Published online May 26, 2021. doi: 10.12998/wjcc.v9.i15.3498
Peer-review started: October 16, 2020
First decision: December 28, 2020
Revised: January 8, 2021
Accepted: March 17, 2021
Article in press: March 17, 2021
Published online: May 26, 2021
Processing time: 207 Days and 7.5 Hours
Cholesterol gallstone (CG) is a common, frequent biliary system disease in China, with a complex and multifactorial etiology. Declined gallbladder motility reportedly contributes to CG pathogenesis. Furthermore, interstitial Cajal-like cells (ICLCs) are reportedly present in human and guinea pig gallbladder tissue. ICLCs potentially contribute to the regulation of gallbladder motility, and aberrant conditions involving the loss of ICLCs and/or a reduction in its pacing potential and reactivity to cholecystokinin may promote CG pathogenesis. This review discusses the association between ICLCs and CG pathogenesis and provides a basis for further studies on the functions of ICLCs and the etiologies of CG.
Core Tip: Interstitial Cajal-like cells (ICLCs) in the gallbladder have been reported to play an important role in the regulation of gallbladder motility. Loss and/or dysfunction of ICLCs may contribute to motion abnormality of the gallbladder and promote cholesterol gallstone (CG) formation. However, the underlying mechanism is still unclear. This mini-review highlights recent findings on the association between gallbladder ICLCs and CG formation.
- Citation: Fu BB, Zhao JN, Wu SD, Fan Y. Cholesterol gallstones: Focusing on the role of interstitial Cajal-like cells. World J Clin Cases 2021; 9(15): 3498-3505
- URL: https://www.wjgnet.com/2307-8960/full/v9/i15/3498.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i15.3498
Cholesterol gallstone (CG) has an incidence rate of approximately 10%-15% among adults in Western countries[1] and 4.42%-11% among Chinese individuals[2], displaying a significantly increasing trend. CG has a complex etiology, including supersaturation of bile cholesterol, acceleration of monohydrate nucleation in bile, and gallbladder dysmotility, among which gallbladder dysmotility is the key factor in CG pathogenesis. Biliary dysmotility reportedly spatiotemporally facilitates the separation of cholesterol crystals from cholesterol-supersaturated bile and gradual enlarging after separation[3]. Numerous studies on the regulation of gallbladder motility have mostly focused on the reduction in gallbladder sensitivity to cholecystokinin (CCK), the reduction in gallbladder smooth muscle functions, dysfunction of CCK receptors, and regulation of the dysfunction of the extrahepatic biliary system. An increasing number of studies on interstitial Cajal cells (ICCs) in the digestive tract have reported that ICCs are extensively present in the digestive tract of humans and other mammals[4,5]. ICCs exterior to the digestive tract are called interstitial Cajal-like cells (ICLCs), and some studies have referred to them as “telocytes”[6] to distinguish them from other interstitial cells. Lavoie et al[7] reported ICLCs in guinea pig gallbladders. Pasternak et al[8] reported ICLCs in human gallbladder tissue and speculated that they are associated with the ability of the gallbladder to generate and transmit a spontaneous rhythm. Therefore, the functions of gallbladder ICLCs and their association with CG formation have received increasing attention. This review discusses the relevant findings and summarizes the current advancements on this topic.
Sun et al[9] discovered ICLCs in rat gallbladders through transmission electron microscopic imaging, reverse transcription-polymerase chain reaction, and Western blot analysis and described them as having a reticular structure with well-developed perinuclear endoplasmic reticulum, free ribosomes, and abundant mitochondria. Lavoie et al[7] reported the presence of ICLCs similar to gastrointestinal ICCs in guinea pig gallbladders and biliary tract systems through immunohistochemical, transmission electron microscopic, and laser-scanning confocal microscopic analyses and described them as extending along the muscle bundles and nerve fibers of gallbladder smooth muscles. Furthermore, spontaneous rhythmic electrical activity of the gallbladder muscularis is reportedly inhibited by Kit receptor tyrosine kinase inhibitors, indicating that ICLCs play an important role in the generation and conduction of rhythmic excitation signals in the gallbladder muscularis.
