Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3607
Peer-review started: January 3, 2021
First decision: February 11, 2021
Revised: February 25, 2021
Accepted: March 12, 2021
Article in press: March 12, 2021
Published online: May 26, 2021
Processing time: 127 Days and 23.4 Hours
Although several trials have shown that the addition of antifoaming agents to polyethylene glycol (PEG) can improve bowel preparation, whether PEG plus antifoaming agents have a beneficial role in the detection of benign tumors during colonoscopy has yet to be confirmed. Our aim was to clarify whether adding simethicone to PEG solution could improve the detection of benign colorectal tumors.
To clarify whether adding simethicone to PEG solution could improve the detection of benign colorectal tumors.
The PubMed, EMBASE, and Cochrane Library databases were searched for articles published prior to September 2019. The outcomes included the detection rates of colorectal adenomas and polyps.
Twenty studies were eligible. Although there was no difference in the colorectal adenoma detection rate (ADR), a significant effect of simethicone for diminutive adenomas (< 10 mm) was revealed in the group taking simethicone. We also found that simethicone could significantly improve the ADR in the proximal colon but did not affect the colorectal polyp detection rate. Furthermore, the subgroup analyses revealed a beneficial effect of simethicone on the ADR among Asians (P = 0.005) and those with an ADR < 25% (P = 0.003). Moreover, it was a significant finding that the low dose simethicone was as effective as the high dose one with respect to the detection of benign colorectal tumors.
In summary, the addition of simethicone to PEG might improve the detection of diminutive adenomas in the right colon by colonoscopy in Asia. Low-dose simethicone was recommended for the detection of benign colorectal tumors. However, large clinical trials are necessary to validate our results and determine the ideal dose of simethicone.
Core Tip: The addition of simethicone to polyethylene glycol might improve the detection of diminutive adenomas in the right colon by colonoscopy in Asia. Low-dose simethicone was recommended for the detection of benign colorectal tumors.
- Citation: Zhang H, Gong J, Ma LS, Jiang T, Zhang H. Effect of antifoaming agent on benign colorectal tumors in colonoscopy: A meta-analysis. World J Clin Cases 2021; 9(15): 3607-3622
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3607.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3607
Colorectal cancer (CRC) is a common cancer worldwide. The incidence and mortality of CRC have been rapidly increasing in Asian countries[1,2]. Early diagnosis is associated with better survival and quality of life. Currently, colonoscopy is a standard first-line tool for the screening, surveillance, and prevention of colorectal tumors[3,4]. The colorectal adenoma detection rate (ADR) is regarded as the most important indicator of colonoscopy. Polyethylene glycol (PEG) is recommended as the preferred choice for bowel preparation[5]. However, up to a quarter of patients have shown inadequate bowel preparation[6]. Inadequate bowel preparation is related to an increased risk of missed benign colorectal tumors and more discomfort for patients[7-9].
Simethicone, which prevents bubble formation and gas retention to alleviate bloating, is an effective and safe antifoaming agent for use during endoscopic procedures. A combination of simethicone and PEG has been shown to improve the visualization of the bowel for colonoscopy. Thus, simethicone could have a theoretical benefit in the detection of benign tumors in colonoscopy, especially diminutive lesions.
A large number of previous studies have evaluated the effect of simethicone in ADR during colonoscopy, but the results have been inconsistent. Hence, a recent meta-analysis is necessary. However, whether simethicone plus PEG has a beneficial role in the detection of benign tumors during colonoscopy has yet to be confirmed. Therefore, we performed a meta-analysis to investigate its effect on the detection of benign colorectal tumors.
The PubMed, EMBASE, and Cochrane Central databases (up to September 1, 2019) were searched using the keywords “colonoscopy”, “antifoaming agent” or “simethicone”, and “randomized”. We also performed a manual search of the reference lists of the published articles.
(1) Study design: randomized studies as full manuscripts; (2) Language: limited to English; (3) Population: patients who underwent a colonoscopy; (4) Controls: PEG without simethicone for bowel preparation; (5) Intervention: PEG with simethicone for bowel preparation; and (6) Outcomes: primary endpoints: colorectal ADR and polyp detection rate (PDR) and secondary endpoint: adverse events.
(1) Bowel preparation without PEG or simethicone; (2) Nonhuman studies; (3) Duplicate publications; and (4) Studies without available data.
The data were extracted by 3 investigators (HZ, JG, and LM) independently. Disagreements were resolved by consensus. The data included the author, year, number of patients, country or region, detailed information on interventions and controls (ADR and PDR), and adverse events.
The Cochrane Collaboration’s risk of bias tool[10] was used to evaluate the quality of the randomized studies. The quality scale was assessed as “low risk of bias”, “unclear risk of bias”, and “high risk of bias”.
The odds ratio (OR) was used for discrete variables, and the mean difference and standardized difference in mean were used for continuous variables. The pooled ORs and 95% confidence intervals (CIs) were calculated from the studies using either a fixed-effects model or a random-effects model. When the heterogeneity was significant, the random-effects model was used for the pooled data; otherwise, a fixed-effects model was used. Heterogeneity among the studies was assessed using the I2 statistic or the χ2 test. I2 > 50% or P < 0.10 was considered to indicate heterogeneity. Publication bias was evaluated by Egger’s test, where P < 0.10 in a two-tailed test was regarded as positive. In the subgroup analyses, P < 0.05 for the χ2 test indicated statistically significant heterogeneity. By excluding one or more studies each time, sensitivity analysis was conducted to evaluate the robustness of the pooled results[11]. All of the statistical analyses and plots were performed using Review Manager statistical software, version 5.0 (the Cochrane Collaboration, Copenhagen, Denmark) and Stata software, version 12.0 (Stata Corp LLC, Texas, United States).
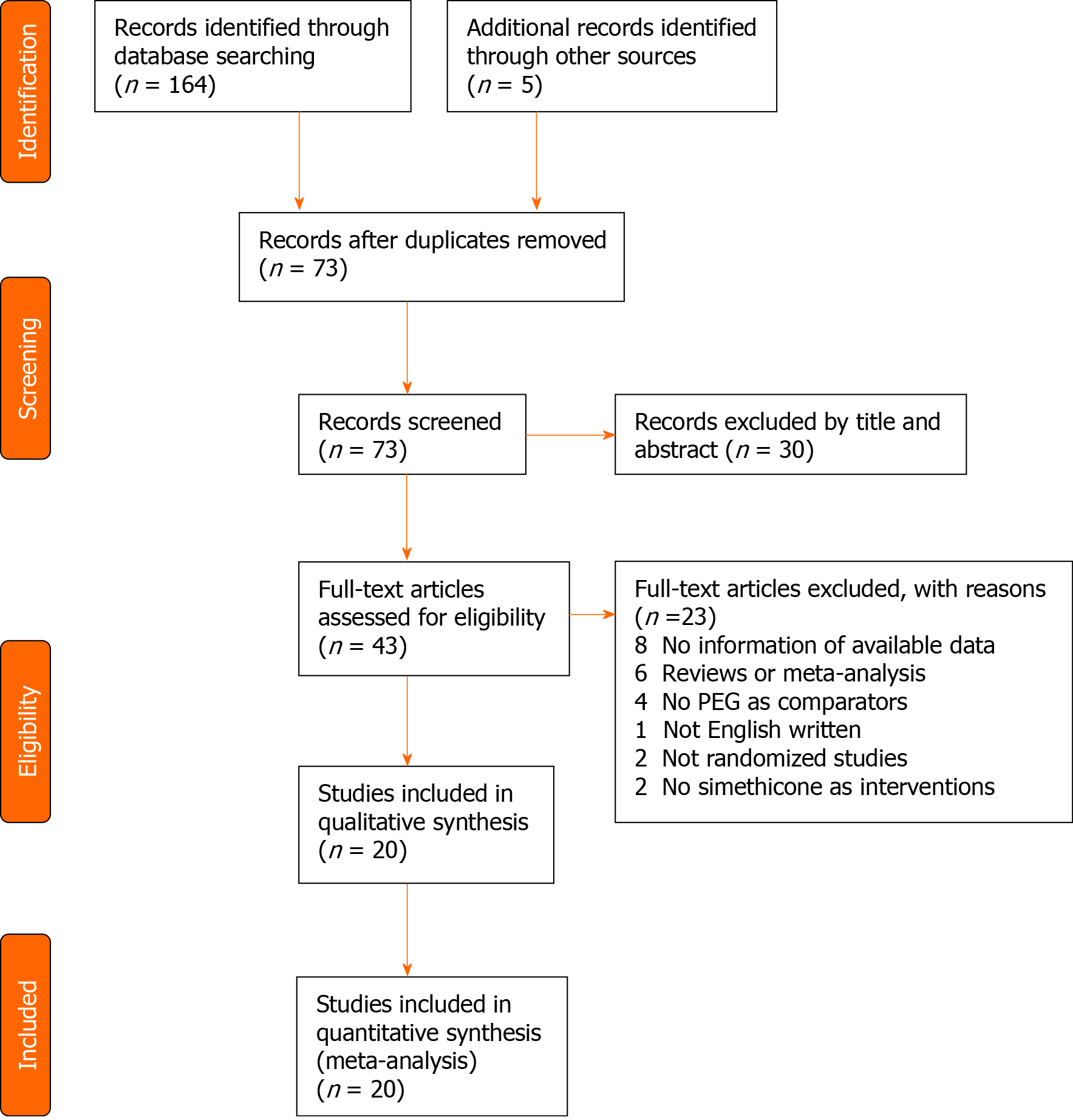
The literature search retrieved 169 citations, 96 of which were excluded due to duplication. Of the 73 eligible studies, 53 studies were excluded, and 20 studies focused on comparing PEG with and without simethicone to evaluate the effects on ADR and PDR. This meta-analysis was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis[12] (Figure 1).
The 20 studies[13-32] included 6306 patients, of whom 3162 and 3144 patients were assigned to the PEG plus simethicone group and PEG group, respectively (Tables 1 and 2). These studies were performed in five countries (China, South Korea, Italy, United States, and Netherlands).
| Ref. | Groups | n | Dose of simethicone in mg | Bubble score | Insertion time in min | Withdraw time in min | Adverse events | |||||
| Bloating | Nausea | Vomiting | Abdominal pain | Sleep disorder | ||||||||
| Rishi et al[32] (2019) | NS | 2L | 84 | 200 | 1.77 ± 1.00 | 5.48 ± 2.82 | 11.23 ± 3.99 | NR | 20 | 6 | 12 | NR |
| S | 2L + Sim | 84 | 1.20 ± 0.60 | 6.06 ± 3.55 | 11.73 ± 5.52 | NR | 13 | 4 | 10 | NR | ||
| Morave et al[31] (2019) | NS | 4L | 139 | 480 | 2.10 ± 2.15 | 6.19 ± 4.62 | 6.65 ± 1.28 | NR | NR | NR | NR | NR |
| S | 4L + Sim | 129 | 0.10 ± 0.15 | 6.06 ± 3.71 | 6.60 ± 1.15 | NR | NR | NR | NR | NR | ||
| Zhang et al[13] (2018) | NS | 2L | 290 | 1200 | 2.5 ± 0.7 | 7.5 ± 5.1 | NR | 59 | 57 | 20 | 24 | 57 |
| S | 2L + Sim | 289 | 2.8 ± 0.5 | 6.3 ± 3.1 | NR | 34 | 61 | 24 | 21 | 53 | ||
| Bai et al[14] (2018) | NS | 2L | 286 | 1200 | 3.98 ± 2.50 | 7.55 ± 4.19 | 6.87 ± 2.03 | 57 | 38 | 27 | 9 | NR |
| S | 2L + Sim | 290 | 1.00 ± 1.26 | 7.84 ± 5.12 | 6.47 ± 1.80 | 23 | 39 | 30 | 11 | NR | ||
| Yoo et al[15] (2016) | NS | 2L | 130 | 400 | NR | 6.75 ± 5.13 | 17.29 ± 13.17 | 71 | 51 | 15 | 31 | 39 |
| S | 2L + Sim | 130 | NR | 6.78 ± 3.78 | 13.35 ± 7.86 | 31 | 54 | 8 | 7 | 36 | ||
| Zorzi et al[16] (2016) | NS | 2L | 924 | NR | NR | NR | 10.4 ± 29.9 | NR | NR | NR | NR | NR |
| S | 2L + Sim | 940 | NR | NR | 10.6 ± 30.0 | NR | NR | NR | NR | NR | ||
| Kump et al[17] (2018) | NS | 2L | 193 | NR | NR | NR | NR | 28 | 26 | 3 | 37 | NR |
| S | 2L + Sim | 194 | NR | NR | NR | 26 | 26 | 1 | 34 | NR | ||
| Parente et al[18] (2015) | NS | 4L | 189 | NR | NR | 12 ± 7 | 10 ± 3 | NR | NR | NR | NR | 43 |
| S | 2L + Sim | 193 | NR | 13 ± 7 | 11 ± 6 | NR | NR | NR | NR | 37 | ||
| Mussetto et al[19] (2015) | NS | 4L | 60 | NR | NR | 7.8 ± 5.1 | 13.8 ± 9.6 | 21 | 20 | NR | 6 | 26 |
| S | 2L + Sim | 60 | NR | 6.5 ± 3.5 | 11.4 ± 9.4 | 15 | 23 | NR | 9 | 17 | ||
| Leone et al[20] (2013) | NS | 4L | 79 | NR | NR | 9.8 ± 3.6 | NR | 1 | 7 | 2 | 2 | 3 |
| S | 2L + Sim | 78 | NR | 10.9 ± 6.1 | NR | 1 | 5 | 6 | 5 | 7 | ||
| Valiante et al[21] (2013) | NS | 4L | 126 | 160 | NR | NR | NR | 33 | 26 | NR | 5 | NR |
| S | 2L + Sim | 138 | NR | NR | NR | 11 | 27 | NR | 13 | NR | ||
| Cesaro et al[22] (2013) | NS | 4L | 51 | 160 | NR | 9.5 ± 5.8 | 7.0 ± 1.8 | 12 | 23 | NR | 2 | NR |
| S | 2L + Sim | 50 | NR | 8.1 ± 3.8 | 7.6 ± 2.4 | 4 | 10 | NR | 6 | NR | ||
| Gentile et al[23] (2013) | NS | 2L | 60 | 160 | NR | NR | NR | NR | 6 | 3 | 1 | 0 |
| S | 4L + Sim | 60 | NR | NR | NR | NR | 12 | 4 | 1 | 0 | ||
| Matro et al[24] (2012) | NS | 2L | 61 | 400 | NR | NR | NR | 32 | 18 | 3 | 21 | 16 |
| S | 2L + Sim | 62 | NR | NR | NR | 25 | 22 | 3 | 17 | 16 | ||
| Repici et al[25] (2012) | NS | 2L | 190 | 160 | NR | 7.3 ± 3.5 | NR | 43 | 57 | NR | 30 | NR |
| S | 2L + Sim | 187 | NR | 7.9 ± 3.7 | NR | 47 | 60 | NR | 34 | NR | ||
| Jansen et al[26] (2011) | NS | 2L | 102 | NR | NR | NR | NR | NR | NR | NR | 12 | NR |
| S | 2L + Sim | 86 | NR | NR | NR | NR | NR | NR | 9 | NR | ||
| Pontone et al[27] (2011) | NS | 2L | 72 | 160 | NR | NR | NR | NR | 7 | 4 | 2 | 1 |
| S | 4L + Sim | 72 | NR | NR | NR | NR | 16 | 5 | 1 | 1 | ||
| Lazzaroni et al[28] (1993) | NS | 4L | 48 | 120 | NR | NR | NR | 26 | 23 | NR | 15 | 21 |
| S | 4L + Sim | 57 | NR | NR | NR | 26 | 20 | NR | 13 | 11 | ||
| McNally et al[29] (1989) | NS | NR | 12 | 160 | 0.778 ± 0.278 | NR | NR | NR | NR | NR | NR | NR |
| S | NR | 14 | 0.180 ± 0.054 | NR | NR | NR | NR | NR | NR | NR | ||
| McNally et al[30] (1988) | NS | NR | 48 | 80 | 0.696 ± 0.112 | NR | NR | NR | NR | NR | NR | NR |
| S | NR | 49 | 0.114 ± 0.050 | NR | NR | NR | NR | NR | NR | NR | ||
| Ref. | Country | Groups | N | Adenoma | Polyp | |||||||||||
| n | % | Left colon | Right colon | < 10 mm | ≥ 10 mm | n | % | Left colon | Right colon | < 10 mm | ≥ 10 mm | |||||
| Rishi et al[32] (2019) | United States | NS | 2L | 84 | NR | NR | NR | NR | NR | NR | 46 | 54.8 | NR | NR | NR | NR |
| S | 2L + Sim | 84 | NR | NR | NR | NR | NR | NR | 47 | 56.0 | NR | NR | NR | NR | ||
| Morave et al[31] (2019) | United States | NS | 4L | 139 | 54 | 38.8 | NR | NR | NR | NR | 69 | 49.6 | NR | NR | NR | NR |
| S | 4L + Sim | 129 | 43 | 33.3 | NR | NR | NR | NR | 60 | 46.5 | NR | NR | NR | NR | ||
| Zhang et al[13] (2018) | China | NS | 2L | 290 | 45 | 15.5 | 22 | 30 | 46 | 6 | 93 | 32.1 | 64 | 46 | NR | NR |
| S | 2L + Sim | 289 | 64 | 22.1 | 36 | 48 | 78 | 6 | 98 | 33.9 | 67 | 62 | NR | NR | ||
| Bai et al[14] (2018) | China | NS | 2L | 286 | 41 | 14.3 | 35 | 32 | 60 | 7 | 85 | 29.7 | NR | NR | NR | NR |
| S | 2L + Sim | 290 | 61 | 21.0 | 49 | 85 | 122 | 12 | 109 | 37.6 | NR | NR | NR | NR | ||
| Yoo et al[15] (2016) | Korea | NS | 2L | 130 | 60 | 46.2 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| S | 2L + Sim | 130 | 65 | 50.0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Zorzi et al[16] (2016) | Italy | NS | 2L | 924 | 346 | 37.4 | NR | NR | NR | NR | 569 | 61.6 | NR | NR | 403 | 166 |
| S | 2L + Sim | 940 | 322 | 34.3 | NR | NR | NR | NR | 542 | 57.7 | NR | NR | 380 | 162 | ||
| Kump et al[17] (2018) | Austria | NS | 2L | 193 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| S | 2L + Sim | 194 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Parente et al[18] (2015) | Italy | NS | 4L | 189 | NR | NR | NR | NR | NR | NR | 89 | 49.2 | NR | NR | 61 | NR |
| S | 2L + Sim | 193 | NR | NR | NR | NR | NR | NR | 91 | 48.1 | NR | NR | 59 | NR | ||
| Mussetto et al[19] (2015) | Italy | NS | 4L | 60 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| S | 2L + Sim | 60 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Leone et al[20] (2013) | Italy | NS | 4L | 79 | 34 | 44.7 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| S | 2L + Sim | 78 | 34 | 43.6 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Valiante et al[21] (2013) | Italy | NS | 4L | 126 | NR | NR | NR | NR | NR | NR | 71 | 56.3 | NR | NR | 55 | 16 |
| S | 2L + Sim | 138 | NR | NR | NR | NR | NR | NR | 105 | 76.1 | NR | NR | 84 | 21 | ||
| Cesaro et al[22] (2013) | Italy | NS | 4L | 51 | 17 | 34.7 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| S | 2L + Sim | 50 | 17 | 32.7 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Gentile et al[23] (2013) | Italy | NS | 2L | 60 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| S | 4L + Sim | 60 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Matro et al[24] (2012) | United States | NS | 2L | 61 | 20 | 32.8 | NR | NR | NR | NR | 29 | 47.5 | NR | NR | NR | NR |
| S | 2L + Sim | 62 | 15 | 24.2 | NR | NR | NR | NR | 23 | 37.1 | NR | NR | NR | NR | ||
| Repici et al[25] (2012) | Italy | NS | 2L | 190 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| S | 2L + Sim | 187 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Jansen et al[26] (2011) | Netherlands | NS | 2L | 102 | NR | NR | NR | NR | NR | NR | 14 | 13.7 | NR | NR | NR | NR |
| S | 2L + Sim | 86 | NR | NR | NR | NR | NR | NR | 23 | 26.7 | NR | NR | NR | NR | ||
| Pontone et al[27] (2011) | Italy | NS | 2L | 72 | 9 | 12.5 | 8 | 1 | NR | NR | 13 | 18.1 | NR | NR | NR | NR |
| S | 4L + Sim | 72 | 12 | 16.7 | 5 | 7 | NR | NR | 22 | 30.6 | NR | NR | NR | NR | ||
| Lazzaroni et al[28] (1993) | Italy | NS | 4L | 48 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| S | 4L + Sim | 57 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| McNally et al[29] (1989) | United States | NS | PEG | 12 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| S | PEG + Sim | 14 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| McNally et al[30] (1988) | United States | NS | PEG | 48 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| S | PEG + Sim | 49 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
The quality of the randomized studies was evaluated by the Cochrane Collaboration’s risk of bias tool. Although all of the studies were single-blind to the examiner, the blinding of outcome assessments was not affected. Therefore, the risk bias of selective reporting of each trial was considered low risk. The quality assessment of the randomized studies is shown in Supplementary Table 1.
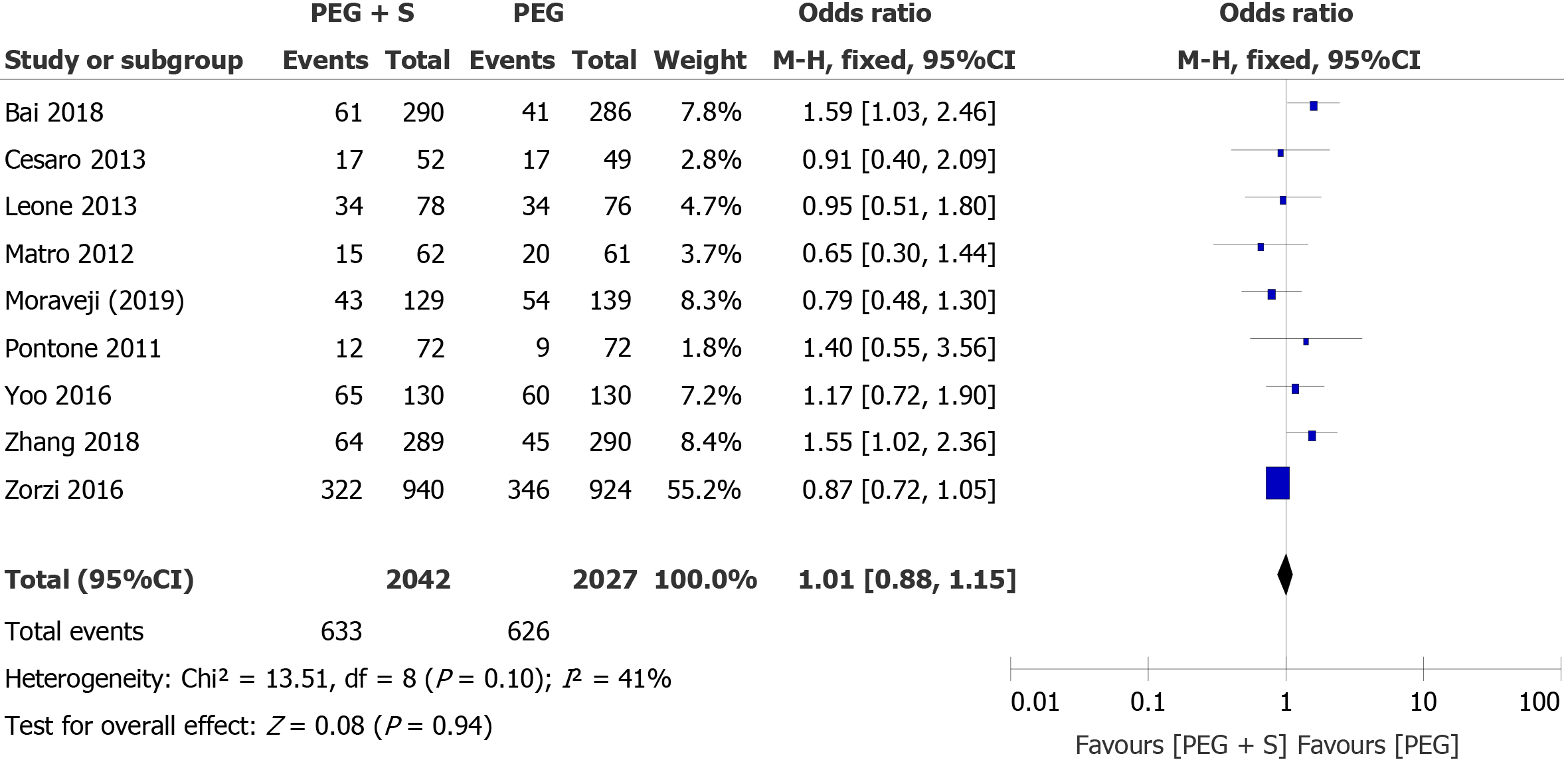
ADR: For the primary endpoint, nine studies reported data on the ADR, including 4069 patients (2042 patients treated with PEG plus simethicone and 2027 patients treated with PEG). The overall ADR during colonoscopy was similar in both groups: 30.9% in the PEG group and 31.0% in the PEG plus simethicone group. The heterogeneity among the studies was not significant (I² = 41%; P = 0.10). According to the fixed-effects model, the pooled OR was not significant (OR = 1.01; 95%CI: 0.88-1.15; P = 0.94), suggesting that there was no statistically significant difference in the ADR during colonoscopy between the two groups (Figure 2). Begg’s funnel plots and Egger’s regression test revealed that there was no significant effect of publication bias on the overall ADR (P = 0.307).
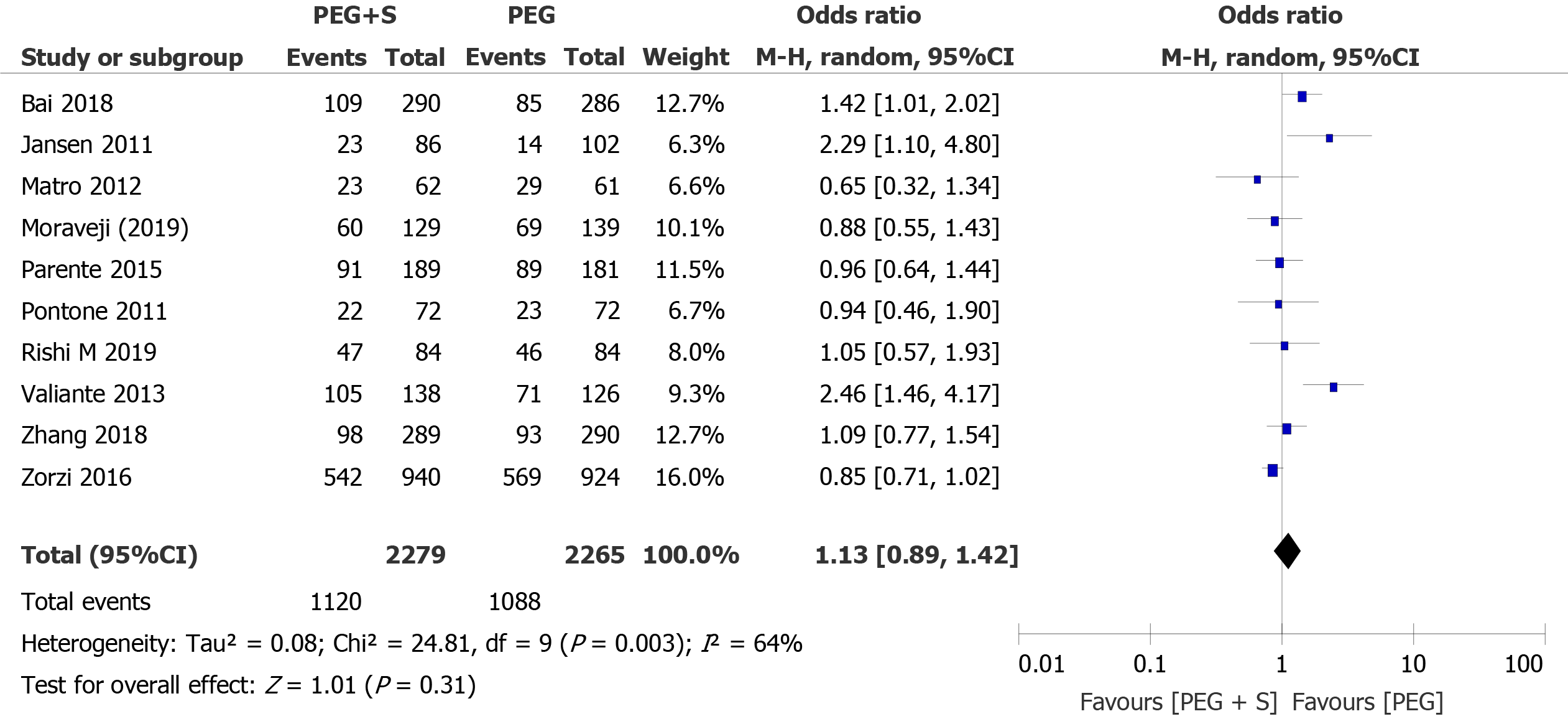
PDR: Overall, the PDR was available in 10 studies, including 4544 patients (2279 patients treated with PEG plus simethicone and 2265 patients treated with PEG). The overall PDR was higher in the group treated with simethicone during colonoscopy (49.1% vs 48.0%). The heterogeneity among the studies was significant (I² = 64%; P = 0.003). The pooled OR, according to a random-effects model for PDR (OR = 1.13; 95%CI: 0.89-1.42; P = 0.31), was not significantly different between the two groups (Figure 3). Egger’s regression test revealed that there was no significant effect of publication bias on the overall PDR (P = 0.221).
Adverse events: Sixteen studies reported data on adverse events, including bloating, vomiting, nausea, abdominal pain, and sleep disturbance. Simethicone significantly reduced the incidence of bloating (15.8% vs 25.3%) (OR = 0.52; 95%CI: 0.44-0.63, P < 0.00001). There were no statistically significant differences in other adverse events. Egger’s regression test revealed that there was no significant effect of publication bias.
Sensitivity analyses: We performed further sensitivity analyses to assess the impact on the heterogeneity by the exclusion of one or more studies at a time. There was statistically significant heterogeneity for the ADR in the right colon (heterogeneity P = 0.09, I2 = 58%). When Bai et al[14] was excluded, it no longer showed heterogeneity for the ADR (heterogeneity P = 0.18, I² = 45%). The other two outcomes had significant heterogeneity, including the PDR and adverse events of bloating. When Valiante et al[21] was excluded, they no longer showed heterogeneity of the PDR. The studies associated with the heterogeneity of each outcome are listed in Table 3.
| Number of trials | Number of patients | OR/MD (95%CI) | P value | I2 | Study associated with heterogeneity | |
| Primary outcome | ||||||
| ADR | 9 | 4069 | 1.01 (0.88-1.15) | 0.94 | 41% | - |
| Proportion of ADR | ||||||
| < 25% | 3 | 1299 | 1.55 (1.16-2.07) | 0.003 | 0% | - |
| ≥ 25% | 6 | 2770 | 0.88 (0.76-1.03) | 0.12 | 0% | - |
| Dose of simethicone | ||||||
| ≥ 400 mg | 5 | 1806 | 1.21 (0.97-1.50) | 0.09 | 50% | - |
| < 400 mg and NR | 4 | 2263 | 0.89 (0.75-1.06) | 0.20 | 0% | |
| Size of adenoma | ||||||
| < 10 mm | 2 | 1155 | 2.36 (1.79-3.10) | < 0.00001 | 29% | - |
| ≥ 10 mm | 2 | 1155 | 1.39 (0.67-2.86) | 0.38 | 0% | - |
| Location of adenoma | ||||||
| Right colon | 3 | 1299 | 2.61 (1.43-4.76) | 0.002 | 58% | Bai 2018 (I2 = 45%) |
| Left colon | 3 | 1299 | 1.44 (1.02-2.02) | 0.04 | 23% | - |
| Regions of the populations | ||||||
| Asia | 3 | 1415 | 1.45 (1.12-1.87) | 0.005 | 0% | - |
| Not-Asia | 5 | 2386 | 0.88 (0.74-1.04) | 0.14 | 0% | - |
| PDR | 10 | 4544 | 1.13 (0.89-1.42) | 0.31 | 64% | Valiante 2013 (I2 = 41%) |
| Dose of simethicone | ||||||
| ≥ 400 mg | 4 | 1546 | 1.06 (0.80-1.41) | 0.67 | 40% | |
| < 400 mg and NR | 6 | 2998 | 1.23 (0.85-1.79) | 0.28 | 74% | Valiante 2013 (I2 = 41%) |
| Size of adenoma | ||||||
| < 10 mm | 3 | 2498 | 0.93 (0.79-1.09) | 0.37 | 46% | - |
| ≥ 10 mm | 2 | 2128 | 0.98 (0.78-1.22) | 0.84 | 0% | - |
| Proportion of PDR | ||||||
| < 40% | 4 | 1487 | 1.29 (0.97-1.72) | 0.08 | 31% | - |
| ≥ 40% | 6 | 3057 | 1.03 (0.75-1.41) | 0.86 | 67% | Valiante 2013 (I2 = 0%) |
| Regions of the populations | ||||||
| Asia | 2 | 1155 | 1.24 (0.95-1.62) | 0.11 | 14% | - |
| Not-Asia | 8 | 3389 | 1.10 (0.82-1.47) | 0.53 | 66% | Valiante 2013 (I2 = 22%) |
| Secondary outcome | ||||||
| Adverse events | ||||||
| Bloating | 11 | 3049 | 0.51 (0.36-0.73) | 0.0002 | 67% | Repici 2012 (I² = 49%) |
| Nausea | 14 | 3397 | 1.03 (0.87-1.22) | 0.69 | 33% | - |
| Vomiting | 9 | 2514 | 1.02 (0.75-1.40) | 0.89 | 0% | - |
| Abdominal pain | 15 | 3669 | 0.89 (0.72-1.10) | 0.29 | 42% | - |
| Sleep disturbance | 9 | 1990 | 0.81 (0.64-1.01) | 0.06 | 25% | - |
Subgroup analyses: The results of the subgroup analyses for the ADR and PDR in relation to sites of colorectal adenomas or polyps (right or left colon), sizes of adenomas or polyps (≥ 10 mm or < 10 mm), populations (Asian or non-Asian), dose of simethicone (≥ 400 mg or < 400 mg and NR), and proportion of ADR (≥ 25% or < 25%) are shown in Table 3.
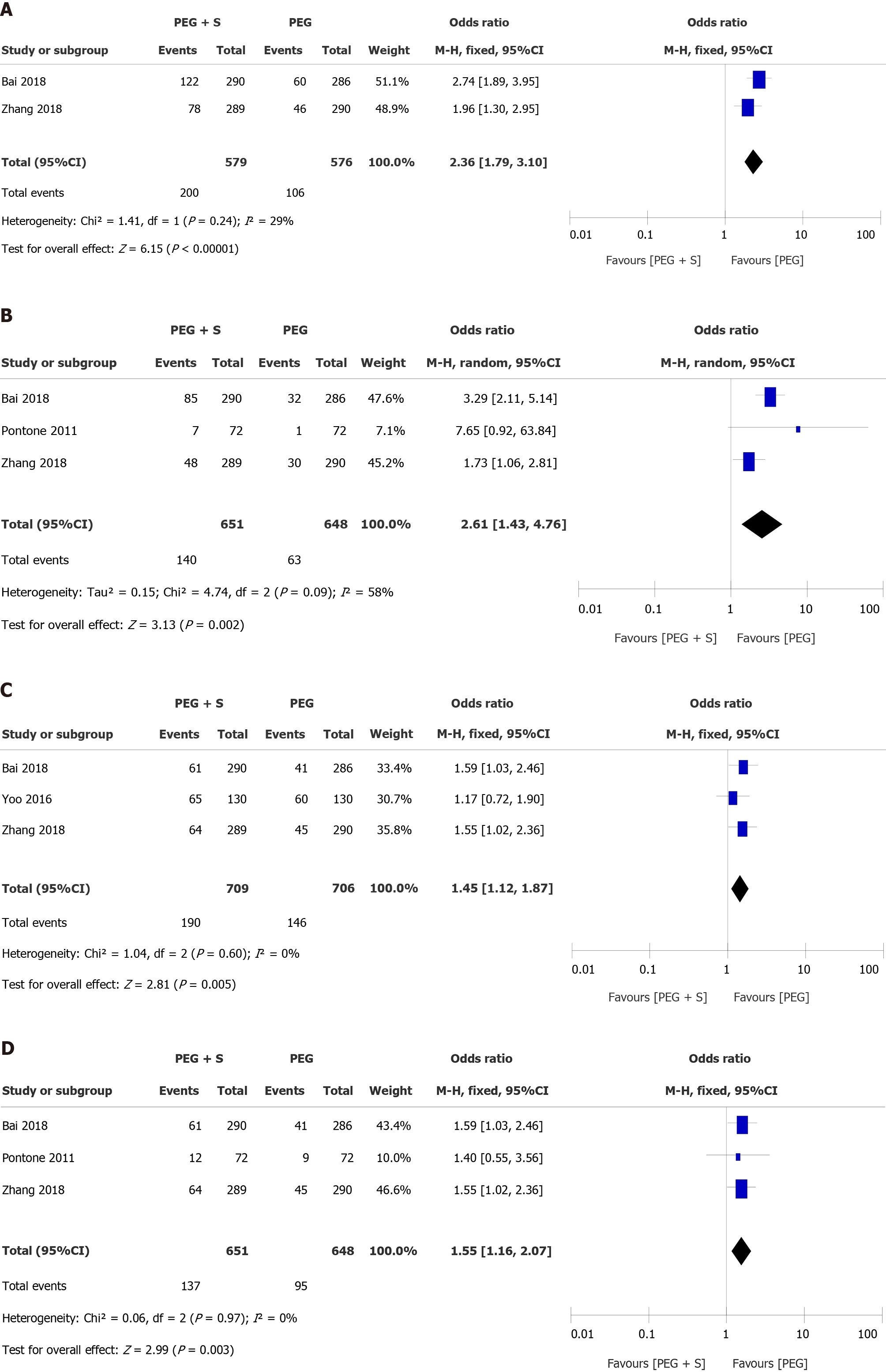
The analysis separately revealed that there was no significant difference (OR = 1.39, 95%CI: 0.67-2.86, P = 0.38) or heterogeneity (P = 0.48, I² = 0%) between the two groups for ADR ≥ 10 mm. However, our study displayed a significant increase in the ADR for small adenomas (< 10 mm) during colonoscopy in the group treated with simethicone (OR = 2.36; 95%CI: 1.79-3.10; P < 0.00001) (Figure 4A).
When analyzed separately, a significantly larger proportion of ADR in the right colon was present in the PEG plus simethicone group (21.5% vs 9.7%, OR = 2.61, 95%CI: 1.43-4.76, P = 0.002) (Figure 4B). In addition, the ADR in the left colon was also higher than that in the PEG group, with borderline statistical significance (13.8% vs 10.0%, P = 0.04).
The subgroup analysis revealed a significant increase in the ADR in the studies from Asia in the PEG with simethicone group (26.8% vs 20.7%, OR= 1.45, 95%CI: 1.12-1.87, P = 0.005) (Figure 4C), and a baseline ADR < 25% of the studies included was associated with a significant benefit of simethicone (OR = 1.55, 95%CI: 1.16-2.07, P = 0.003) (Figure 4D). In addition, our analysis revealed that there was no significant difference in ADR between the two groups with respect to the dose of simethicone, suggesting that the low dose of simethicone was as effective as the high dose with respect to the detection of benign colorectal tumors.
The comparison of PDR between the two groups showed no differences in the proportion of PDR, dose of simethicone, size of polyps, or populations when simethicone was added.
The effectiveness of colonoscopy could significantly reduce the incidence and mortality of CRC[33], depending on adequate bowel preparation and removal of colorectal precancerous lesions[34]. Inadequate bowel preparation increases economic costs, prolongs procedure times, and increases the likelihood of potential lesions being missed, especially those in the proximal colon[35].
Simethicone is an effective antifoaming agent used during endoscopic procedures. The Gastroenterological Society of Australia consensus panel found that the current evidence supported the use of simethicone for improving visibility and that it likely facilitates adenoma detection at colonoscopy[36]. Although simethicone addition to PEG solution could improve bowel cleanness and mucosal visibility[37], our results found that simethicone did not affect the total ADR or PDR. This outcome might be related to the possible explanation that solid stool was unlikely to be cleaned, although simethicone could improve the overall bowel cleanness.
The ADR has been recognized as the most important indicator of colonoscopy quality. The current international guidelines have recommended that the ADR should be ≥ 25% overall as the minimal requirement for surveillance colonoscopy[38]. In the subgroup analysis, we revealed a positive effect of simethicone with statistical significance in the low ADR group (< 25%). An interesting finding in our study was that the population of the low ADR group was Asian. This phenomenon might be related to the genes, diet, and/or microbiota of Asians.
The most important finding in our study was that simethicone could significantly improve detection of small adenomas (< 10 mm) of the proximal colon. The main reason is that simethicone can improve bowel preparation, especially in the right colon[39]. Because bubbles usually present in the ascending colon, bubble elimination could enhance the ability to detect smaller proximal adenomas. A previous study revealed that missed cancers in the proximal colon were more often found with poor bowel preparation[40]. A previous study reported that CRC in Eastern China has undergone a rightward change in the site distribution over the past two decades[41]. Therefore, improving the effectiveness of right-sided cleansing plays a key role in improving compliance with screening programs, which is crucial for screening efficiency in CRC prevention. However, simethicone did not significantly affect the ADR in the left colon, which might be associated with the small samples in the studies included. Therefore, further large clinical trials are necessary to confirm our results.
Although a recent study reported a 10% increase in the detection rate of colorectal polyps when simethicone was added to the water pump during colonoscopy[42], residual simethicone in biopsy channels could promote biofilm formation[43]. In addition, endoscopists with higher ADRs likely spent more time cleaning the colon. Simethicone addition to PEG solution could decrease the infection risk from endoscope transmission[31]. However, the optimal dose of simethicone has yet to be ascertained[44]. The addition of 2–3 mL of 120 mg/mL simethicone to lavage fluid was recommended [33]. In the subgroup analysis, we compared the effect of low-dose simethicone (< 400 mg) to that of high-dose simethicone (≥ 400 mg) for the ADR and PDR. Our results revealed that simethicone at a high or low dose made no significant difference in terms of ADR and PDR, suggesting that the low dose was not inferior to the high dose, similar to the study of Li et al[45]. Further research is required to determine the optimal dose of simethicone in clinical practice.
The strengths were as follows in our study. First, subgroup analyses and sensitivity analyses were conducted to seek potential reasons. To reduce possible bias, we conducted sensitivity analyses to assess the impact on the heterogeneity by excluding one or more studies at a time and performing subgroup analyses according to the site and size of colorectal benign tumors, the population included, and the proportion of ADR. There was no significant heterogeneity found in the meta-analysis of the ADR, except for right-side ADR. When Valiante et al[21] study was excluded, it no longer showed heterogeneity of the PDR. Second, our results of the subgroup analyses for the ADR and PDR included the population included and the dose of simethicone before colonoscopy. Third, 20 studies were included in our meta-analysis. This large number of studies allowed for firm conclusions and adequate subgroup analyses. Therefore, the results of our study are convincing.
There are several limitations to our meta-analysis. First, our meta-analysis was restricted to publications written in English, which might have produced potential selection bias. Second, all of the included studies were single blinded for outcome assessment; therefore, further double-blind randomized controlled trials should be conducted to confirm the positive effects of simethicone. Third, demographic and procedure data, such as race, diet, microbiota, and genes, might have been interesting to evaluate, but these data were not analyzed due to the limited condition. Fourth, although the endoscopists were trained adequately, the effects of observer bias cannot be ignored.
In conclusion, we believe that simethicone might improve small ADRs, especially in the proximal colon, for colonoscopy in Asians with low baseline ADRs. Simethicone at a low dose was not inferior to that at a high dose with respect to the detection of benign colorectal tumors. Additional large clinical trials are necessary to validate our results and to evaluate the ideal dose of simethicone.
The incidence and mortality of colorectal cancer have been rapidly increasing in Asian countries, and inadequate bowel preparation is related to an increased risk of missed benign colorectal tumors and more discomfort for patients.
Simethicone is an effective and safe antifoaming agent for use during endoscopic procedures. A combination of simethicone and polyethylene glycol has been shown to improve the visualization of the bowel for colonoscopy.
We performed a meta-analysis to investigate its effect on the detection of benign colorectal tumors.
The PubMed, EMBASE, and Cochrane Library databases were searched for articles published.
A significant effect of simethicone for diminutive adenomas (< 10 mm) and the adenoma detection rate in the proximal colon were revealed in the group taking simethicone. Moreover, it was a significant finding that the low dose simethicone was as effective as the high dose one with respect to the detection of colorectal benign tumors.
The addition of simethicone to polyethylene glycol might improve the detection of diminutive adenomas in the right colon by colonoscopy in Asia. Low-dose simethicone was recommended for the detection of benign colorectal tumors.
We believe that simethicone might improve small adenoma detection rates, especially in the proximal colon for colonoscopy in Asians with low baseline adenoma detection rates.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Panteris V S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LL
| 1. | Ng SC, Wong SH. Colorectal cancer screening in Asia. Br Med Bull. 2013;105:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, Kim DY, Oh JH. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat. 2012;44:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Meester RG, Doubeni CA, Lansdorp-Vogelaar I, Jensen CD, van der Meulen MP, Levin TR, Quinn VP, Schottinger JE, Zauber AG, Corley DA, van Ballegooijen M. Variation in Adenoma Detection Rate and the Lifetime Benefits and Cost of Colorectal Cancer Screening: A Microsimulation Model. JAMA. 2015;313:2349-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1551] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 5. | Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE; American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 7. | Clark BT, Protiva P, Nagar A, Imaeda A, Ciarleglio MM, Deng Y, Laine L. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150:396-405; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 8. | Bugajski M, Wieszczy P, Hoff G, Rupinski M, Regula J, Kaminski MF. Modifiable factors associated with patient-reported pain during and after screening colonoscopy. Gut. 2018;67:1958-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Horton N, Garber A, Hasson H, Lopez R, Burke CA. Impact of Single- vs. Split-Dose Low-Volume Bowel Preparations on Bowel Movement Kinetics, Patient Inconvenience, and Polyp Detection: A Prospective Trial. Am J Gastroenterol. 2016;111:1330-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24651] [Article Influence: 1760.8] [Reference Citation Analysis (3)] |
| 11. | Higgins JP. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37:1158-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 730] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 12. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15786] [Article Influence: 1578.6] [Reference Citation Analysis (1)] |
| 13. | Zhang S, Zheng D, Wang J, Wu J, Lei P, Luo Q, Wang L, Zhang B, Wang H, Cui Y, Chen M. Simethicone improves bowel cleansing with low-volume polyethylene glycol: a multicenter randomized trial. Endoscopy. 2018;50:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Bai Y, Fang J, Zhao SB, Wang D, Li YQ, Shi RH, Sun ZQ, Sun MJ, Ji F, Si JM, Li ZS. Impact of preprocedure simethicone on adenoma detection rate during colonoscopy: a multicenter, endoscopist-blinded randomized controlled trial. Endoscopy. 2018;50:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Yoo IK, Jeen YT, Kang SH, Lee JH, Kim SH, Lee JM, Choi HS, Kim ES, Keum B, Chun HJ, Lee HS, Kim CD. Improving of bowel cleansing effect for polyethylene glycol with ascorbic acid using simethicone: A randomized controlled trial. Medicine (Baltimore). 2016;95:e4163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 16. | Zorzi M, Valiante F, Germanà B, Baldassarre G, Coria B, Rinaldi M, Heras Salvat H, Carta A, Bortoluzzi F, Cervellin E, Polo ML, Bulighin G, Azzurro M, Di Piramo D, Turrin A, Monica F; TriVeP Working Group. Comparison between different colon cleansing products for screening colonoscopy. A noninferiority trial in population-based screening programs in Italy. Endoscopy. 2016;48:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Kump P, Hassan C, Spada C, Brownstone E, Datz C, Haefner M, Renner F, Schoefl R, Schreiber F. Efficacy and safety of a new low-volume PEG with citrate and simethicone bowel preparation for colonoscopy (Clensia): a multicenter randomized observer-blind clinical trial vs. a low-volume PEG with ascorbic acid (PEG-ASC). Endosc Int Open. 2018;6:E907-E913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Parente F, Vailati C, Bargiggia S, Manes G, Fontana P, Masci E, Arena M, Spinzi G, Baccarin A, Mazzoleni G, Testoni PA. 2-Litre polyethylene glycol-citrate-simethicone plus bisacodyl versus 4-litre polyethylene glycol as preparation for colonoscopy in chronic constipation. Dig Liver Dis. 2015;47:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Mussetto A, Frazzoni L, Paggi S, Dari S, Laterza L, Radaelli F, Hassan C, Triossi O, Fuccio L. Split dosing with a low-volume preparation is not inferior to split dosing with a high-volume preparation for bowel cleansing in patients with a history of colorectal resection: a randomized trial. Endoscopy. 2015;47:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | de Leone A, Tamayo D, Fiori G, Ravizza D, Trovato C, De Roberto G, Fazzini L, Dal Fante M, Crosta C. Same-day 2-L PEG-citrate-simethicone plus bisacodyl vs split 4-L PEG: Bowel cleansing for late-morning colonoscopy. World J Gastrointest Endosc. 2013;5:433-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Valiante F, Bellumat A, De Bona M, De Boni M. Bisacodyl plus split 2-L polyethylene glycol-citrate-simethicone improves quality of bowel preparation before screening colonoscopy. World J Gastroenterol. 2013;19:5493-5499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 22. | Cesaro P, Hassan C, Spada C, Petruzziello L, Vitale G, Costamagna G. A new low-volume isosmotic polyethylene glycol solution plus bisacodyl versus split-dose 4 L polyethylene glycol for bowel cleansing prior to colonoscopy: a randomised controlled trial. Dig Liver Dis. 2013;45:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Gentile M, De Rosa M, Cestaro G, Forestieri P. 2 L PEG plus ascorbic acid versus 4 L PEG plus simethicon for colonoscopy preparation: a randomized single-blind clinical trial. Surg Laparosc Endosc Percutan Tech. 2013;23:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Matro R, Tupchong K, Daskalakis C, Gordon V, Katz L, Kastenberg D. The effect on colon visualization during colonoscopy of the addition of simethicone to polyethylene glycol-electrolyte solution: a randomized single-blind study. Clin Transl Gastroenterol. 2012;3:e26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Repici A, Cestari R, Annese V, Biscaglia G, Vitetta E, Minelli L, Trallori G, Orselli S, Andriulli A, Hassan C. Randomised clinical trial: low-volume bowel preparation for colonoscopy - a comparison between two different PEG-based formulations. Aliment Pharmacol Ther. 2012;36:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Jansen SV, Goedhard JG, Winkens B, van Deursen CT. Preparation before colonoscopy: a randomized controlled trial comparing different regimes. Eur J Gastroenterol Hepatol. 2011;23:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Pontone S, Angelini R, Standoli M, Patrizi G, Culasso F, Pontone P, Redler A. Low-volume plus ascorbic acid vs high-volume plus simethicone bowel preparation before colonoscopy. World J Gastroenterol. 2011;17:4689-4695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Lazzaroni M, Petrillo M, Desideri S, Bianchi Porro G. Efficacy and tolerability of polyethylene glycol-electrolyte lavage solution with and without simethicone in the preparation of patients with inflammatory bowel disease for colonoscopy. Aliment Pharmacol Ther. 1993;7:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 29. | McNally PR, Maydonovitch CL, Wong RK. The effect of simethicone on colonic visibility after night-prior colonic lavage. A double-blind randomized study. J Clin Gastroenterol. 1989;11:650-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | McNally PR, Maydonovitch CL, Wong RK. The effectiveness of simethicone in improving visibility during colonoscopy: a double-blind randomized study. Gastrointest Endosc. 1988;34:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Moraveji S, Casner N, Bashashati M, Garcia C, Dwivedi A, Zuckerman MJ, Carrion A, Ladd AM. The role of oral simethicone on the adenoma detection rate and other quality indicators of screening colonoscopy: a randomized, controlled, observer-blinded clinical trial. Gastrointest Endosc. 2019;90:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Rishi M, Kaur J, Ulanja M, Manasewitsch N, Svendsen M, Abdalla A, Vemala S, Kewanyama J, Singh K, Singh N, Gullapalli N, Osgard E. Randomized, double-blinded, placebo-controlled trial evaluating simethicone pretreatment with bowel preparation during colonoscopy. World J Gastrointest Endosc. 2019;11:413-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M; Italian Multicentre Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 497] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 34. | Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG 2nd, Park WG, Rizk MK, Sawhney MS, Shaheen NJ, Wani S, Weinberg DS. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 832] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 35. | Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 698] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 36. | Devereaux BM, Taylor ACF, Athan E, Wallis DJ, Brown RR, Greig SM, Bailey FK, Vickery K, Wardle E, Jones DM. Simethicone use during gastrointestinal endoscopy: Position statement of the Gastroenterological Society of Australia. J Gastroenterol Hepatol. 2019;34:2086-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Pan P, Zhao SB, Li BH, Meng QQ, Yao J, Wang D, Li ZS, Bai Y. Effect of supplemental simethicone for bowel preparation on adenoma detection during colonoscopy: A meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2019;34:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson DJ. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153:307-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 509] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 39. | Benmassaoud A, Parent J. Canadian Association of Gastroenterology Position Statement on the Impact of Simethicone on Endoscope Reprocessing. J Can Assoc Gastroenterol. 2018;1:40-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Doubeni CA, Corley DA, Quinn VP, Jensen CD, Zauber AG, Goodman M, Johnson JR, Mehta SJ, Becerra TA, Zhao WK, Schottinger J, Doria-Rose VP, Levin TR, Weiss NS, Fletcher RH. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2018;67:291-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 281] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 41. | Zhang S, Cui Y, Weng Z, Gong X, Chen M, Zhong B. Changes on the disease pattern of primary colorectal cancers in Southern China: a retrospective study of 20 years. Int J Colorectal Dis. 2009;24:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Kutyla M, O'Connor S, Gurusamy SR, Gururatsakul M, Gould K, Whaley A, Kendall BJ, Hourigan L, Holtmann GJ. Influence of Simethicone Added to the Rinse Water during Colonoscopies on Polyp Detection Rates: Results of an Unintended Cohort Study. Digestion. 2018;98:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Barakat MT, Huang RJ, Banerjee S. Simethicone is retained in endoscopes despite reprocessing: impact of its use on working channel fluid retention and adenosine triphosphate bioluminescence values (with video). Gastrointest Endosc. 2019;89:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 44. | Hassan C, East J, Radaelli F, Spada C, Benamouzig R, Bisschops R, Bretthauer M, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Fuccio L, Awadie H, Gralnek I, Jover R, Kaminski MF, Pellisé M, Triantafyllou K, Vanella G, Mangas-Sanjuan C, Frazzoni L, Van Hooft JE, Dumonceau JM. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:775-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 347] [Article Influence: 57.8] [Reference Citation Analysis (4)] |
| 45. | Li DF, Luo MH, Du QQ, Zhang HY, Tian YH, Liu TT, Shi RY, Xiong F, Lai MG, Li YX, Luo S, Song Y, Wu BH, Xu ZL, Zhang DG, Yao J, Wang LS. Efficacy of low-dose versus high-dose simethicone with polyethylene glycol for bowel preparation: A prospective randomized controlled trial. J Gastroenterol Hepatol. 2020;35:1488-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |