Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3623
Peer-review started: January 5, 2021
First decision: January 17, 2021
Revised: January 28, 2021
Accepted: March 3, 2021
Article in press: March 3, 2021
Published online: May 26, 2021
Processing time: 126 Days and 7.5 Hours
Osteochondritis dissecans (OCD) is a rare disease of unclear cause characterized by subchondral bone damage and overlying cartilage defects. The current report presents the results of subchondral bone as a novel target for implantation of peripheral blood stem cells (PBSCs) in the treatment of OCD.
A 16-year-old patient diagnosed with OCD underwent subchondral bone implantation of PBSCs. Four months later, the patient's visual analog scale scores, Western Ontario and McMaster University osteoarthritis index, and whole-organ magnetic resonance imaging score improved significantly, and regeneration of cartilage and subchondral bone was observed on magnetic resonance imaging.
This is the first case of OCD treated with subchondral bone as an implantation target of PBSCs, which highlights the importance of subchondral bone for cartilage repair. This treatment could be a potential option for articular cartilage and subchondral bone recovery in OCD.
Core Tip: Osteochondritis dissecans is a relatively rare disease, and we used an unreported treatment that focused on subchondral bone as a novel target for implantation using peripheral blood stem cells. Magnetic resonance imaging at 4 mo postoperatively showed repair of both cartilage and subchondral bone, and the patient's knee function and pain improved.
- Citation: Zhang SY, Xu HH, Xiao MM, Zhang JJ, Mao Q, He BJ, Tong PJ. Subchondral bone as a novel target for regenerative therapy of osteochondritis dissecans: A case report. World J Clin Cases 2021; 9(15): 3623-3630
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3623.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3623
Extensive cartilage and subchondral bone lesions in osteochondritis dissecans (OCD) appear to be difficult to regenerate with repeated friction on exposed articular surfaces, causing persistent pain and dysfunction[1].
Mesenchymal stromal stem cell therapy is a recently emerging approach that has been applied to diseases associated with cartilage damage based on stem cell multi-differentiation potential (osteogenic, chondrogenic, etc.)[2]. Although there are clinical cases where stem cells injected into the joint cavity have been reported to relieve pain and improve function in patients with OCD[3], it is easily overlooked that the distribution of commonly used intra-articular implanted stem cells in the joint cavity is unclear. Markides et al[4] tracked superparamagnetic iron oxide nanoparticle-labeled stem cells by magnetic resonance imaging (MRI) and found that stem cells implanted via the joint cavity did not accumulate at the site of the cartilage defect. Therefore, anchoring stem cells to the defective surface via a biological scaffold may increase the likelihood that stem cells will function, but this procedure typically involves a highly invasive and prolonged surgery and has so far failed to produce sufficient clinical results to support clinical adoption[5,6]. A recent study revealed that resident skeletal stem cells present in the subchondral bone that undergo activation from minor injuries (such as microfractures) can regenerate cartilage[7]. However, OCD often has large subchondral bone defects and large subchondral bone marrow lesions (BMLs) accompanied by a consequent impact on resident skeletal stem cell function[8], thus increasing the probability of regenerative failure. Since no studies have shown that stem cells injected into the joint cavity can enter the subchondral bone, we hypothesized that when exogenous stem cells are implanted directly into the subchondral bone, these cells can concentrate more at the site of injury to repair BMLs and act as resident skeletal stem cells for cartilage regeneration.
Previous studies have shown that autologous peripheral blood stem cells (PBSCs) also contain CD34+ cells (CD34 is an antigen expressed on stem and progenitor cells) and have the advantages of being easy to collect and safe to use with few complications[9]. Therefore, in this case, we ultimately implanted PBSCs into the subchondral bone in an attempt to exogenously replenish stem cells into the subchondral bone and to assist in the regeneration of cartilage. This study was approved by an institutional review board and registered (registration number ChiCTR-OCN-15006356), and the patient who participated in this study provided informed consent.
The patient, a 16-year-old man, presented to our orthopedic outpatient clinic with right knee pain and occasional locking after one basketball game 3 mo ago.
The pain appeared first after one basketball game, was followed by locking, and progressively worsened over 3 mo. During this period, the patient received pain medication and physical therapy, which failed to relieve his symptoms. The patient presented with right knee pain and occasional locking and underwent an MRI examination at the outpatient clinic.
The patient had tenderness and swelling in the right knee, which was worse when the limb was moved with flexion or extension. The neurovascular status was normal.
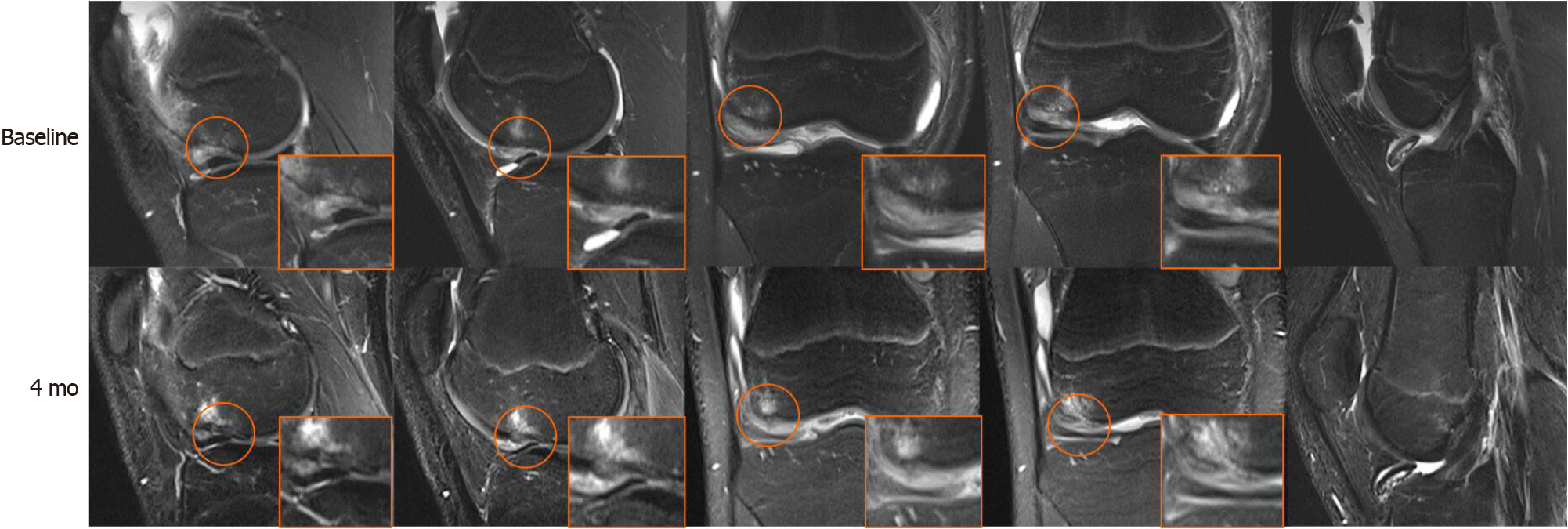
MRI (3.0 T) was used to assess the lesions of the right knee. T2-weighted imaging revealed localized cartilage and corresponding subchondral bone defects in the lower right femur with BMLs below and in front of the femoral condyle. The joint cavity and suprapatellar capsule had a large amount of fluid. The loose body was formed from partially low-signal subchondral bone and medium- to high-signal cartilaginous material (Figure 1).
The patient was diagnosed with OCD based on symptoms, physical examination, and imaging and was scheduled for subchondral bone implantation combined with intra-articular injection of PBSCs. The patient's right knee was braced and braked with the knee-aiding system preoperatively. The loose body was not removed prior to injection due to the patient's lack of surgical intent.
The patient was subcutaneously injected with recombinant human granulocyte stimulating factor (150 μg each time, twice a day for 5 d). When the patient's white blood cell count was > 25 × 109, 50 mL of peripheral blood stem cell suspension was collected with a COM. TEC blood cell separator (Fresenius HemoCare Company). Forty milliliters of stem cell suspension and an equal volume of normal saline were mixed and diluted 1:1, and the diluted stem cell suspension was packed in 16 split-core tubes in equal volume. Five milliliters of human peripheral blood lymphocyte separation solution was added into each split-core tube and centrifuged at 2000 r/min for 20 min at room temperature (eccentric radius: 7.5 cm). The sucked stem cell layer was transferred to another centrifuge tube, and centrifuged at room temperature at 1800 r/min for 20 min (centrifugal radius: 7.5 cm). The supernatant was discarded to obtain relatively pure stem cells. The CD34+ cell count in the purified solution of peripheral blood stem cells was 3 × 107/L.
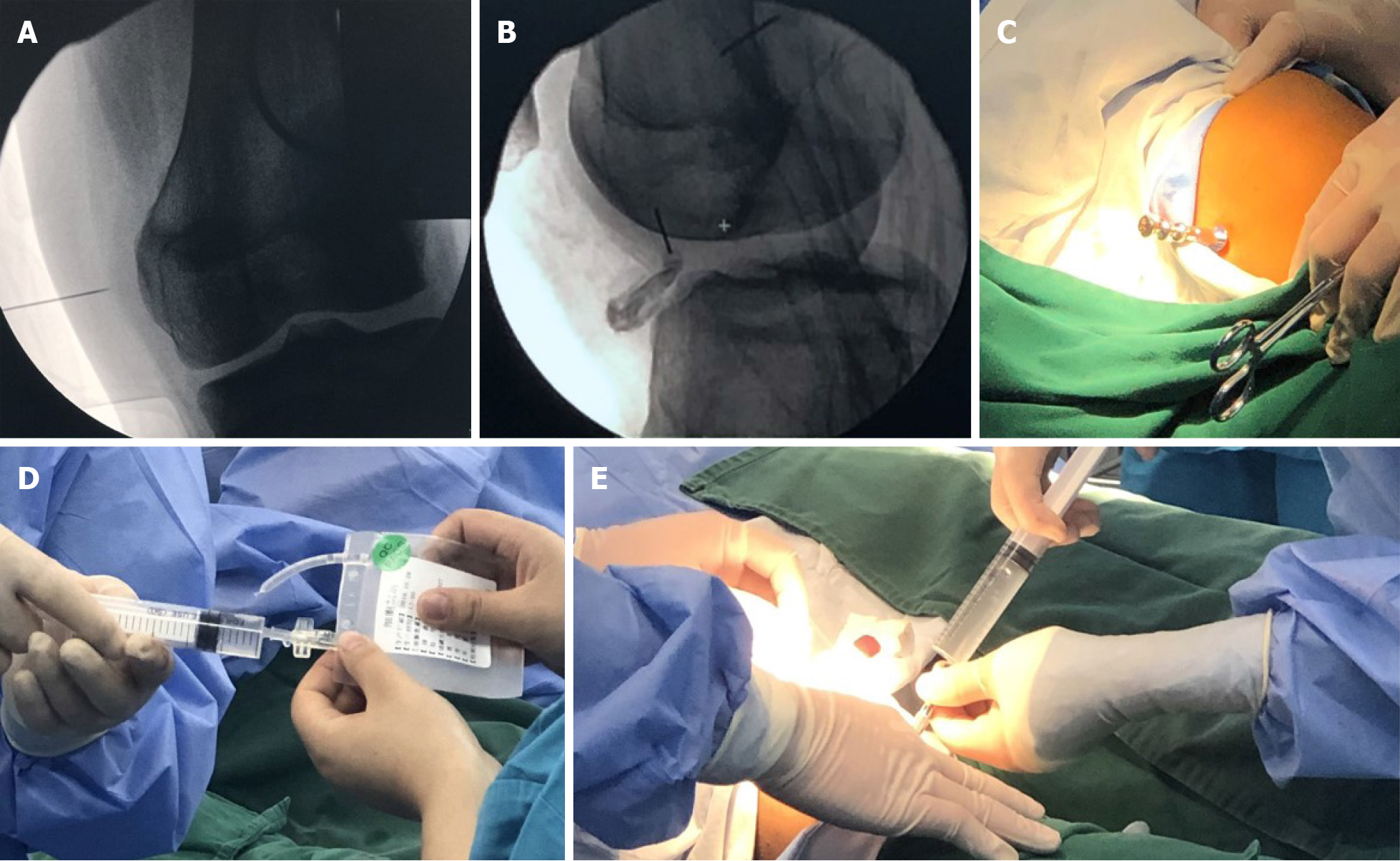
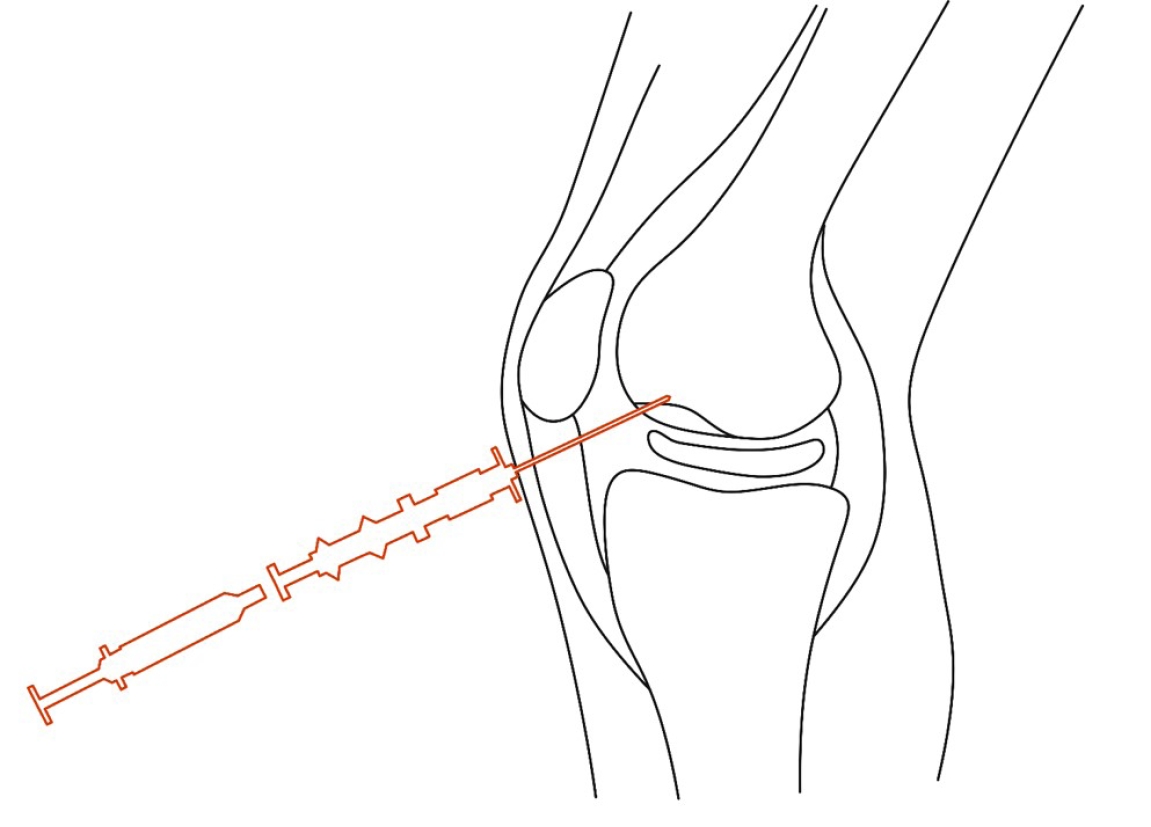
Based on the MRI results, the location of the lesion area was marked with fine metal wires on the surface of the knee in both the frontal and lateral positions. With routine disinfection and anesthesia of the surgical area, a bone marrow needle was percutaneously punctured to the subchondral bone site under C-arm fluoroscopy, as shown by MRI via the lateral knee approach. When the needle made contact with the subchondral bone, the needle was rotated to the left and right, slowly drilling into the bone. After the puncture needle was fixed in the subchondral bone, the needle core was removed, and the syringe was attached to inject PBSCs into the subchondral bone through the needle channel. Removing the needle, pressure was applied to the puncture point to stop bleeding for 5 min. The operation is illustrated in Figures 2 and 3.
The patient's right knee was braced with the knee-aiding system to prevent further damage due to knee instability for 1 mo. During this period, the patient was advised to complete 20-30 min of a range of motion knee flexion and extension exercises once a day. Then, the patient was allowed to partly bear weight and gradually transitioned to weight-bearing as tolerated. After 2 mo, the patient was allowed to conduct mild physical activity within tolerable limits and gradually increased activity levels.
The patient was diagnosed with OCD with a total cartilage defect, an underlying subchondral bone defect, and an articular loose body.
The patient was treated with PBSCs via minimal C-arm X-ray-guided subchondral bone implantation.
The patient was followed 4 mo postoperatively with no adverse effects. No other treatment was used during this period, and no adverse effects occurred. The Western Ontario and McMaster University osteoarthritis indexes preoperatively and postoperatively were 23 and 6, respectively, with pain, stiffness, and function scores dropping from 9, 4, and 10 to 2, 0, and 4, respectively. The pain subsided with visual analog scale scores decreasing from 4 to 1. The range of motion in knee flexion and extension increased from 0°-80° to 0°-130°. The patient has resumed mild physical activity. It should be mentioned that the patient still disagreed with further arthroscopic surgery to remove the loose body because of satisfactory pain relief.
Significant improvement was observed in MRI. Compared to the previous area, in the defect area, the subchondral bone was thickened, and normal signal cartilage could be seen on the coronal surface. Initial integration was observed between regenerative and native subchondral bone and cartilage. The area of BMLs and fluid were reduced. The loose body was still noted in the right knee intraarticular space, and subchondral bone continuity seemed to be disrupted (Figure 1). The preoperative whole-organ magnetic resonance imaging scores[10] were 25 and 14, in which cartilage scores were 13 and 9, subchondral bone attrition scores were 5 and 3, and bone marrow abnormality scores were 4 and 1 (Table 1).
| Baseline | 4 mo | |
| Cartilage | 13 | 9 |
| Marrow abnormality | 4 | 1 |
| Bone cysts | 0 | 0 |
| Bone attrition | 5 | 3 |
| Osteophytes | 0 | 0 |
| Menisci | 0 | 0 |
| Ligaments | 0 | 0 |
| Synovitis | 3 | 1 |
| Total | 25 | 14 |
Due to the lack of blood supply to the cartilage and undifferentiated cells that can migrate, proliferate, and participate in the repair response, restoring the structure and function of the cartilage becomes a challenge in OCD treatment[11,12]. There are drawbacks to these procedures used to regenerate cartilage, such as ACI and OAT. Both damage the cartilage in the sampling area and have limitations in the effectiveness of the treatment when the cartilage defect extends to the subchondral bone[1,13]. The subchondral bone consists of the subchondral bone plate and the trabecular, vascular, and intertrabecular spaces beneath it, which are essential functional units of articular cartilage and provide mechanical and metabolic protection for the cartilage[14]. Current studies suggest that subchondral bone plays an important role in cartilage degeneration and that complete subchondral bone, especially tidemark leveling, appears to be necessary for cartilage regeneration[15,16]. We hypothesize that this mechanism is partly due to shear forces from the formation of uneven subchondral bone that disrupt cartilage regeneration and may partially be due to inadequate recruitment and activation of skeletal stem cells in the extensively damaged subchondral bone[7]. Therefore, OCD repair techniques should first restore the subchondral bone both structurally and functionally. Second, when the damaged subchondral bone is insufficient to recruit and activate sufficient skeletal stem cells to regenerate cartilage, exogenous stem cell input may be able to compensate. These are the two main reasons why subchondral bone can be a precise therapeutic target, unlike the aimlessness of intra-articular injection.
The use of PBSCs for cartilage repair and regeneration has been demonstrated to be safe in animal and human studies[17]. In this study, PBSCs were applied for the first time to treat OCD, thus avoiding more tedious collection and in vitro expansion, such as those of adipose-and bone marrow-derived stem cells. Four months after surgery, the subchondral bone had regained some thickness and was nearly complete with signs of cartilage regeneration on the subchondral surface. As a result, whole-organ magnetic resonance imaging scores, which reflect the overall condition of the knee, were improved, as were knee symptom scores (Western Ontario and McMaster University and visual analog scale). Another advantage of puncture injection is that it avoids the trauma of open surgery.
In the coronal T2 image at 4 mo, there seemed to be an interruption in the continuity of the subchondral bone. Nevertheless, there were signals similar to the new cartilage in the interruption. One of the bone formation methods is endochondral ossification[18]. Therefore, we presume that the disrupted area was still in the process of repair but slower than the surrounding repair due to the tiny puncture damage in the center. The patient's pain relief seemed to go so far beyond what we would have expected from the MRI improvement that he ultimately decided not to undergo further surgery. Reviewing the literature, we found that BMLs are necrotic and fibrotic according to pathology slides and release inflammatory factors that can cause pain[19,20]. The reduction of the BML areas (the BMLs in front of the femoral condyles have largely disappeared) and fluid was more intuitive than incomplete repair of the cartilage, which may be a function of paracrine cytokines, exosomes, and other active substances of stem cells[21]. In addition to pain, previous studies found dysfunction of resident stem cells in the BML region, exhibiting lower proliferation and mineralization capacity, which may lead to abnormal bone remodeling and thus affect the overlying cartilage[8,22]. This finding suggests that the reduction in BMLs by subchondral bone mesenchymal stem cell manipulation may be a secondary therapeutic target for OCD.
We report a novel treatment for osteochondritis dissecans based on the multi-differentiation ability of peripheral blood stem cells with subchondral bone implantation, which effectively restored cartilage and subchondral bone and relieved the patient's symptoms in a short period. We suggest that repair of subchondral bone damage is the basis for good cartilage regeneration. However, randomized controlled studies with larger samples are still needed to demonstrate the effectiveness and safety of this approach.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vynios D S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Paatela T, Vasara A, Sormaala M, Nurmi H, Kautiainen H, Kiviranta I. Chondral and Osteochondritis Dissecans Lesions Treated by Autologous Chondrocytes Implantation: A Mid- to Long-Term Nonrandomized Comparison. Cartilage. 2020: 1947603520935953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Kong L, Zheng LZ, Qin L, Ho KKW. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Translat. 2017;9:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 3. | Freitag J, Shah K, Wickham J, Li D, Norsworthy C, Tenen A. Evaluation of autologous adipose-derived mesenchymal stem cell therapy in focal chondral defects of the knee: a pilot case series. Regen Med. 2020;15:1703-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Markides H, Newell KJ, Rudorf H, Ferreras LB, Dixon JE, Morris RH, Graves M, Kaggie J, Henson F, El Haj AJ. Ex vivo MRI cell tracking of autologous mesenchymal stromal cells in an ovine osteochondral defect model. Stem Cell Res Ther. 2019;10:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Chimutengwende-Gordon M, Ahmad MA, Bentley G, Brammah J, Carrington R, Miles J, Donaldson J. Stem cell transplantation for the treatment of osteochondral defects of the knee: Operative technique for a single-stage transplantation procedure using bone marrow-derived mesenchymal stem cells. Knee. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Guerra E, Fabbri D, Cavallo M, Marinelli A, Rotini R. Treatment of Capitellar Osteochondritis Dissecans With a Novel Regenerative Technique: Case Report of 3 Patients After 4 Years. Orthop J Sports Med. 2018;6:2325967118795831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Murphy MP, Koepke LS, Lopez MT, Tong X, Ambrosi TH, Gulati GS, Marecic O, Wang Y, Ransom RC, Hoover MY, Steininger H, Zhao L, Walkiewicz MP, Quarto N, Levi B, Wan DC, Weissman IL, Goodman SB, Yang F, Longaker MT, Chan CKF. Articular cartilage regeneration by activated skeletal stem cells. Nat Med. 2020;26:1583-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 8. | Campbell TM, Churchman SM, Gomez A, McGonagle D, Conaghan PG, Ponchel F, Jones E. Mesenchymal Stem Cell Alterations in Bone Marrow Lesions in Patients With Hip Osteoarthritis. Arthritis Rheumatol. 2016;68:1648-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Saw KY, Anz A, Siew-Yoke Jee C, Merican S, Ching-Soong Ng R, Roohi SA, Ragavanaidu K. Articular cartilage regeneration with autologous peripheral blood stem cells vs hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29:684-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 10. | Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1155] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 11. | Fang J, Wang X, Jiang W, Zhu Y, Hu Y, Zhao Y, Song X, Zhao J, Zhang W, Peng J, Wang Y. Platelet-Rich Plasma Therapy in the Treatment of Diseases Associated with Orthopedic Injuries. Tissue Eng Part B Rev. 2020;26:571-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Olstad K, Shea KG, Cannamela PC, Polousky JD, Ekman S, Ytrehus B, Carlson CS. Juvenile osteochondritis dissecans of the knee is a result of failure of the blood supply to growth cartilage and osteochondrosis. Osteoarthritis Cartilage. 2018;26:1691-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Masquijo J, Kothari A. Juvenile osteochondritis dissecans (JOCD) of the knee: current concepts review. EFORT Open Rev. 2019;4:201-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Pan J, Wang B, Li W, Zhou X, Scherr T, Yang Y, Price C, Wang L. Elevated cross-talk between subchondral bone and cartilage in osteoarthritic joints. Bone. 2012;51:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Hernigou P, Bouthors C, Bastard C, Flouzat Lachaniette CH, Rouard H, Dubory A. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: what better postpone knee arthroplasty at fifteen years? Int Orthop. 2021;45:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Smaida R, Pijnenburg L, Irusta S, Himawan E, Mendoza G, Harmouch E, Idoux-Gillet Y, Kuchler-Bopp S, Benkirane-Jessel N, Hua G. Potential Implantable Nanofibrous Biomaterials Combined with Stem Cells for Subchondral Bone Regeneration. Materials (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Chen YR, Yan X, Yuan FZ, Ye J, Xu BB, Zhou ZX, Mao ZM, Guan J, Song YF, Sun ZW, Wang XJ, Chen ZY, Wang DY, Fan BS, Yang M, Song ST, Jiang D, Yu JK. The Use of Peripheral Blood-Derived Stem Cells for Cartilage Repair and Regeneration In Vivo: A Review. Front Pharmacol. 2020;11:404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Jing Y, Zhou X, Han X, Jing J, von der Mark K, Wang J, de Crombrugghe B, Hinton RJ, Feng JQ. Chondrocytes Directly Transform into Bone Cells in Mandibular Condyle Growth. J Dent Res. 2015;94:1668-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Zhou J, Xiong W, Gou P, Chen Z, Guo X, Huo X, Xue Y. Clinical effect of intramuscular calcitonin compared with oral celecoxib in the treatment of knee bone marrow lesions: a retrospective study. J Orthop Surg Res. 2020;15:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Higuchi J, Yamagami R, Matsumoto T, Terao T, Inoue K, Tsuji S, Maenohara Y, Matsuzaki T, Chijimatsu R, Omata Y, Yano F, Tanaka S, Saito T. Associations of clinical outcomes and MRI findings in intra-articular administration of autologous adipose-derived stem cells for knee osteoarthritis. Regen Ther. 2020;14:332-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020;11:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 22. | Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 601] [Article Influence: 40.1] [Reference Citation Analysis (0)] |