Early studies on ICCs have often used methods including methylene blue staining, argentaffin staining, and the Champy-Maillet method to observe the morphology and distribution of ICCs[4]; however, these methods do not have high specificity. The recent discovery of c-Kit expression by ICCs[10] has rendered c-Kit (CD117) an ICC-specific marker. Through immunohistochemical staining using the anti-c-Kit antibody has high specificity, thus furthering the understanding of ICCs.
ICCs express tyrosine kinase receptor c-Kit as a specific marker, whose ligand is stem cell factor (SCF). The SCF/c-Kit signaling pathway plays an important role in the development, differentiation, and phenotype maintenance of ICCs. For animals harboring spontaneous c-Kit mutations, ICCs do not normally occur and develop owing to the marked decline in c-Kit activity. Similarly, inhibition of c-Kit activity with anti-c-Kit antibodies exerts the same effect. After intraperitoneally injecting c-Kit-neutralizing antibody ACK-II in newly born mice, disorder in normal phasic contractions, obliteration of slow-wave activity, and absence of ICCs were observed in the mouse intestines[11]. Fan et al[12] concluded from the study that decreased number of ICCs and decreased expression of c-Kit and SCF in the terminal ileum were present in guinea pigs fed a high cholesterol diet. Simultaneously, SCF levels should be adequate in the ICC microenvironment during culture, since it is important for the occurrence, development, and phenotype maintenance of ICCs[13]. There are five currently known downstream pathways of c-Kit/SCF: The Src family kinase pathway, the phosphatidylinositol 3-kinase pathway, the phospholipase C pathway, the Ras/Raf-1/MAP pathway, and the Jak/STAT pathway. The roles of these pathways in the development, phenotype maintenance, and function regulation of ICCs need further study.
ICCs in the digestive system primarily maintain the pace of smooth muscle motility, enhance electrical activity transmission, and mediate and regulate neurotrans
Pacing cells for slow-wave potentials: Gastrointestinal smooth muscles have slow-wave potentials and functional potentials. Slow-wave potentials are also called basic electrical rhythms. ICCs, as the pacing points of the gastrointestinal tract rhythm, regulate cycles, spontaneous depolarization, and slow-wave potential generation[18]. A study reported that upon the removal of ICCs from smooth muscles, the remaining smooth muscle tissue completely or almost completely lost slow-wave potentials[19]. Balemba et al[20] reported that ICLCs, as the pacing cells of the gallbladder contraction rhythm, help generate slow-wave potentials. Fan et al[21] reported a significant reduction in the amplitude and frequency of slow waves in gallbladder muscle strips with damaged ICLCs, suggesting that ICLCs play an important role in generating and conducting rhythmic excitation of gallbladder smooth muscles.
Conducting cells for slow-wave potentials: Gap junctions widely connect ICCs with neurons and smooth muscle cells, serving as the structural basis for intercellular signal conduction. A related study reported that upon elimination of distal ICCs while retaining proximal ICCs, slow-wave potentials were observed at the proximal end, while distal slow-wave potentials were obliterated, suggesting that ICCs conduct slow-wave potentials, which cannot be conducted among smooth muscle cells[22]. It is currently believed that slow-wave potentials are conducted among smooth muscle cells through the network structure of ICCs to regulate their contraction[23].
Mediating the conduction of neural signals: ICCs are located between autonomic neuron terminals and muscle cells, forming connections similar to classical synapses with external neurons. This structure contains receptors coordinated with tachykinin released by excitatory neurons[24] and is sensitive to nitric oxide released by inhibitory neurons. Faussone-Pellegrini[25] accurately measured the connection distances from ICCs to smooth muscle cells and neurons to be only 20-30 nm, which is much smaller than the regular synaptic cleft of 50-100 nm at regular neuromuscular junctions, suggesting that ICCs potentially play an important role in conducting neural signals.
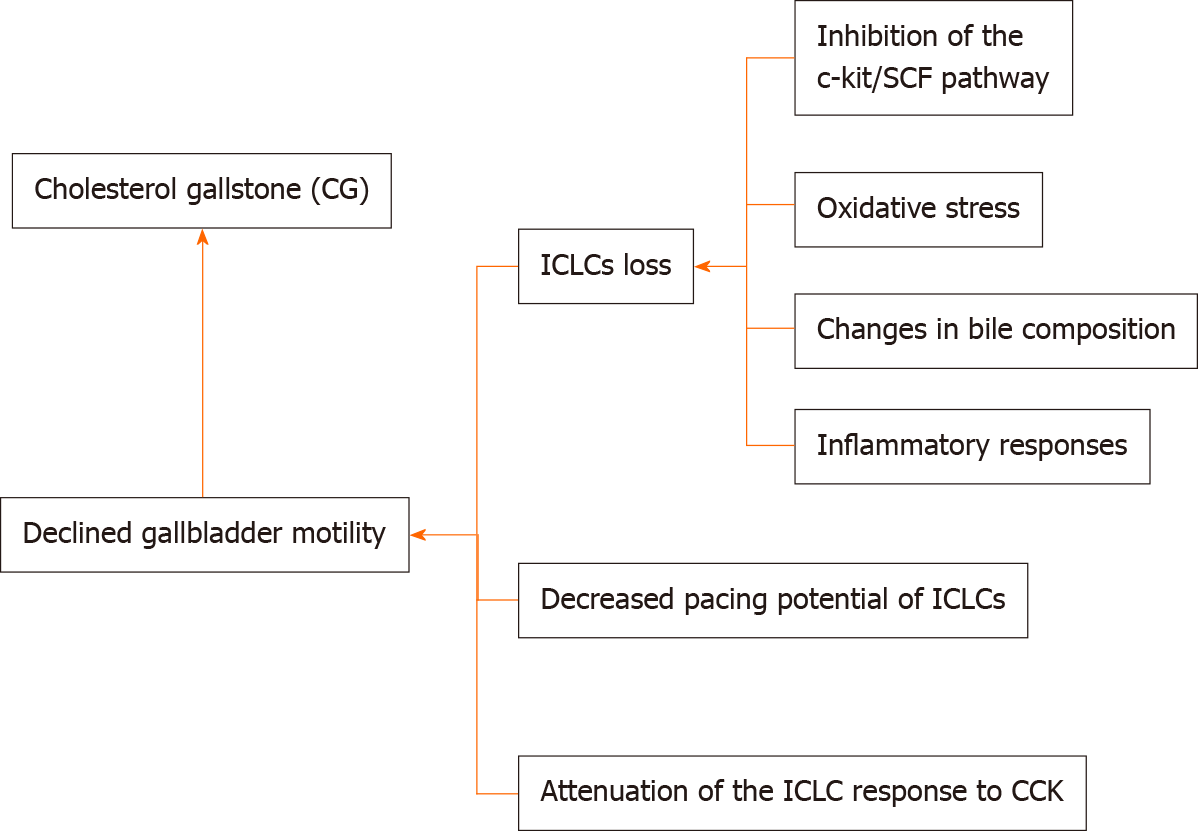
The reduction in gallbladder ICLCs weakens their role in regulating gallbladder motility, resulting in gallbladder dysmotility. Pasternak et al[26] conducted a comparative study in 30 patients with cholecystolithiasis and 25 patients without cholecystolithiasis and reported that ICLCs were significantly fewer in the gallbladder muscularis in the cholecystolithiasis group than in the control group. Furthermore, Tai et al[27] conducted a comparative study including 54 patients with cholecystolithiasis and 49 patients without cholecystolithiasis and reported that the gallbladder contraction rate and number of gallbladder ICLCs in the cholecystolithiasis group were significantly reduced relative to those in the control group. Huang et al[28] divided 30 guinea pigs into an experimental group administered a high-cholesterol diet and a control group administered a standard diet and reported that during CG formation, the number of ICLCs from the neck to the bottom of the gallbladder significantly decreased, and the number of apoptotic cells significantly increased, suggesting that this change potentially affects gallbladder ICLC function. Franks[29] further reported that the number of ICLCs in gallbladder smooth muscles in the cholecystolithiasis group was significantly lower than that in the normal group; however, the specific cause for the reduction in ICLCs during GC pathogenesis remains unclear. A previous study reported a potential phenotypic transformation in ICCs[30].
Further studies have focused on the causes of ICLC loss during CG pathogenesis, primarily considering the following aspects.
ICLC loss caused by oxidative stress: During CG pathogenesis, the oxidative stress response in the gallbladder tissue may lead to apoptosis of ICLCs and decrease their number. Tan[31] reported that the number of ICLCs was significantly lower in a rabbit model of GC than in healthy control rabbits, and the ultrastructure of ICLCs was altered and their network was damaged. Subsequently, cholesterol was supplemented at different concentrations to ICLCs, and with a gradual increase in the cholesterol concentration, the antioxidant stress indices, including superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX) activities, of ICLCs gradually decreased and the apoptotic rate of ICLCs in each group also significantly increased, both in a dose-dependent manner. When cholesterol scavengers of different concentrations were supplemented in culture solutions with corresponding cholesterol concentration, the antioxidant stress indices SOD and GSH-PX activities of ICLCs gradually increased, while the apoptotic rate of ICCs gradually decreased, suggesting that during CG pathogenesis, the increase in cholesterol concentration induces an oxidative stress response in gallbladder tissue, resulting in continuous apoptosis in ICLCs and a significant reduction in the number of ICLCs in gallbladder tissue. However, Kaji et al[32] conducted a study on murine jejunal ICCs and reported that ICC dysfunction mediated by interferon (IFN)-γ and lipopolysaccharide (LPS) largely results from NO-induced oxidative stress. However, the NO pathway only downregulated the ICC markers but did not cause apoptosis in ICCs or damage their ultrastructure. The association between oxidative stress response and gallbladder dysmotility requires further study.
ICLC loss caused by inhibition of the c-kit/SCF pathway: High cholesterol concentrations inhibit the c-Kit/SCF pathway, thereby affecting the development of ICLCs and reducing their number. Tan et al[33] performed a study including 18 patients undergoing laparoscopic cholecystectomy (LC) owing to symptoms similar to those in the experimental group and 14 patients undergoing surgical treatment for pancreatic head tumor as the control group. The gallbladder emptying fraction (GEF) of the two groups was determined through presurgical ultrasonic examination, and the GEF of the experimental group was lower than that of the control group. Gallbladder specimens were intraoperatively harvested and subjected to immunohistochemical analysis, revealing that the number of ICLCs in the experimental group was significantly lower than that in the control group. Reverse transcription-polymerase chain reaction and Western blot analyses revealed that c-Kit/SCF were significantly downregulated in the experimental group, indicating that the suppression of the c-Kit/SCF signaling pathway helps reduce the number of ICLCs. Fan et al[21] reported that c-Kit and SCF were significantly decreased in animal models administered a high-cholesterol diet, indicating that a high-cholesterol diet may cause ICLC damage by affecting the development of ICLCs. High cholesterol concentrations can inhibit the proliferation of ICLCs and promote apoptosis. This decrease in the ICLC proliferation rate might be caused by the inhibition of the SCF/c-Kit signaling pathway[34]. Feng et al[35] reported that compared with the Artemisia capillaris-treated group, the c-Kit expression and gallbladder motility in the high-cholesterol diet group significantly decreased, suggesting that Artemisia capillaris retains gallbladder motility by upregulating c-Kit. Another study reported that pluripotent stem cells can repair damaged ICCs in the digestive system, promote their occurrence and development, and restore their functions[36], thus potentially suggesting a novel therapeutic target for cholecystolithiasis and digestive tract diseases at the cellular level.
ICLC loss caused by changes in bile composition: Different bile components influence changes in the number of gallbladder ICLCs. Pasternak et al[37] performed a study involving 30 patients who underwent LC owing to CG and 25 patients who underwent cholecystectomy owing to other diseases as the experimental group and control group, respectively, and reported that the number of ICLCs in the gallbladder tissue was significantly lower in the experimental group than in the control group. Furthermore, glycocholic acid and taurocholic acid levels in the bile in the experimental group significantly decreased and were directly proportional to the number of ICLCs, suggesting that glycocholic acid and taurocholic acid in bile protect ICLCs. The toxicity of supersaturated bile may reduce the number of ICLCs as both levels decrease during lithogenesis. Therefore, bile composition potentially helps reduce the gallbladder ICLC density. Subsequently, Pasternak et al[38] conducted a study involving 25 CG patients undergoing LC as the experimental group and 15 patients undergoing surgical treatment for pancreatic head tumor as the control group and reported that the number of ICLCs on the gallbladder wall was significantly lower in the experimental group than in the control group. However, analysis of bile composition revealed a significant increase in the cholesterol saturation index (an indicator of bile lithogenicity) in the crystallization group, a significant reduction in the average glycocholic acid and taurocholic acid concentrations in the experimental group, and a significant increase in the concentration of polyunsaturated fatty acids (PUFAs) in the phospholipid fraction. No difference in ω-3 PUFA levels was observed between the two groups, while the ω-6 PUFA concentration and ω-6/ω-3 PUFA ratio were significantly increased in the experimental group, suggesting that the number of ICLCs in the muscularis propria of the gallbladder is potentially correlated with total PUFA and ω-6 PUFA levels and the ω-6/ω-3 PUFA ratio. Some observational studies reported that a high intake of saturated fatty acids and trans fatty acids can increase the incidence of cholecystolithiasis[39,40], while a high intake of PUFAs and monounsaturated fatty acids can reduce its risk[41]. An increase in ω-6 PUFA levels in lithogenic bile is an important factor affecting the density of gallbladder ICLCs and may be one of the pathophysiological factors in the CG progression. The reduction in the number of ICLCs may result from the toxic effect of supersaturated bile, while other bile components including ω-3 PUFA, glycocholic acid, and taurocholic acid protect ICLCs, thus potentially influencing the factors regulating gallbladder and extrahepatic bile duct motility.
ICLC loss caused by inflammatory responses: Chronic inflammation in the gallbladder wall may be another factor promoting ICLC-related decreases. Using an acute cholecystitis model, Lin et al[42] reported that the number of ICCs was reduced in each part of the gallbladder in the experimental group. A study reported that protection of gallbladder ICLCs was markedly greater through a reduction in tumor necrosis factor alpha (TNF-α) levels and inflammatory cell infiltration upon administration of a high-cholesterol diet rather than upon treatment with ursodeoxycholic acid, suggesting that supersaturated bile and hydrophobic bile acid promote gallbladder inflammation, and that the release of pro-inflammatory cytokine TNF-α can stimulate the TNF-α/Caspase8/Caspase3 cascade, thus inducing apoptosis in ICLCs[43]. Portincasa et al[44] reported that damage to gallbladder motility results from mild inflammation. Inflammatory factors alter the ICC phenotype by affecting their microenvironment, wherein Toll-like receptor-4, lipopolysaccharide, and TNF-α are involved[45,46]. However, Pasternak et al[26] reported that ICLC-related diseases are not associated with the grade of inflammation or number of mast cells. The association between the effects of inflammatory cells and the reduction in the ICLCs requires further study.
Disorder of intracellular calcium ions and inflammatory responses can weaken the ICLC pacing potential, resulting in gallbladder dysmotility. During gallbladder lithogenesis, excessive cholesterol in the cellular caveolae potentially reduces membrane fluidity[47], further causing a disorder in the intracytoplasmic calcium balance. Calcium ions reportedly contribute to slow wave generation in individual ICCs, and inositol triphosphate receptors are upregulated in ICCs, which regulate pacing currents by regulating the release of calcium ions[48]. Therefore, a high concentration of calcium ions is necessary for spontaneous electrical activity, and a disorder in such a balance between membrane and intracytoplasmic calcium will lead to swing disorder in the membrane potential of ICLCs, thus affecting the pacing function of ICLCs[49]. Furthermore, inflammation affects the pacing activity of ICC. Kaji et al[32] reported that inflammation mediated by IFN-γ and LPS in murine jejunal ICCs activates pro-inflammatory cytokines, thereby reducing the pacing activity of ICCs.
During gallstone formation, the low response of gallbladder ICLCs to CCK decreases gallbladder motility. Xu et al[50] reported that gallbladder ICLCs expressed CCK-A receptors in guinea pigs, and gallbladder tissue displayed a marked contractile response to CCK-A upon in vitro CCK excitation, while under the same conditions, the contractile response of gallbladder tissue to CCK-A significantly decreased upon eliminating ICLCs, indicating that ICLCs may mediate the contractile effect of CCK on gallbladder tissue. Fan et al[21] further confirmed that gallbladder ICLCs contribute to gallbladder contraction induced by CCK-8. However, a reduction in ICLCs in lithic gallbladders may affect the contractile effect of CCK on gallbladder tissue, thus causing gallbladder dysmotility. However, bile with a high cholesterol content can downregulate CCK-A receptors in the gallbladder, suggesting that high cholesterol levels reduce the ICLC response to CCK by downregulating CCK-A receptors in ICLCs and smooth muscles, resulting in gallbladder dysmotility[51].
The specific mechanisms of causes of CG have not been fully clarified. The decline in gallbladder motility is one of the most important factors of causes of CG. The decline in gallbladder motility caused by a high-cholesterol diet may largely result from further damage to the gallbladder ICLC network owing to the down-regulation of SCF and c-Kit. However, the mechanism underlying the regulation of SCF and c-Kit by high-cholesterol diet and the mechanism underlying the reduction in ICLCs and specific cellular alterations remain unclear. Furthermore, it remains unclear whether a high-cholesterol diet only affects ICLCs or also affects gallbladder smooth muscles and gallbladder contraction and whether it affects ICLCs or smooth muscles at the level of neuroregulation or hormonal regulation. The specific mechanism underlying the reduction in ICLCs during CG pathogenesis, the association between ICLC numbers and downstream SCF/c-Kit pathways, and the specific mechanisms of action of ICLCs during neuroregulation and hormonal regulation require further study.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta R S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6:172-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 739] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 2. | Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39:157-169, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 3. | Reshetnyak VI. Concept of the pathogenesis and treatment of cholelithiasis. World J Hepatol. 2012;4:18-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (5)] |
| 4. | Blair PJ, Rhee PL, Sanders KM, Ward SM. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil. 2014;20:294-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94:859-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 6. | Matyja A, Gil K, Pasternak A, Sztefko K, Gajda M, Tomaszewski KA, Matyja M, Walocha JA, Kulig J, Thor P. Telocytes: new insight into the pathogenesis of gallstone disease. J Cell Mol Med. 2013;17:734-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Lavoie B, Balemba OB, Nelson MT, Ward SM, Mawe GM. Morphological and physiological evidence for interstitial cell of Cajal-like cells in the guinea pig gallbladder. J Physiol. 2007;579:487-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Pasternak A, Gajda M, Gil K, Matyja A, Tomaszewski KA, Walocha JA, Kulig J, Thor P. Evidence of interstitial Cajal-like cells in human gallbladder. Folia Histochem Cytobiol. 2012;50:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Sun X, Yu B, Xu L, Dong W, Luo H. Interstitial cells of Cajal in the murine gallbladder. Scand J Gastroenterol. 2006;41:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Al-Shboul OA. The importance of interstitial cells of cajal in the gastrointestinal tract. Saudi J Gastroenterol. 2013;19:3-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:G602-G611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Fan Y, Wu SD, Fu BB, Weng C, Wang XP. Decreased number of interstitial cells of Cajal play an important role in the declined intestinal transit during cholesterol gallstone formation in guinea pigs fed on high cholesterol diet. Int J Clin Exp Med. 2014;7:1262-1268. [PubMed] |
| 13. | Nakahara M, Isozaki K, Vanderwinden JM, Shinomura Y, Kitamura Y, Hirota S, Matsuzawa Y. Dose-dependent and time-limited proliferation of cultured murine interstitial cells of Cajal in response to stem cell factor. Life Sci. 2002;70:2367-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Huizinga JD, Chen JH. Interstitial cells of Cajal: update on basic and clinical science. Curr Gastroenterol Rep. 2014;16:363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Okada T, Sasaki F, Honda S, Cho K, Matsuno Y, Itoh T, Kubota KC, Todo S. Disorders of interstitial cells of Cajal in a neonate with segmental dilatation of the intestine. J Pediatr Surg. 2010;45:e11-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Moraveji S, Bashashati M, Elhanafi S, Sunny J, Sarosiek I, Davis B, Torabi A, McCallum RW. Depleted interstitial cells of Cajal and fibrosis in the pylorus: Novel features of gastroparesis. Neurogastroenterol Motil. 2016;28:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Zani-Ruttenstock E, Zani A, Paul A, Diaz-Cano S, Ade-Ajayi N. Interstitial cells of Cajal are decreased in patients with gastroschisis associated intestinal dysmotility. J Pediatr Surg. 2015;50:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Pasternak A, Szura M, Gil K, Matyja A. Interstitial cells of Cajal - systematic review. Folia Morphol (Warsz). 2016;75:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Fintl C, Hudson NP. The interstitial cells of Cajal of the equine gastrointestinal tract: what we know so far. Equine Vet J. 2010;42:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Balemba OB, Bartoo AC, Nelson MT, Mawe GM. Role of mitochondria in spontaneous rhythmic activity and intracellular calcium waves in the guinea pig gallbladder smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2008;294:G467-G476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Fan Y, Wu S, Fu B, Weng C, Wang X. The role of interstitial Cajal-like cells in the formation of cholesterol stones in guinea pig gallbladder. Hepatol Int. 2015;9:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Zhu YF, Wang XY, Lowie BJ, Parsons S, White L, Kunze W, Pawelka A, Huizinga JD. Enteric sensory neurons communicate with interstitial cells of Cajal to affect pacemaker activity in the small intestine. Pflugers Arch. 2014;466:1467-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Takayama I, Horiguchi K, Daigo Y, Mine T, Fujino MA, Ohno S. The interstitial cells of Cajal and a gastroenteric pacemaker system. Arch Histol Cytol. 2002;65:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Wouters MM, Farrugia G, Schemann M. 5-HT receptors on interstitial cells of Cajal, smooth muscle and enteric nerves. Neurogastroenterol Motil. 2007;19 Suppl 2:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Faussone-Pellegrini MS. Histogenesis, structure and relationships of interstitial cells of Cajal (ICC): from morphology to functional interpretation. Eur J Morphol. 1992;30:137-148. [PubMed] |
| 26. | Pasternak A, Gil K, Matyja A, Gajda M, Sztefko K, Walocha JA, Kulig J, Thor P. Loss of gallbladder interstitial Cajal-like cells in patients with cholelithiasis. Neurogastroenterol Motil. 2013;25:e17-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Tai JX, Xu BH, Li HL, Qi SY. Study on the number of Cajal interstitial cells in gallbladder tissue and its correlation with contractile function in patients with cholecystolithiasis. J Clin Surg. 2018;26:865-867. |
| 28. | Huang ZP, Qiu H, Yu BP. Distribution changes of interstitial cells of Cajal during cholesterol gallstone formation in guinea pigs fed a high cholesterol diet. Int J Clin Exp Patho. 2018;11:1653-1659. |
| 29. | Franks I. Gallbladder: Loss of interstitial Cajal-like cells in the gallbladder might contribute to gallstone formation. Nat Rev Gastroenterol Hepatol. 2012;9:689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 248] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Tan YY. Studies on the role of Cajal interstitial cell in cholecystolithiasis and surgical methodology of endoscopic minimal invasive cholecystolithotomy. Dongnan Daxue. 2015;58-91. |
| 32. | Kaji N, Horiguchi K, Iino S, Nakayama S, Ohwada T, Otani Y, Firman, Murata T, Sanders KM, Ozaki H, Hori M. Nitric oxide-induced oxidative stress impairs pacemaker function of murine interstitial cells of Cajal during inflammation. Pharmacol Res. 2016;111:838-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Tan YY, Ji ZL, Zhao G, Jiang JR, Wang D, Wang JM. Decreased SCF/c-kit signaling pathway contributes to loss of interstitial cells of Cajal in gallstone disease. Int J Clin Exp Med. 2014;7:4099-4106. [PubMed] |
| 34. | Fu BB, Xu JH, Wu SD, Fan Y. Effect of cholesterol on in vitro cultured interstitial Cajal-like cells isolated from guinea pig gallbladders. World J Gastrointest Surg. 2020;12:226-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Feng H, Wang F, Wang C. C-Kit expression in the gallbladder of guinea pig with chronic calculous cholecystitis and the effect of Artemisia capillaris Thunb on interstitial cells of Cajal. Iran J Basic Med Sci. 2016;19:720-725. [PubMed] |
| 36. | Meng W, Zhou J, Elliott R, Murphy P, Ho V, O'Connor M. Is there a role for human pluripotent stem cells in modelling interstitial cells of Cajal and gut motility disorders? Curr Stem Cell Res Ther. 2015;10:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Pasternak A, Szura M, Matyja M, Tomaszewski KA, Matyja A. Does bile protect or damage interstitial Cajal-like cells in the human gallbladder? Folia Med Cracov. 2013;53:47-59. [PubMed] |
| 38. | Pasternak A, Bugajska J, Szura M, Walocha JA, Matyja A, Gajda M, Sztefko K, Gil K. Biliary Polyunsaturated Fatty Acids and Telocytes in Gallstone Disease. Cell Transplant. 2017;26:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Long-term intake of trans-fatty acids and risk of gallstone disease in men. Arch Intern Med. 2005;165:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Long-chain saturated fatty acids consumption and risk of gallstone disease among men. Ann Surg. 2008;247:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. The effect of long-term intake of cis unsaturated fats on the risk for gallstone disease in men: a prospective cohort study. Ann Intern Med. 2004;141:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Lin MJ, Chen L, Huang ZP, Qiu H, Yu BP. Neutrophils injure gallbladder interstitial Cajal-like cells in a guinea pig model of acute cholecystitis. J Cell Physiol. 2019;234:4291-4301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Wan JF, Chu SF, Zhou X, Li YT, He WB, Tan F, Luo P, Ai QD, Wang Q, Chen NH. Ursodeoxycholic acid protects interstitial Cajal-like cells in the gallbladder from undergoing apoptosis by inhibiting TNF-α expression. Acta Pharmacol Sin. 2018;39:1493-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Portincasa P, Di Ciaula A, Vendemiale G, Palmieri V, Moschetta A, Vanberge-Henegouwen GP, Palasciano G. Gallbladder motility and cholesterol crystallization in bile from patients with pigment and cholesterol gallstones. Eur J Clin Invest. 2000;30:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Wei J, Li N, Xia X, Chen X, Peng F, Besner GE, Feng J. Effects of lipopolysaccharide-induced inflammation on the interstitial cells of Cajal. Cell Tissue Res. 2014;356:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Zuo DC, Choi S, Shahi PK, Kim MY, Park CG, Kim YD, Lee J, Chang IY, So I, Jun JY. Inhibition of pacemaker activity in interstitial cells of Cajal by LPS via NF-κB and MAP kinase. World J Gastroenterol. 2013;19:1210-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Lavoie B, Nausch B, Zane EA, Leonard MR, Balemba OB, Bartoo AC, Wilcox R, Nelson MT, Carey MC, Mawe GM. Disruption of gallbladder smooth muscle function is an early feature in the development of cholesterol gallstone disease. Neurogastroenterol Motil. 2012;24:e313-e324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Zheng H, Park KS, Koh SD, Sanders KM. Expression and function of a T-type Ca2+ conductance in interstitial cells of Cajal of the murine small intestine. Am J Physiol Cell Physiol. 2014;306:C705-C713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Chen L, Yu B. Telocytes and interstitial cells of Cajal in the biliary system. J Cell Mol Med. 2018;22:3323-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Xu D, Yu BP, Luo HS, Chen LD. Control of gallbladder contractions by cholecystokinin through cholecystokinin-A receptors on gallbladder interstitial cells of Cajal. World J Gastroenterol. 2008;14:2882-2887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Zhu J, Han TQ, Chen S, Jiang Y, Zhang SD. Gallbladder motor function, plasma cholecystokinin and cholecystokinin receptor of gallbladder in cholesterol stone patients. World J Gastroenterol. 2005;11:1685-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |