Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3675
Peer-review started: November 30, 2020
First decision: February 28, 2021
Revised: March 3, 2021
Accepted: March 23, 2021
Article in press: March 23, 2021
Published online: May 26, 2021
Processing time: 162 Days and 0.3 Hours
Infiltrating ductal breast carcinoma with monoclonal gammopathy of undetermined significance (MGUS) is rare and easily misdiagnosed. Most patients are first diagnosed with MGUS. We report a rare case of MGUS secondary to infiltrating ductal breast carcinoma. We also review the literature to analyze the clinical characteristics and diagnostic methods.
A 51-year-old woman underwent modified radical mastectomy for infiltrating ductal carcinoma of the right breast and was then treated with radiation and chemotherapy. A decreased platelet count was found on routine blood examination, and MGUS was subsequently diagnosed. This is the first report of the occurrence of MGUS after breast cancer surgery.
Vigilance is required to distinguish this rare comorbidity from breast plasmacytoma.
Core Tip: With age, the risk of monoclonal gammopathy of undetermined significance increases, ranging from 2% to 3% over the age of 50 and approximately 5% over the age of 70. In addition, obesity increases the risk relatively. The cause is unclear. Genetic factors, environmental factors, radiation and chemicals (such as pesticides, herbicides) may have a certain impact on the occurrence of this disease. The risk of infection is higher than normal, the risk of osteoporosis and fractures is increased, and the risk of thrombosis is increased. Tumors with monoclonal gammopathy of undetermined significance should be given more attention.
- Citation: Ma Y, Cui S, Yin YJ. Infiltrating ductal breast carcinoma with monoclonal gammopathy of undetermined significance: A case report. World J Clin Cases 2021; 9(15): 3675-3679
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3675.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3675
Plasma cell tumors account for 3% of all plasma cell diseases and can be characterized by single or multiple lesions. The most common manifestation is bone marrow-based disseminated tumors. But in rare cases, they can migrate to soft tissue with the help of adhesion molecules, resulting in extra medullary plasmacytoma[1,2]. Polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes, adenopathy and extensive skin patch overlying a plasmacytoma are paraneoplastic syndromes associated with plasma cell neoplasms[3]. To date, extramedullary plasmacytoma located on both sides of the breast have been reported, and pathological examination confirmed the existence of plasma cell infiltration in the breast[4].
However, there are no reports of infiltrating ductal breast carcinoma (not breast plasmacytoma) complicated by monoclonal gammopathy of undetermined significance (MGUS). We report the first case of infiltrating ductal breast carcinoma with MGUS.
A 51-year-old woman had undergone breast cancer surgery 18 mo ago, and she had a decrease in platelets for more than 9 mo.
The patient attended our hospital on September 9, 2020. She had a right breast tumor as the initial diagnosis, and pathology after modified radical mastectomy showed infiltrating ductal carcinoma stage I, pT1N1M0, IIA of the right breast. From April to July 2020, the patient received six cycles of docetaxel and Adriamycin chemotherapy followed by radiation therapy and finally two cycles of docetaxel and Adriamycin chemotherapy. On September 8, 2020, she had a routine blood examination that showed a platelet count of 4 × 109/L. She denied any uncomfortable symptoms.
She had type 2 diabetes.
The patient did not have any special history.
Hemorrhage was observed over the body, mainly on both lower limbs. The right breast was absent. A surgical scar approximately 15 cm in length was present. The left breast was normal without a palpable mass.
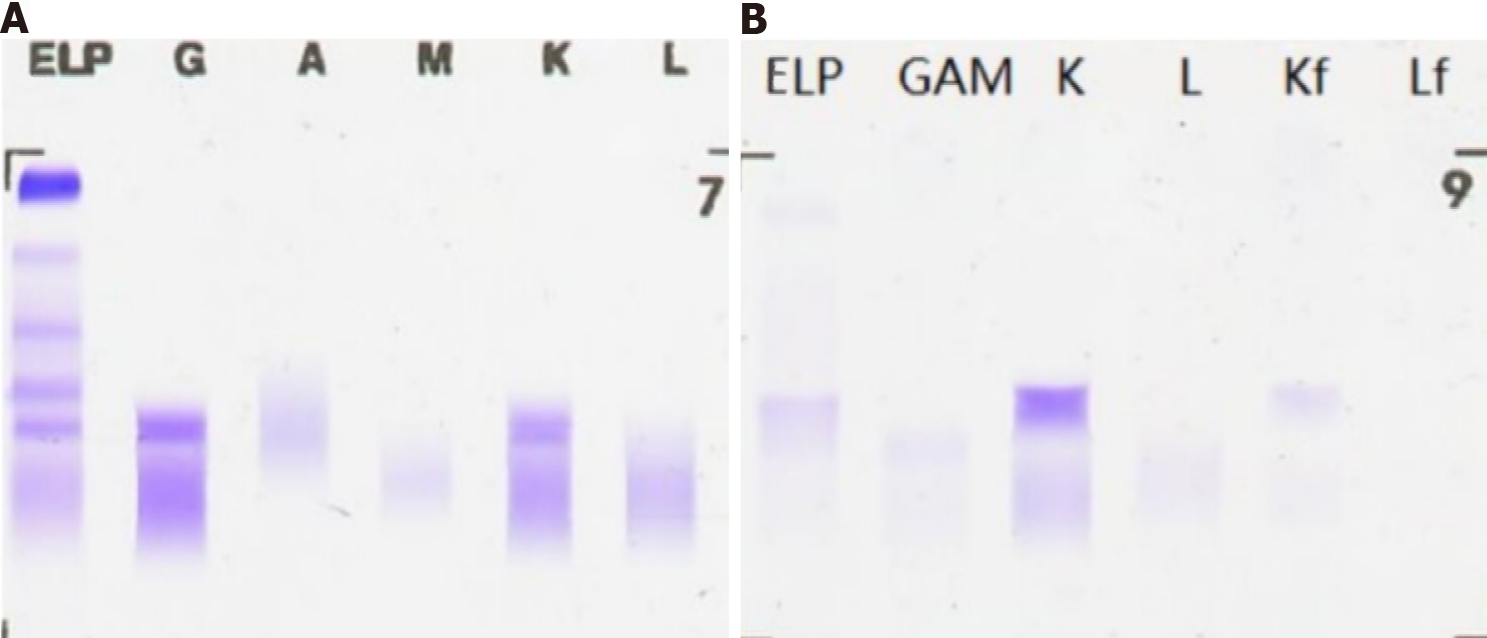
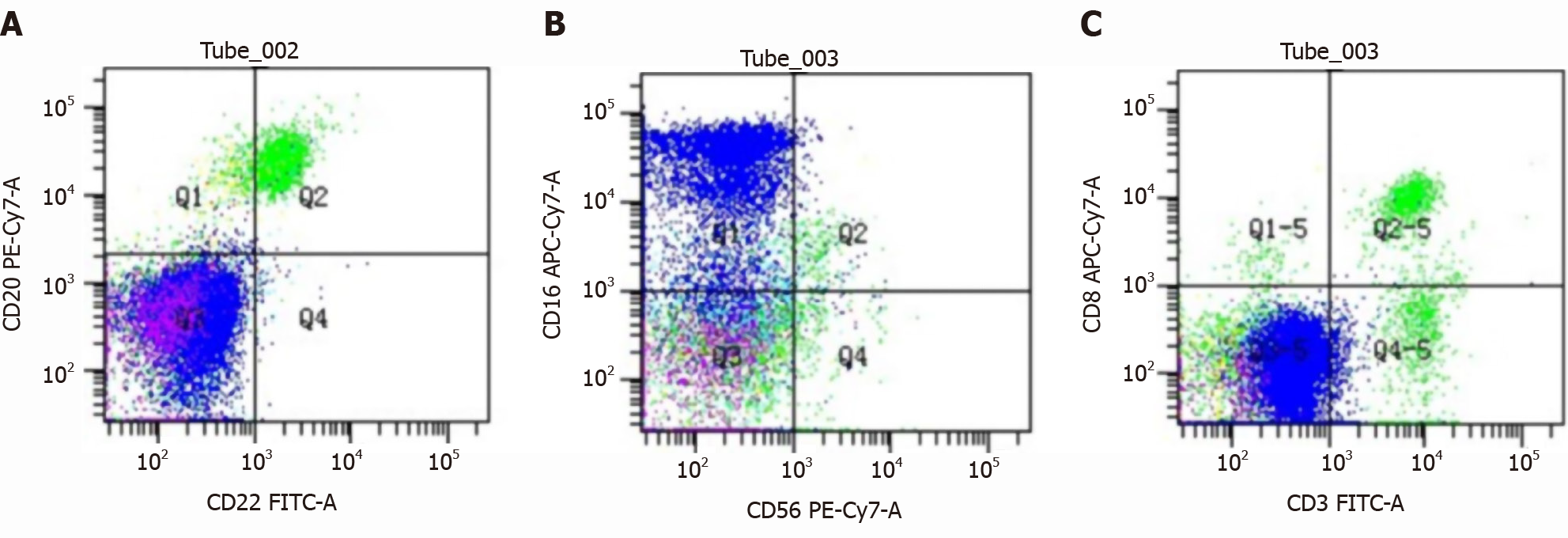
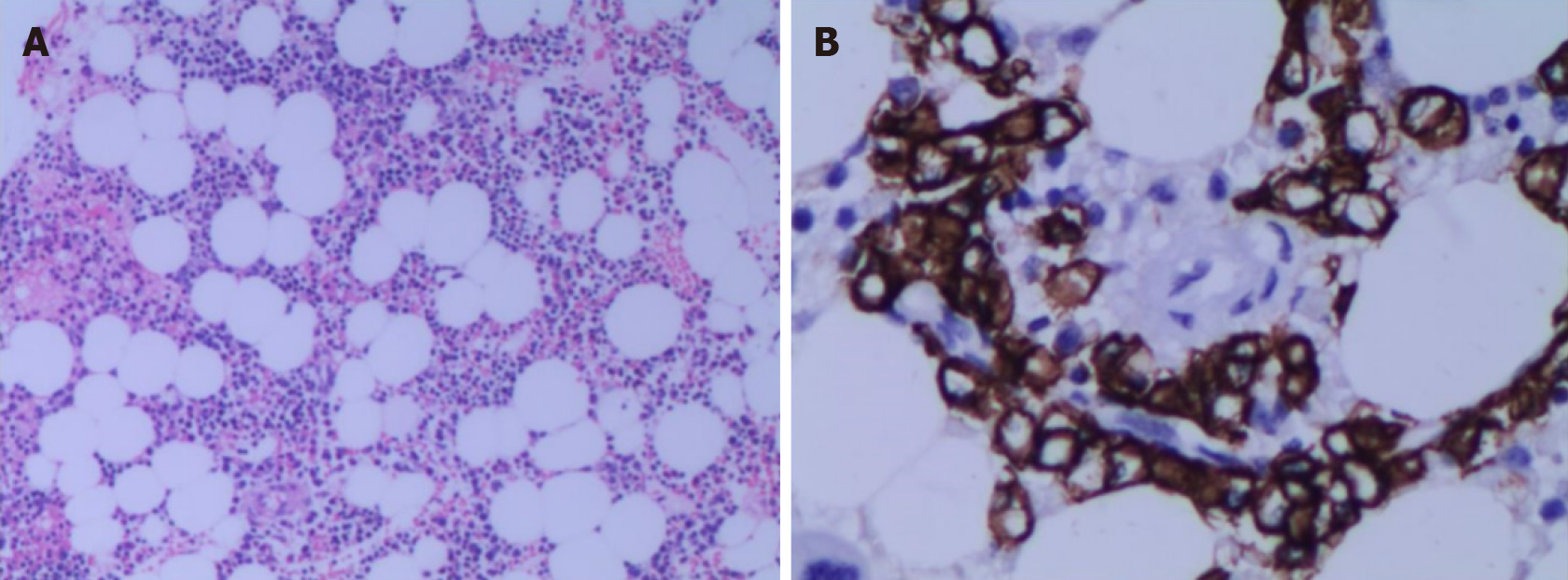
Liver fibrosis index showed type III procollagen peptide 88.75 ng/mL, type IV collagen IV-C 63.01 ng/mL and hyaluronic acid 208.90 ng/mL. Liver biopsy showed liver parietal hepatocyte dysplasia, reticular fiber disorder; negative Epstein-Barr virus DNA and, negative hepatitis C virus core antigen and antibody. Bone marrow cytology showed few platelets and megakaryocytes. Hypersplenism was also observed. Serum and urine protein electrophoresis showed M protein 8.6%, immunoglobulin (Ig)G-kappa M proteinemia and positive Bence-Jones protein in urine, which was 0.37 g/L (Figure 1). The serum-free light chain assay showed κ chain 233 mg/L, λ chain 27 mg/L and κ/λ 8.630, considering monoclonal gamma globulin disorder. The urine-free light chain assay showed κ chain 1170 mg/L, λ chain 92 mg/L, and κ/λ 12.717. The L265P mutation of MYD88 gene was negative. Tumor immunophenotyping showed that lymphocytes accounted for approximately 20% of nuclear cells, of which B lymphocytes accounted for a large proportion of the lymphocytes and no obvious light chain restriction (Figure 2). Bone marrow biopsy showed active hematopoietic tissue proliferation, scattered granulocytes and thrombocytosis and an increased proportion of erythroblasts. Scattered or small clusters of plasma cells accounted for approximately 5%-10% and were CD3 (+), CD20 (+), CD56 (-) and CD13 (+) (Figure 3).
The patient was diagnosed with infiltrating ductal breast carcinoma and MGUS.
The patient’s condition was stable after chemotherapy, radiotherapy and breast cancer surgery. The treatment principle for MGUS was observation, long-term close follow-up and no special treatment because MGUS showed no clinical manifestations of malignant plasma cell disease and did not reach the indications for monoclonal immunoglobulin therapy. The patient received a platelet transfusion and thrombopoietin for symptomatic treatment.
The patient was discharged from hospital when her platelet count was normal and was contacted by phone 1 mo after discharge. She had no skin or mucous membrane bleeding.
MGUS is an asymptomatic precancerous plasma cell disease, which is essentially a precancerous lesion and does not always progress to an obvious malignant tumor. It is characterized by a serum M protein lower than 30 g/L, bone marrow clonal plasma cells less than 10%, no plasma cell myeloma-related terminal organ damage, including hypercalcemia, renal insufficiency, anemia and bone lesions and no B-cell lymphoma or other diseases known to produce M protein. About 3% of people aged over 50 years have MGUS, and these individuals are more likely to have recurrent infections, ischemic heart disease, peripheral neuropathy and kidney disease[5]. Serum protein electrophoresis can be used to diagnose MGUS. The prevalence of MGUS in the general population tends to increase with age, with a higher proportion in males and Black races[6].
There are three different types of MGUS: (1) Non-IgM MGUS, which accounts for the majority of MGUS and can progress to multiple myeloma; (2) IgM-MGUS is characterized by monoclonal plasma cells. Non-IgM-MGUS can develop into IgM-MGUS and usually develops into lymphocytic lymphoma, Waldenstrom macroglobulinemia, and other lymphoproliferative diseases. The probability of malignant transformation of IgM-MGUS is significantly higher than that of non-IgM-MGUS; and (3) Light chain MGUS involves a monoclonal protein lacking immunoglo
In a previous case report, multiple myeloma involved the breast, which was characterized by a solitary plasma cell tumor[9]. Patients with MGUS have a higher risk of developing nonhematological malignancies, including nonmelanoma skin cancer, endocrine cancer, breast cancer, renal and urinary cancer, respiratory cancer, male reproductive system cancer and gastrointestinal cancer. The most common malignant tumor is colorectal cancer followed by prostate cancer and thyroid cancer. Breast cancer is relatively rare[10].
However, in this case, the patient was diagnosed with IgG-MGUS and infiltrating ductal breast carcinoma. The initial consideration of thrombocytopenia was because of radiation and chemotherapy for infiltrating ductal breast carcinoma. However, after admission, relevant laboratory tests and examinations confirmed secondary thrombocytopenia caused by MGUS. The patient also had liver cirrhosis and hepatocyte dysplasia but refused abdominal contrast-enhanced computed tomogra
There may be two reasons for the patient’s splenomegaly: (1) Liver cirrhosis can lead to splenomegaly and hypersplenism, aggravating the destruction of platelets; and (2) It is possible that abnormal plasma cells can infiltrate the spleen. In more than 30% of patients with IgG-MGUS or IgA-MGUS, the initial cause may be viral infection, including chronic antigen stimulation caused by Epstein-Barr virus, hepatitis C virus. Compared with IgG-MGUS, IgA-MGUS is relatively rare, and patients with IgA myeloma are more likely to develop bone destruction and extra medullary diseases. Therefore, survival time is shorter, and prognosis is worse[11]. However, Epstein-Barr virus DNA and the core antigen and antibody of hepatitis C virus in this patient were negative, suggesting that there was no potential chronic infection, and MGUS was unlikely to progress.
Vigilance is required to distinguish this rare comorbidity from breast plasmacytoma.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fakhr I, Murdaca G S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Ghiassi-Nejad Z, Ru M, Moshier E, Chang S, Jagannath S, Dharmarajan K. Overall Survival Trends and Clinical Characteristics of Plasmacytoma in the United States: A National Cancer Database Analysis. Clin Lymphoma Myeloma Leuk. 2019;19:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Wang J, Li J, Zhang F, Zhang P. Retroperitoneal extramedullary plasmacytoma: A case report and review of the literature. Medicine (Baltimore). 2018;97:e13281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Cardenas-de la Garza JA, Esquivel-Valerio JA, Arvizu-Rivera RI, Colunga-Pedraza PR, Galarza-Delgado DA. Flushing out a plasmacytoma in a patient with POEMS and AESOP syndromes. Lancet. 2020;396:e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Curtis Martínez C, Oller Navarro I, Porras A, Cansado P, Arroyo Sebastian A. Extramedullary plasmacytoma of the breast: An unusual diagnosis. Breast J. 2020;26:1841-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Lomas OC, Mouhieddine TH, Tahri S, Ghobrial IM. Monoclonal Gammopathy of Undetermined Significance (MGUS)-Not So Asymptomatic after All. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Taino G, Bordini L, Sarto C, Porro S, Chirico F, Oddone E, Imbriani M. [Monoclonal gammopathy of uncertain significance (MGUS) and occupational risk factors: criteria to carry out the health surveillance]. G Ital Med Lav Ergon. 2019;41:202-207. [PubMed] |
| 7. | Kaseb H, Annamaraju P, Babiker HM. Monoclonal Gammopathy Of Undetermined Significance. 2020 Dec 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] |
| 8. | Goldschmidt H, Nitschmann S. [Long-term follow-up of monoclonal gammopathies of undetermined significance (MGUS)]. Internist (Berl). 2018;59:639-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Ali HOE, Nasir Z, Marzouk AMSM. Multiple Myeloma Breast Involvement: A Case Report. Case Rep Radiol. 2019;2019:2079439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Sandecka V, Adam Z, Krejci M, Stork M, Rehak Z, Koukalova R, Sevcikova S, Brozova L, Kral Z, Mayer J, Pour L. Diagnostic relevance of 18F-FDG PET/CT in newly diagnosed patients with monoclonal gammopathy of undetermined significance (MGUS): Single-center experience. Neoplasma. 2020;67:939-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Bosseboeuf A, Seillier C, Mennesson N, Allain-Maillet S, Fourny M, Tallet A, Piver E, Lehours P, Mégraud F, Berthelot L, Harb J, Bigot-Corbel E, Hermouet S. Analysis of the Targets and Glycosylation of Monoclonal IgAs From MGUS and Myeloma Patients. Front Immunol. 2020;11:854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |