, decreased velocity of blood flow, hyperglycemic vaso-constriction, abnormal coagulation cascade, and platelet dysfunction, acted as an additional risk factor for a high-risk patient with T1DM, BICAO, prior stroke, hypertension, and hyperlipidemia to develop catastrophically bilateral ACA and MCA territory infarctions.
The prognosis of bilateral ACA and MCA territories infarction is poor. For type 1 diabetic patients, diet control and good glycemic control are fundamental to prevent DKA, diabetic vasculopathy, and strokes. Hypertension and hyperlipidemia should be controlled well. Additionally, regular aerobic exercise can benefit type 1 diabetic patients by improving cardiopulmonary fitness and endothelial function, as well as decreasing insulin dose and the risk of cardiovascular events[37].
DKA, which is a complication of T1DM, plays an important role in cerebral infarction by increasing oxidative stress, diffuse endothelial injury, decreasing velocity of blood flow, abnormal coagulation cascade, and platelet dysfunction. BICAO is rare and its outcome is grave. For this patient, long-term T1DM, hypertension, hyperlipidemia, BICAO and this DKA episode contributed to fatal bilateral ACA and MCA infarction. Diet control, glycemic control, and regular aerobic exercise for type 1 diabetic patients are important to prevent diabetic complications.
Manuscript source: Unsolicited manuscript
Specialty type: Neurosciences
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Perez MM S-Editor: Zhang H L-Editor: A P-Editor: Liu JH
| 1. | O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, Mondo C, Diener HC, Ryglewicz D, Czlonkowska A, Pogosova N, Weimar C, Iqbal R, Diaz R, Yusoff K, Yusufali A, Oguz A, Wang X, Penaherrera E, Lanas F, Ogah OS, Ogunniyi A, Iversen HK, Malaga G, Rumboldt Z, Oveisgharan S, Al Hussain F, Magazi D, Nilanont Y, Ferguson J, Pare G, Yusuf S; INTERSTROKE investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1081] [Cited by in RCA: 1406] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
| 2. | Yaghi S, Elkind MS. Lipids and Cerebrovascular Disease: Research and Practice. Stroke. 2015;46:3322-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012;11:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 335] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 4. | Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42:1489-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 471] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 5. | Wolfsdorf J, Glaser N, Sperling MA; American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Amin OS. Bilateral atherosclerotic internal carotid artery occlusion and recurrent ischaemic stroke. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Mead GE, Wardlaw JM, Lewis SC, Dennis MS; Lothian Stroke Registry Study Group. No evidence that severity of stroke in internal carotid occlusion is related to collateral arteries. J Neurol Neurosurg Psychiatry. 2006;77:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Ota K, Matsubara N, Takahashi I, Imaoka E, Harada H, Kataoka H, Makino K, Kano T. A Case of Acute Simultaneous Bilateral Internal Carotid artery Occlusion Treated by Thrombectomy. J Neuroendovas Thera. 2018;12:386-392. [DOI] [Full Text] |
| 9. | Griffiths D, Sturm J. Epidemiology and etiology of young stroke. Stroke Res Treat. 2011;2011:209370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Ji R, Schwamm LH, Pervez MA, Singhal AB. Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA Neurol. 2013;70:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K. Mechanisms of diabetic vasculopathy: an overview. Am J Hypertens. 2001;14:475-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 550] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 13. | Flaherty ML, Kissela B, Khoury JC, Alwell K, Moomaw CJ, Woo D, Khatri P, Ferioli S, Adeoye O, Broderick JP, Kleindorfer D. Carotid artery stenosis as a cause of stroke. Neuroepidemiology. 2013;40:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 14. | Takahashi K, Matsui R, Yamagata S, Kobayashi S. Cerebral infarction throughout both internal carotid arteries detected by diffusion-weighted MRI. Stroke. 2001;32:817-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 2971] [Article Influence: 270.1] [Reference Citation Analysis (0)] |
| 16. |
Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Stroke Assocation; American Association of Neuroscience Nurses; American Association of Neurological Surgeons; American College of Radiology; American Society of Neuroradiology; Congress of Neurolgocial Surgeons; Society of Atherosclerosis Imaging and Prevention; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of NeuroInterventional Surgery; Society for Vascular Medicine; Society for Vascular Surgery; American Academy of Neurology and Society of Cardiovascular Computed Tomography. |
| 17. | Umpierrez GE, Kitabchi AE. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. 2003;2:95-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Malone ML, Gennis V, Goodwin JS. Characteristics of diabetic ketoacidosis in older versus younger adults. J Am Geriatr Soc. 1992;40:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 19. | Nyenwe EA, Razavi LN, Kitabchi AE, Khan AN, Wan JY. Acidosis: the prime determinant of depressed sensorium in diabetic ketoacidosis. Diabetes Care. 2010;33:1837-1839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Sprung CL, Rackow EC, Fein IA. Pulmonary edema; a complication of diabetic ketoacidosis. Chest. 1980;77:687-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Misra S, Oliver NS. Diabetic ketoacidosis in adults. BMJ. 2015;351:h5660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Burzynski J. DKA and thrombosis. CMAJ. 2005;173:132; author reply 132-132; author reply 133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Lin JJ, Lin KL, Wang HS, Wong AM, Hsia SH. Occult infarct with acute hemorrhagic stroke in juvenile diabetic ketoacidosis. Brain Dev. 2008;30:91-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Jain SK, McVie R, Bocchini JA Jr. Hyperketonemia (ketosis), oxidative stress and type 1 diabetes. Pathophysiology. 2006;13:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Glaser NS, Wootton-Gorges SL, Marcin JP, Buonocore MH, Dicarlo J, Neely EK, Barnes P, Bottomly J, Kuppermann N. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr. 2004;145:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Evan-Wong LA, Davidson RJ, Stowers JM. Alterations in erythrocytes in hyperosmolar diabetic decompensation: a pathophysiological basis for impaired blood flow and for an improved design of fluid therapy. Diabetologia. 1985;28:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Chang HY, Li X, Karniadakis GE. Modeling of Biomechanics and Biorheology of Red Blood Cells in Type 2 Diabetes Mellitus. Biophys J. 2017;113:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Giugliano D, Marfella R, Coppola L, Verrazzo G, Acampora R, Giunta R, Nappo F, Lucarelli C, D'Onofrio F. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation. 1997;95:1783-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 232] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Carl GF, Hoffman WH, Passmore GG, Truemper EJ, Lightsey AL, Cornwell PE, Jonah MH. Diabetic ketoacidosis promotes a prothrombotic state. Endocr Res. 2003;29:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Hoffman WH, Burek CL, Waller JL, Fisher LE, Khichi M, Mellick LB. Cytokine response to diabetic ketoacidosis and its treatment. Clin Immunol. 2003;108:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 31. | Schalkwijk CG, Poland DC, van Dijk W, Kok A, Emeis JJ, Dräger AM, Doni A, van Hinsbergh VW, Stehouwer CD. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 1999;42:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 212] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Ma SG, Jin Y, Xu W, Hu W, Bai F, Wu XJ. Increased serum levels of ischemia-modified albumin and C-reactive protein in type 1 diabetes patients with ketoacidosis. Endocrine. 2012;42:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Ceriello A, Giugliano D, Quatraro A, Marchi E, Barbanti M, Lefebvre P. Possible role for increased C4b-binding-protein level in acquired protein S deficiency in type I diabetes. Diabetes. 1990;39:447-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Hoffman WH, Cudrici CD, Zafranskaia E, Rus H. Complement activation in diabetic ketoacidosis brains. Exp Mol Pathol. 2006;80:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Ileri NS, Büyükaşik Y, Karaahmetoğlu S, Ozatli D, Sayinalp N, Ozcebe OI, Kirazli S, Müftüoğlu O, Dündar SV. Evaluation of the haemostatic system during ketoacidotic deterioration of diabetes mellitus. Haemostasis. 1999;29:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1090] [Cited by in RCA: 1188] [Article Influence: 74.3] [Reference Citation Analysis (3)] |
| 37. | Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? Diabetologia. 2012;55:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 288] [Article Influence: 22.2] [Reference Citation Analysis (0)] |