Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3741
Peer-review started: January 8, 2021
First decision: February 12, 2021
Revised: February 24, 2021
Accepted: March 25, 2021
Article in press: March 25, 2021
Published online: May 26, 2021
Processing time: 123 Days and 9 Hours
Alopecia areata (AA) is a common autoimmune disease characterized by hair loss. AA appears in extensive forms, such as progressive and diffusing hair loss (diffuse AA), a total loss of scalp hair (alopecia totalis), and complete loss of hair over the entire body (alopecia universalis). Recently, mesenchymal stem cells (MSCs) have been identified as a therapeutic alternative for autoimmune diseases. For this reason, preclinical and case studies of AA and related diseases using MSCs have been conducted.
Case 1: A 55-year-old woman suffered from AA in two areas of the scalp. She was given 15 rounds of minimally manipulated umbilical cord-MSCs (MM-UC-MSCs) over 6 mo. The AA gradually improved 3 mo after the first round. The patient was cured, and AA did not recur. Case 2: A 30-year-old woman, with history of local steroid hormone injections, suffered from AA in one area on the scalp. She was given two rounds of MM-UC-MSCs over 1 mo. The AA immediately improved after the first round. The patient was cured, and AA did not recur. Case 3: A 20-year-old woman, who was diagnosed with alopecia universalis at the age of 12, was given 14 rounds of MM-UC-MSCs over 12 mo. Her hair began to grow about 3 mo after the first round. The patient was cured, and alopecia universalis did not recur.
MM-UC-MSC transplantation potentially treats patients who suffer from AA and related diseases.
Core Tip: Previous studies demonstrated that transplantation of mesenchymal stem cells (MSCs) was effective in treating autoimmune diseases. Alopecia areata (AA) and its related diseases are a representative autoimmune disease. In this case study, we used allogenic, minimally manipulated umbilical cord MSCs for the successful treatment of AA and alopecia universalis. This is the first report of using minimally manipulated umbilical cord MSCs to treat AA and related diseases.
- Citation: Ahn H, Lee SY, Jung WJ, Lee KH. Alopecia treatment using minimally manipulated human umbilical cord-derived mesenchymal stem cells: Three case reports and review of literature. World J Clin Cases 2021; 9(15): 3741-3751
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3741.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3741
Alopecia areata (AA) is an autoimmune disease characterized by varying degrees of hair loss in individuals. It affects more than 6.5 million people in the United States, has a worldwide prevalence of 0.1%-0.2%, and a lifetime risk of 2% in the general population[1-5]. There are three major forms of AA progression: Diffused loss of hair in well-defined patches (diffuse AA), total loss of hair from the scalp (alopecia totalis, AT), and complete loss of body hair (alopecia universalis, AU)[5-7]. Patients with AA suffer from extensive hair loss that can have a devastating impact on their quality of life[8]. Nonetheless, the treatment options for AA are limited, with a poor prognosis. Therapy for hair loss includes the use of glucocorticoids, minoxidil-based drugs, or hair transplantation, all of which present varying side effects[5-7]. Therefore, these options are not usually recommended for patients with diffuse AA, AT, or AU.
Advances in the understanding of AA have led to the development of new treatments. Janus kinase inhibitors as a treatment for AA have been studied since their efficacy for AA in mice was confirmed in 2014[9-13]. Platelet-rich plasma therapies have varied efficacy in the treatment of AA[13-15]. The results of in vitro and in vivo animal tests have shown that mesenchymal stem cell (MSC) transplantation is a powerful alternative to traditional AA treatment[16-20]. The function of MSCs as an immunomodulator has made it a promising potential treatment for AA and related diseases[21-24]. However, because only a limited number of cases for treatment of AA with MSCs have been reported, the effectiveness is not well known[16,25-27]. Therefore, there is a need for sufficient case studies of AA treatment using MSCs.
Minimally manipulated umbilical cord derived-MSCs (MM-UC-MSCs) (used in this study) are isolated from the umbilical cord, immediately frozen, and stored at -197 °C without any other manipulations including cell culture. Minimally manipulated-MSCs (MM-MSCs) have better proliferation and differentiation capacities than cultured MSCs. Therefore, their therapeutic effect is expected to be better[28-30]. Moreover, it is easy to obtain enough cells for transplantation because the umbilical cord has more cells that can be acquired than adipose tissue or bone marrow[31]. It is generally known that MSCs are safe in vivo and do not form tumors[32-34]. Also, long-term culturing of MSCs in vitro has the potential to cause age-related modifications and genetic mutations, and MM-MSCs are safer than manipulated MSCs[35-39]. Based on these previous results, we decided to use MM-UC-MSCs to treating AA and related diseases. The safety and efficacy of MM-UC-MSCs for the treatment of AA and AU will be shown through this case study.
Case 1: On May 29, 2016, a 55-year-old woman, suffering from AA, visited our clinic. She had two areas of complete baldness on the left and right sides of the scalp.
Case 2: On March 19, 2018, a 30-year-old woman, suffering from AA, visited our clinic. She had one area of complete baldness on the top of the scalp.
Case 3: On February 12, 2014, a 20-year-old woman, suffering from AU, visited our clinic. She had very little hair on her body.
Case 1: AA occurred in May 2016 on the two areas of the scalp (left and right).
Case 2: The patient developed AA in six areas on the scalp, with an onset of April 2017. She was given local steroid hormone injections at another hospital for 1 year. As a result, five of the six lesions were cured. However, a lesion at the top of scalp was unaffected by treatment.
Case 3: The patient was diagnosed with AU at the age of 12. After the onset, various medications were prescribed and hair transplantation was performed, both of which were unsuccessful.
All patients had no history of specific illnesses.
All patients had a free personal and family history.
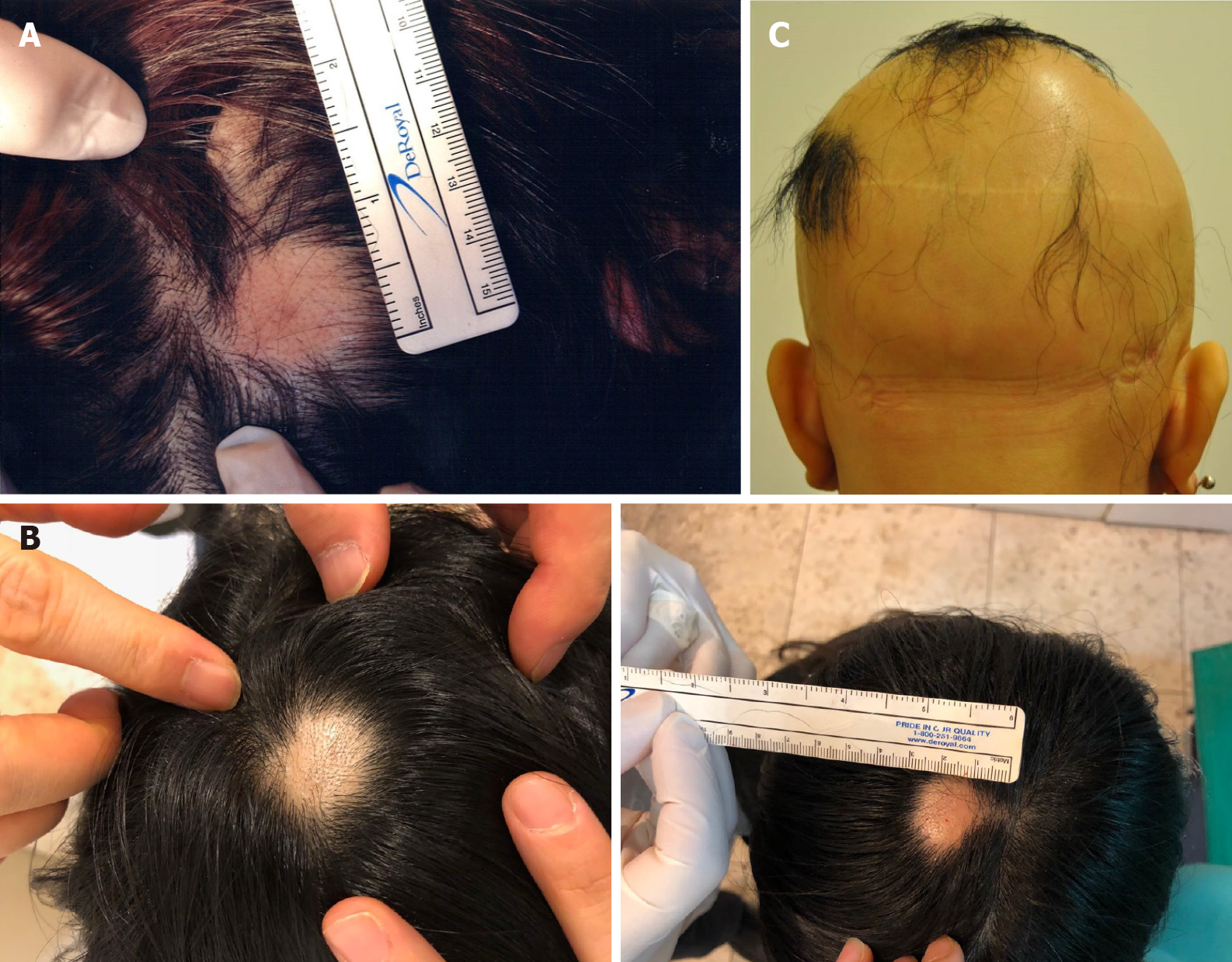
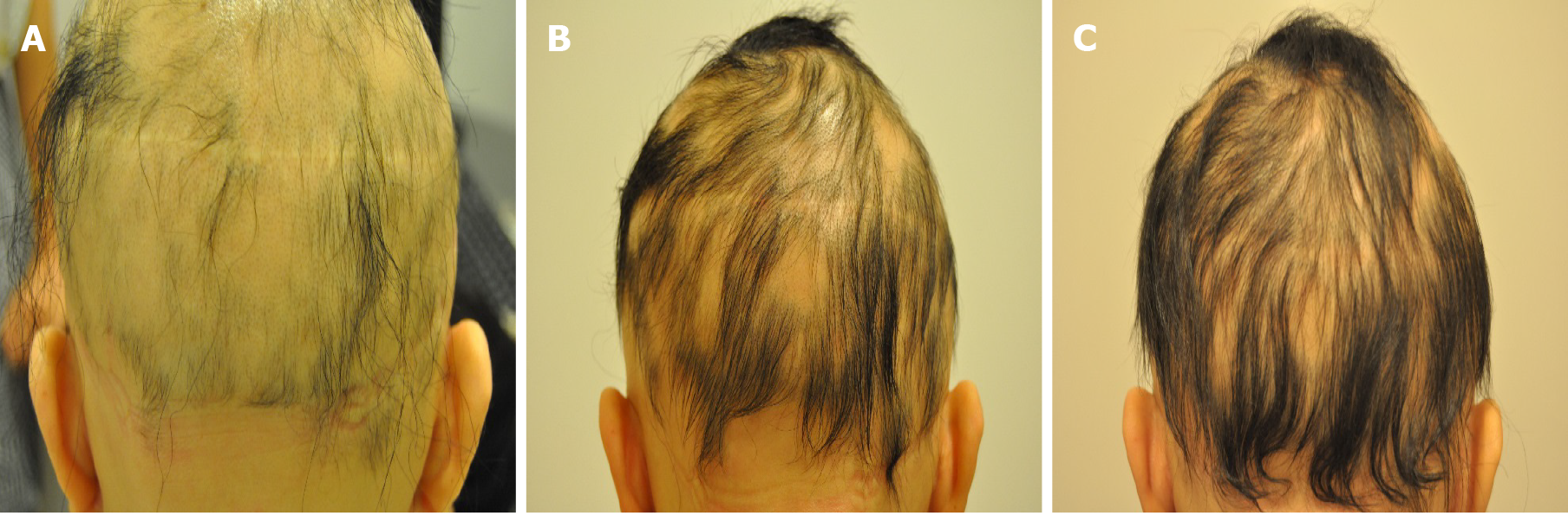
Case 1: The patient presented with two 2.0 cm × 1.0 cm lesions of AA on the left and right sides of the scalp (Figure 1A).
Case 2: The patient presented with a 1.5 cm × 1.5 cm lesion of AA on the top of the scalp (Figure 1B).
Case 3: The patient had little hair on her scalp (Figure 1C).
Laboratory examinations were not performed.
Imaging examinations were not performed.
Cases 1 and 2: The patients were diagnosed with AA based on the formation of a round or oval-shaped area of baldness.
Case 3: The patient was diagnosed with AU because she had little hair on her body.
Umbilical cords were donated by the Obstetrics and Gynecology Department at Lynn Woman’s Hospital, after disinfection. An agreement for an umbilical cord donation was obtained from the mother. We performed a total of seven blood and urine tests from the mother, including for hepatitis B surface antigen, hepatitis B surface antibody, hepatitis C antigen, hepatitis C antibody, human immunodeficiency virus, syphilis rapid plasma reagin, and human T-cell lymphotropic virus type I and II antibody, to confirm the safety of the donated umbilical cord.
The donated umbilical cords were 20-30 cm in length, and (2.5-7.0) × 108 cells were obtained from each umbilical cord[40]. In the process of isolating cells from the umbilical cord, the amnion and three blood vessels of the donated umbilical cord were removed. Next, the umbilical cord tissue was cut using operating scissors and ground using a disposable tissue grinder. Then, the ground tissue was treated with an enzyme mixture of collagenase and hyaluronidase and placed in a 37 °C, 50 mL/L CO2 incubator for 0.5-1.0 h. After that, the solutions were filtered through a 100 μm cell strainer and centrifuged to obtain cells. The cells were immediately frozen and stored at -197 °C.
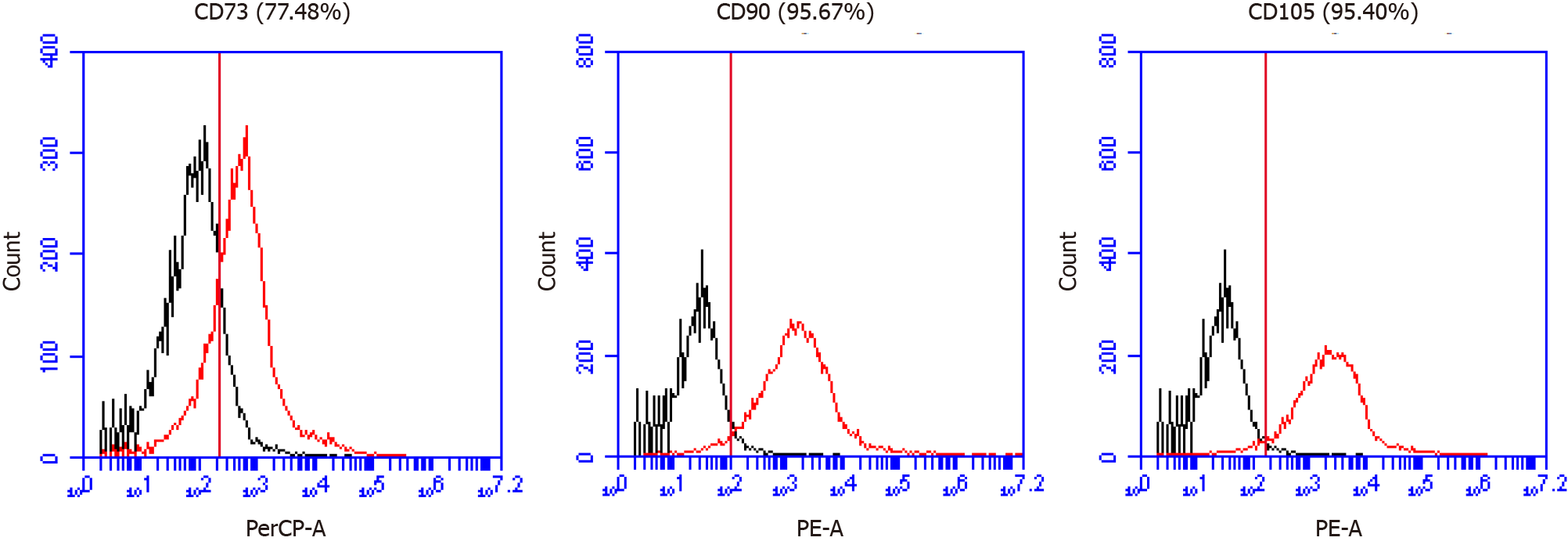
We confirmed the expression of MSC markers such as CD73, CD90, and CD105 from MM-UC-MSCs using a using a CyFlow® Cube 6 (Sysmex) and FCS Express 5 software (Figure 2). We confirmed the isolated MM-UC-MSCs expressed MSC markers CD73 (77.48%), CD90 (95.67%), and CD105 (95.40%). We determined that the isolated MM-UC-MSCs were of the same quality as our previous results because the MM-UC-MSCs isolated by the same method uniformly expressed CD73 (70%-80%), CD90 (90%-100%), and CD105 (90%-100%). The cells were assessed using a sterility test, mycoplasma test, endotoxin test, and testing for adventitious agents of biological products, according to regulations from the Ministry of Food and Drug Safety in the Republic of Korea (data not shown).
After thawing the frozen MM-UC-MSCs, the cells were obtained by centrifugation. The cells were resuspended in 0.9% physiological saline at a concentration of 1 × 106 cells/mL. Then, 1 mL of the solution was filled into an Ultra-FineTM II Insulin Syringe (BD Biosciences, Franklin Lakes, NJ, United States).
Case 1: The injection solution containing MM-UC-MSCs was injected in and around each lesion site at 1 cm intervals. Each injection site received 0.25 mL of the solution for a total of 1 mL at each AA lesion. The patient received a total of 15 treatments over a period of 6 mo (Table 1).
| Treatment number | Date | Concentration of injection solution | Volume of injection solution | Number of lesion sites |
| 1 | May 29, 2016 | 1 × 106 cells/mL | 1 mL/lesion site | 2 (right, left) |
| 2 | June 7, 2016 | |||
| 3 | June 22, 2016 | |||
| 4 | June 30, 2016 | |||
| 5 | July 8, 2016 | |||
| 6 | July 20, 2016 | |||
| 7 | July 27, 2016 | |||
| 8 | August 3, 2016 | |||
| 9 | August 16, 2016 | |||
| 10 | August 29, 2016 | |||
| 11 | September 15, 2016 | |||
| 12 | October 11, 2016 | |||
| 13 | October 26, 2016 | |||
| 14 | November 8, 2016 | |||
| 15 | November 22, 2016 |
Case 2: The injection solution containing MM-UC-MSCs was injected in and around the lesion site at 1 cm intervals. Each injection site received 0.25 mL of the solution for a total of 1 mL at each AA lesion. The patient received a total of two treatments over a period of 1 mo (Table 2).
| Treatment number | Date | Concentration of injection solution | Volume of injection solution | Number of lesion sites |
| 1 | March 19, 2018 | 1 × 106 cells/mL | 1 mL/lesion site | 1 |
| 2 | April 12, 2018 |
Case 3: The injection solution containing MM-UC-MSCs was injected into the entire scalp at 2-3 cm intervals. Each injection site received 0.25 mL of the solution. The patient received a total of fourteen treatments over a period of 12 mo (Table 3).
| Treatment number | Date | Concentration of injection solution | Volume of injection solution |
| 1 | February 12, 2014 | 1 × 106 cells/mL | 6 mL |
| 2 | February 26, 2014 | 10 mL | |
| 3 | March 12, 2014 | 10 mL | |
| 4 | March 28, 2014 | 12 mL | |
| 5 | April 14, 2014 | 12 mL | |
| 6 | June 23, 2014 | 12 mL | |
| 7 | July 21, 2014 | 36 mL | |
| 8 | August 6, 2014 | 12 mL | |
| 9 | September 2, 2014 | 12 mL | |
| 10 | September 25, 2014 | 12 mL | |
| 11 | October 10, 2014 | 12 mL | |
| 12 | November 5, 2014 | 12 mL | |
| 13 | January 16, 2015 | 24 mL | |
| 14 | February 10, 2015 | 12 mL |
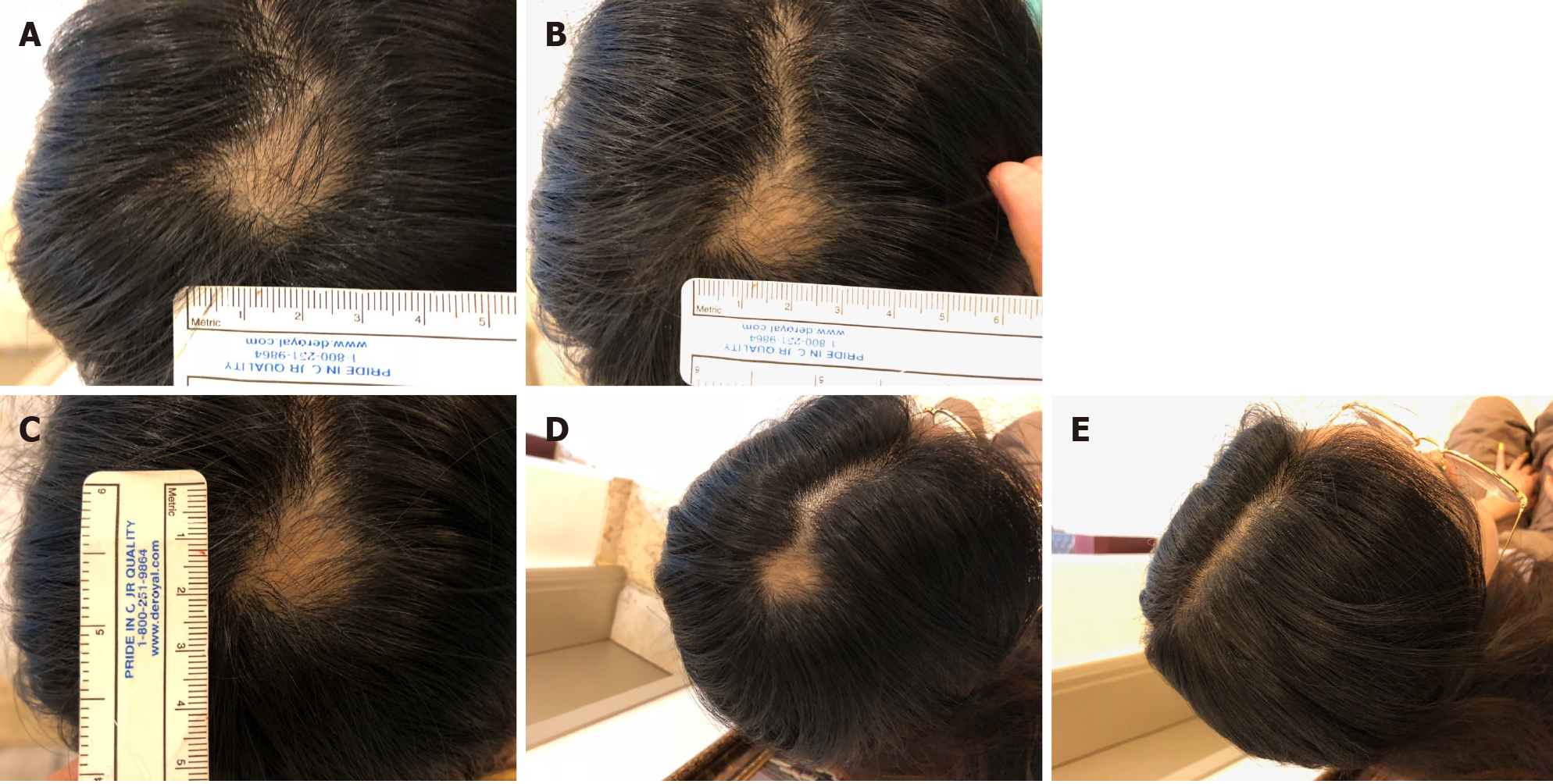
No visible change was observed until 3 mo after the first treatment (Figure 3A and B). Approximately 5 mo after the first treatment, hair grew to cover the lesion site (Figure 3C). Twenty-two months after the final treatment, there was no recurrence of AA (Figure 3D). Four years after treatment, the patient’s mean hair densities at the previous AA lesion sites were 169.33 hairs/cm2 (right lesion) and 168.00 hairs/cm2 (left lesion) (Table 4).
| Lesion | No. | Hair density (hairs/cm2) | Mean (hairs/cm2) |
| Right side | 1 | 169 | 169.33 ± 1.53 |
| 2 | 168 | ||
| 3 | 171 | ||
| Left side | 1 | 165 | 168.00 ± 2.65 |
| 2 | 169 | ||
| 3 | 170 |
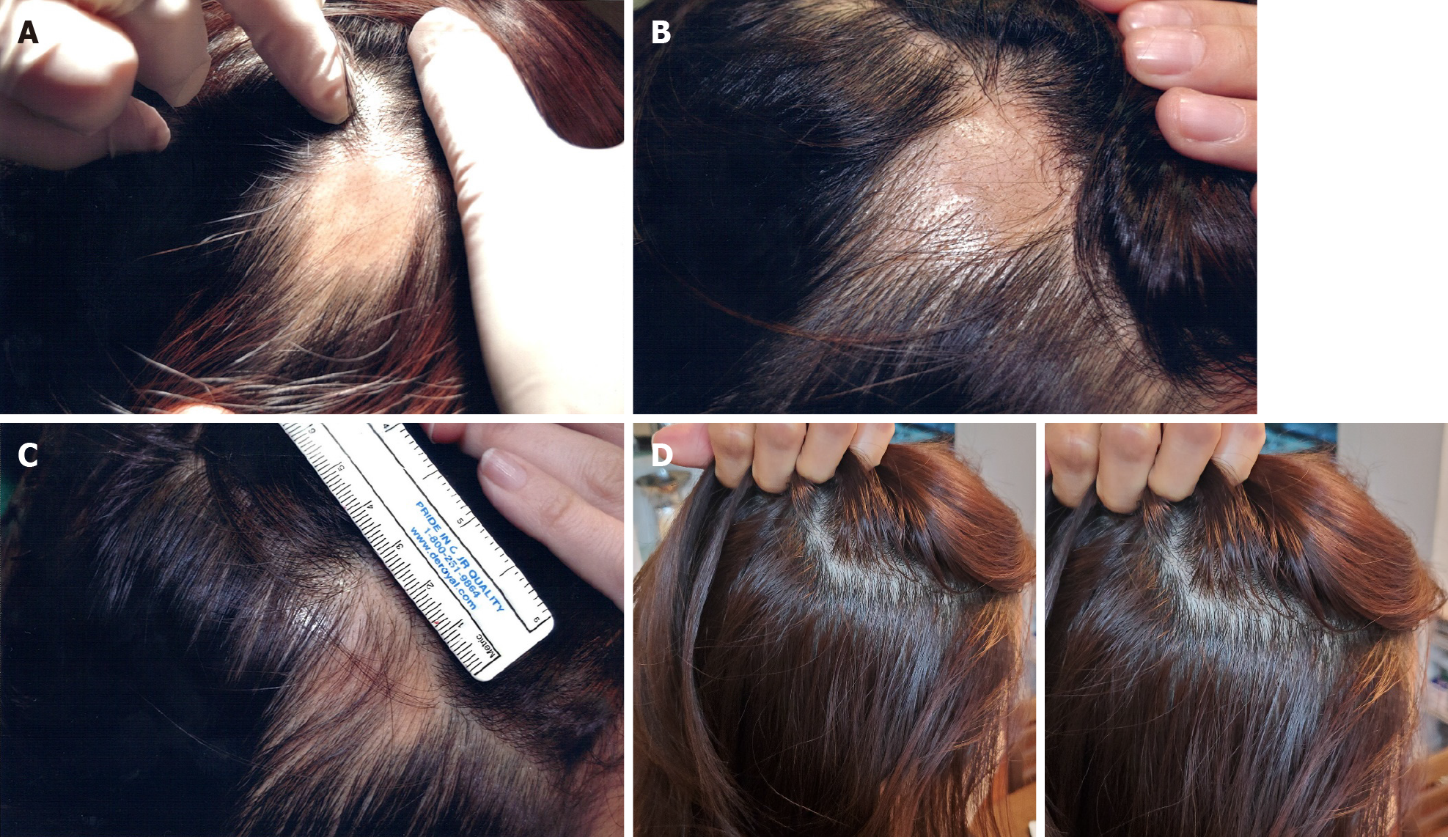
The lesion area decreased after the first transplantation, and AA was completely cured approximately 3 mo after the second transplantation (Figure 4). There was no recurrence of AA in the patient for 2 years after the final transplant. Three years after the final transplant, the patient’s mean hair density at the previous AA lesion site was 162.00 hairs/cm2 (Table 5).
| Lesion | No. | Hair density (hairs/cm2) | Mean (hairs/cm2) |
| Top of scalp | 1 | 163 | 162.00 ± 2.65 |
| 2 | 159 | ||
| 3 | 164 |
The patient’s hair began to grow after approximately 3 mo after the first transplant (Figure 5). Three months after the final transplantation, the patient stopped wearing a wig. The condition was maintained at the 1-year follow-up (data not shown).
During the treatment and follow-up duration, no adverse reactions related to dermatitis were observed or reported by the patients. The patients experienced no symptoms such as fever, chill, or nausea that can frequently appear in patients who receive MSC transplantation. No other side effects were reported by the patients.
AA is a well-known autoimmune disease that can have a devastating impact on a patient’s quality of life[5,8]. Currently, various drug treatments have been attempted. However, they only slowed down the progression of the disease, with limited benefit to the patient[5-7]. Hair transplantation has little effect in cases of diffuse AA, AT, or AU. Therefore, new treatment strategies for AA and related diseases are needed. Various studies have been attempted for the treatment of AA. Among them is the use of MSCs, which has had remarkable success[18,25,26]. MSCs are efficacious in the treatment of autoimmune diseases because they have anti-inflammatory and immunomodulatory properties[21-23]. In addition, MSC treatment is suitable as a new alternative treatment method for AA because MSCs promote hair growth and strengthen the hair[18]. Minimally manipulated MSCs are known to preserve the characteristics of MSCs compared to cultured MSCs[29,30,38,39]. Based on these results, we transplanted MM-UC-MSCs to treat AA in 2 patients and AU in 1 patient.
In the cases of 1 and 2, it was observed that AA was cured, and no recurrence was observed for more than 1 year. It was confirmed that transplanting MM-UC-MSCs has high efficacy in the treatment of AA. Interestingly, case 1 had no visible change in the lesion sites for about 3 mo, while case 2 had a noticeable visible change after the first transplantation. We hypothesize that the treatment of case 2 with local steroid hormone injections for a year prior to transplantation resulted in a synergistic effect that shortened the time for the MSCs to engraft and exert their immune function to regrow the hair.
In the case of case 3, the hair grew on the scalp at levels similar to normal, and she reported no recurrence until 1 year after treatment. We relied on the patients to submit their follow-up pictures. Unfortunately, we have no other images from case 3 after day 226. Based on the result of this case, it was confirmed that transplanting MM-UC-MSCs has high efficacy for the treatment of AU as well. Interestingly, case 3, like case 1, did not see hair growth immediately after the first transplant. Hair regrowth was observed after approximately 3 mo (similar to case 1). This supports our hypothesis that prior steroid treatment can enhance the engraftment and functionality of the MM-UC-MSCs. Without the steroid use, we hypothesize that it will take about 3 mo for the MM-UC-MSCs to engraft and exert effects on the immune system. Further studies are needed to prove this hypothesis.
MM-UC-MSCs are expected to be a safer agent than cultured MSCs, embryonic stem cells, and induced pluripotent stem cells in vivo because minimally manipulated MSCs have little tumorigenicity, genetic mutations, and aging-related modifica
Based on our results, MM-UC-MSC transplantation was safe and effective to treat AA and AU. Further studies should be conducted to confirm the safety and effectiveness of this treatment before adopting this approach to treat a greater number of patients with AA and related diseases.
The therapeutic effect of MM-UC-MSCs on AA and related diseases is very high as shown in the patients presented here. Recurrence of AA and AU and side effects did not occur during the treatment and follow-up duration of at least 1 year. Based on these results, we expect that MM-UC-MSC transplantation will be a safe and efficient alternative for the treatment of AA, AT, and AU. However, it is necessary to conduct clinical trials with a greater number of patients.
Manuscript source: Unsolicited manuscript
Specialty type: Dermatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salim A, Sukumaran A S-Editor: Liu M L-Editor: A P-Editor: Yuan YY
| 1. | WALKER SA, ROTHMAN S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 56] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 132] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ 3rd. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 353] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Mirzoyev SA, Schrum AG, Davis MDP, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Pratt CH, King LE Jr, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 468] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 6. | Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 409] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 8. | Fabbrocini G, Panariello L, De Vita V, Vincenzi C, Lauro C, Nappo D, Ayala F, Tosti A. Quality of life in alopecia areata: a disease-specific questionnaire. J Eur Acad Dermatol Venereol. 2013;27:e276-e281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, de Jong A, Harel S, DeStefano GM, Rothman L, Singh P, Petukhova L, Mackay-Wiggan J, Christiano AM, Clynes R. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 676] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 10. | Jabbari A, Dai Z, Xing L, Cerise JE, Ramot Y, Berkun Y, Sanchez GA, Goldbach-Mansky R, Christiano AM, Clynes R, Zlotogorski A. Reversal of Alopecia Areata Following Treatment With the JAK1/2 Inhibitor Baricitinib. EBioMedicine. 2015;2:351-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Phan K, Sebaratnam DF. JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2019;33:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 12. | Hamilton CE, Craiglow BG. JAK Inhibitors for the Treatment of Pediatric Alopecia Areata. J Investig Dermatol Symp Proc. 2020;20:S31-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Barbulescu CC, Goldstein NB, Roop DR, Norris DA, Birlea SA. Harnessing the Power of Regenerative Therapy for Vitiligo and Alopecia Areata. J Invest Dermatol. 2020;140:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Almohanna HM, Ahmed AA, Griggs JW, Tosti A. Platelet-Rich Plasma in the Treatment of Alopecia Areata: A Review. J Investig Dermatol Symp Proc. 2020;20:S45-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Pototschnig H, Madl MT. Successful Treatment of Alopecia Areata Barbae with Platelet-rich Plasma. Cureus. 2020;12:e7495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Elmaadawi IH, Mohamed BM, Ibrahim ZAS, Abdou SM, El Attar YA, Youssef A, Shamloula MM, Taha A, Metwally HG, El Afandy MM, Salem ML. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J Dermatolog Treat. 2018;29:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Hye Kim J, Gyu Park S, Kim WK, Song SU, Sung JH. Functional regulation of adipose-derived stem cells by PDGF-D. Stem Cells. 2015;33:542-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Kim JE, Oh JH, Woo YJ, Jung JH, Jeong KH, Kang H. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models. Exp Dermatol. 2020;29:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Shi J, Chi S, Xue J, Yang J, Li F, Liu X. Emerging Role and Therapeutic Implication of Wnt Signaling Pathways in Autoimmune Diseases. J Immunol Res. 2016;2016:9392132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Gentile P, Garcovich S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells. 2019;8:466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 21. | Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk HD, Reichenspurner H, Robbins RC, Schrepfer S. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011;20:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 22. | De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 488] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 23. | Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 24. | Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 25. | Nilforoushzadeh MA, Lotfi E, Heidari-Kharaji M. Autologous adipose transplantation an effective method to treat alopecia after trauma: a case report. Clin Cosmet Investig Dermatol. 2019;12:647-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Anderi R, Makdissy N, Azar A, Rizk F, Hamade A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res Ther. 2018;9:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Fukuoka H, Narita K, Suga H. Hair Regeneration Therapy: Application of Adipose-Derived Stem Cells. Curr Stem Cell Res Ther. 2017;12:531-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Jones E, Schäfer R. Biological differences between native and cultured mesenchymal stem cells: implications for therapies. Methods Mol Biol. 2015;1235:105-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 355] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 30. | Gu Y, Li T, Ding Y, Sun L, Tu T, Zhu W, Hu J, Sun X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol Med Rep. 2016;13:5207-5215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Vangsness CT Jr, Sternberg H, Harris L. Umbilical Cord Tissue Offers the Greatest Number of Harvestable Mesenchymal Stem Cells for Research and Clinical Application: A Literature Review of Different Harvest Sites. Arthroscopy. 2015;31:1836-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Kuçi S, Henschler R, Müller I, Biagi E, Meisel R. Basic biology and clinical application of multipotent mesenchymal stromal cells: from bench to bedside. Stem Cells Int. 2012;2012:185943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Zhao J, Wang J, Dang J, Zhu W, Chen Y, Zhang X, Xie J, Hu B, Huang F, Sun B, Bellanti JA, Zheng SG. A preclinical study-systemic evaluation of safety on mesenchymal stem cells derived from human gingiva tissue. Stem Cell Res Ther. 2019;10:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Liang J, Zhang H, Kong W, Deng W, Wang D, Feng X, Zhao C, Hua B, Wang H, Sun L. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: a long-term retrospective study. Stem Cell Res Ther. 2018;9:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 35. | Wu PK, Wang JY, Chen CF, Chao KY, Chang MC, Chen WM, Hung SC. Early Passage Mesenchymal Stem Cells Display Decreased Radiosensitivity and Increased DNA Repair Activity. Stem Cells Transl Med. 2017;6:1504-1514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Bao X, Wang J, Zhou G, Aszodi A, Schönitzer V, Scherthan H, Atkinson MJ, Rosemann M. Extended in vitro culture of primary human mesenchymal stem cells downregulates Brca1-related genes and impairs DNA double-strand break recognition. FEBS Open Bio. 2020;10:1238-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Liu J, Ding Y, Liu Z, Liang X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front Cell Dev Biol. 2020;8:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 38. | Li S, Wang Y, Guan L, Ji M. Characteristics of human umbilical cord mesenchymal stem cells during ex vivo expansion. Mol Med Rep. 2015;12:4320-4325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Zhuang Y, Li D, Fu J, Shi Q, Lu Y, Ju X. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: immunomodulatory ability is enhanced in aged cells. Mol Med Rep. 2015;11:166-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Tong CK, Vellasamy S, Tan BC, Abdullah M, Vidyadaran S, Seow HF, Ramasamy R. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |