Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3418
Peer-review started: December 2, 2020
First decision: December 31, 2020
Revised: December 31, 2020
Accepted: February 23, 2021
Article in press: February 23, 2021
Published online: May 16, 2021
Processing time: 147 Days and 10 Hours
Neoadjuvant treatment has become a standard of care for borderline or locally advanced pancreatic cancer and is increasingly considered even for up-front resectable disease. The aim of this article is to present the case of a 62-year-old patient with locally advanced pancreatic adenocarcinoma who was successfully treated with gemcitabine plus nab-paclitaxel after the failure of the first line treatment.
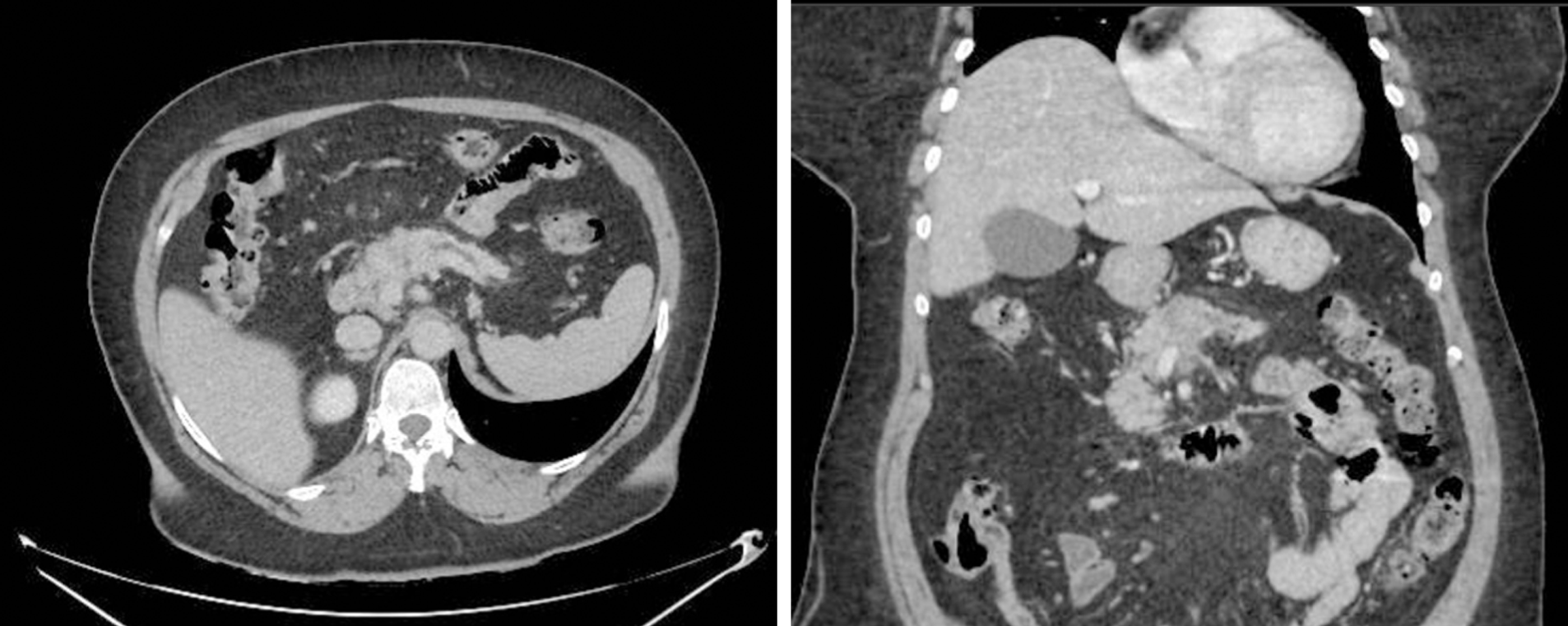
Computerized tomography scan and magnetic resonance imaging demonstrated a nodular lesion of ill-defined limits in the body of the pancreas, measuring approximately 4.2 cm × 2.7 cm, with an infiltrative aspect. The tumor had contact with the superior mesenteric vein, splenomesenteric junction and the proximal segment of the splenic artery, causing focal reduction of its lumens. Due to vascular involvement, neoadjuvant chemotherapy treatment with eight cycles of “folinic acid, 5-fluorouracil, irinotecan and oxa
Gemcitabine plus nab-paclitaxel may be effective as an alternative regimen when FOLFIRINOX fails as the first line of treatment, suggesting the need for further studies to identify which patients would benefit from each type of therapeutic approach.
Core Tip: Neoadjuvant treatment with folinic acid, 5-fluorouracil, irinotecan and oxaliplatine or gemcitabine plus nab-paclitaxel has become a standard of care for borderline or locally advanced pancreatic cancer and is increasingly considered even for up-front resectable disease.
- Citation: Meyer A, Carvalho BJ, Medeiros KA, Pipek LZ, Nascimento FS, Suzuki MO, Munhoz JV, Iuamoto LR, Carneiro-D'Alburquerque LA, Andraus W. Change in neoadjuvant chemotherapy could alter the prognosis of patients with pancreatic adenocarcinoma: A case report. World J Clin Cases 2021; 9(14): 3418-3423
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3418.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3418
Pancreatic cancer is among the most aggressive solid tumors, representing the fourth most common cause of death by cancer in both sexes worldwide[1]. In Brazil, it corresponds to the seventh and fourth cause of death from cancer in men and women, respectively[2]. Among pancreatic tumors, adenocarcinoma is the most common (85%), followed by neuroendocrine tumors (5%). The 5-year overall survival is 5%, but it becomes 2% in the case of metastatic disease. Those low survival rates are partially due to the late diagnosis of this disease. Indeed, 80% of pancreatic cancers are diagnosed at locally advanced or metastatic stages. Therefore, only 20% of cases can be treated definitively by a multimodality treatment[3].
Neoadjuvant treatment with “folinic acid, 5-fluorouracil, irinotecan and oxaliplatine” (FOLFIRINOX) or gemcitabine plus nab-paclitaxel has become a standard of care for borderline or locally advanced pancreatic cancer and is increasingly considered even for up-front resectable disease[4].
The aim of this article is to present the case of a 62-year-old patient with locally advanced pancreatic adenocarcinoma who was successfully treated with gemcitabine plus nab-paclitaxel after the failure of FOLFIRINOX as the first line of neoadjuvant treatment.
A 62-year-old female patient presented at a medical consultation with epigastric pain.
The patient presented with 30 d of epigastric pain with no improvement with the administration of a proton pump inhibitor.
No history of relevant past illness
In her personal history, she had non-insulin dependent type 2 diabetes mellitus and dyslipidemia
No significant findings in physical examination.
The tumor markers were within the reference values (carcinoembryonic antigen, carbohydrate antigen 19-9 and cancer antigen-125). Antibody panel was positive for mismatch repair endonuclease, MutS homolog 6, MutS homolog 2 and human mutL homolog 1 (Table 1).
| PMS2 | MSH6 | MSH2 | MLH1 |
| Clone: MRQ-28 | Clone: 44 | Clone: 25D12 | Clone: G168-728 |
| Reading: + | Reading: + | Reading: + | Reading: + |
In computerized tomography (CT) scan and magnetic resonance imaging (MRI) the pancreas had preserved dimensions, with emphasis on a nodular lesion of ill-defined limits in the body of the pancreas, measuring approximately 4.2 cm × 2.7 cm, with an infiltrative aspect. The tumor had contact with the superior mesenteric vein, splenomesenteric junction and the proximal segment of the splenic artery, causing focal reduction of its lumens. On the other hand, no contact was seen with the portal vein, superior mesenteric artery and the celiac trunk and its branches. Peripancreatic lymph nodes were prominent in number, less than 1.0 cm (Figure 1).
The final diagnosis of the presented case is pancreatic adenocarcinoma.
Due to vascular involvement, neoadjuvant chemotherapy treatment was started. Eight cycles of FOLFIRINOX were performed spaced apart for 2 wk, which consisted of oxaliplatin 85 mg/m² (day 1, for 120 min), calcium folinate 400 mg/m² (day 1, for 120 min), irinotecan 180 mg/m² (day 1, for 90 min) and fluorouracil 400 mg/m² (day 1, for 10 min).
The CT scan of the abdomen performed 4 mo after the start of chemotherapy treatment showed a reduction in size, presenting dimensions of 3.0 cm × 2.6 cm. Contact with the superior mesenteric vein, splenomesenteric junction and proximal segment of the splenic artery persisted, causing the reduction of the lumen of these vessels. The visible peripancreatic lymph nodes remained numerous but still smaller than 1 cm.
At the end of the cycles, surgery was performed to remove the tumor, given the absence of metastases detectable by imaging tests and the reduction of its size. During surgery, lesions suspected of metastasis in segments III and IV of the liver were observed, and a frozen section was performed that revealed infiltration by adenocarcinoma only of the segment III lesion, 1.5 cm in its longest axis. The procedure was interrupted without the removal of the pancreatic tumor.
Positron emission tomography/CT examination performed after an attempt to remove the tumor revealed hyperconcentration of radiopharmaceuticals in hepatic, celiac and hilar lymph nodes together with the emergence of the superior mesenteric artery, suspected of secondary neoplastic involvement. The hyperconcentration of the radiopharmaceutical in hepatic pericapsular regions of segments III, IVB and VI/VII and in muscle-adipose planes of the anterior abdominal wall suggested a probable occurrence of a post-surgical inflammatory process in these areas.
Chemotherapy treatment was restarted in four cycles of gemcitabine 1000 mg/m² (days 1, 8 and 15, for 30 min) plus nab-paclitaxel 125 mg/m² (days 1, 8 and 15, for 30 minutes) every 28 d. After the chemotherapy treatment, positron emission tomography/CT examination found a reduction in the glycolytic metabolism of the pancreatic tumor but with maintenance of its dimensions. No significant radiopharmaceutical concentration was detected in hepatic, celiac and superior mesenteric artery lymph nodes. The reduction of the radiopharmaceutical concentration in the hepatic and abdominal pericapsular regions previously mentioned corroborated the hypothesis of the presence of a post-surgical in
The gastroduodenopancreatectomy was performed 10 d after the last session of gemcitabine plus nab-paclitaxel with complete removal of the pancreatic lesion and hospital discharge on the 6th postoperative day. Anatomopathological examination revealed the absence of invasive neoplasia, presenting only foci of intraepithelial neoplasia with low-grade dysplasia and free margins. The dissected regional lymph nodes were free of neoplasia (ypT0 ypN0/15).
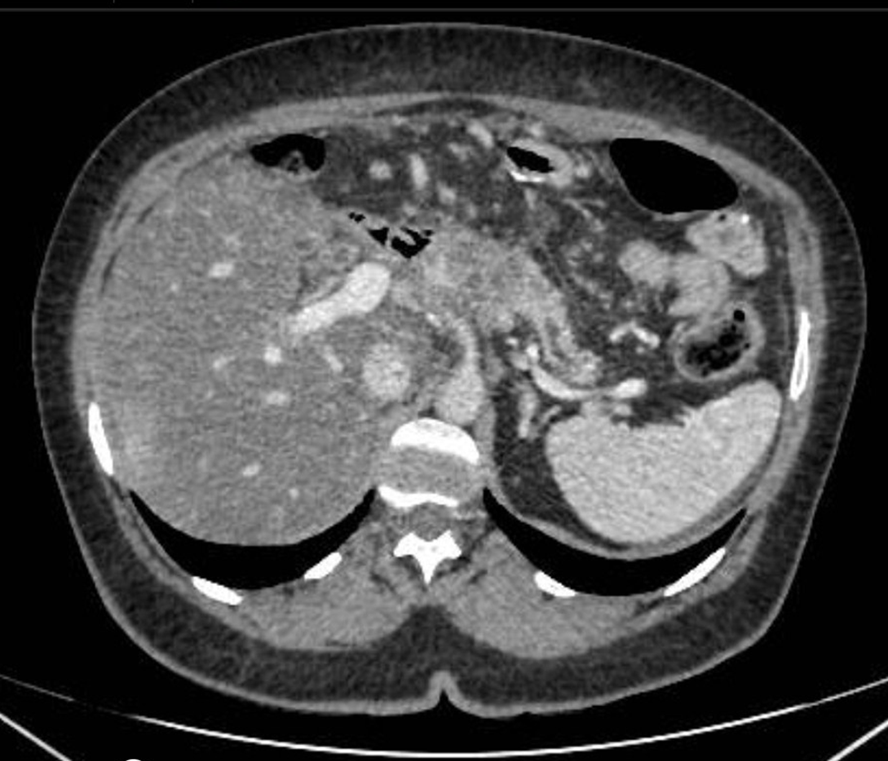
So far, 18 mo since the last surgery, there has been no tumor recurrence or late postoperative complications (Figure 2).
The incidence of pancreatic ductal adenocarcinoma is higher in men and in developed countries, for reasons that are not completely understood, but probably related to behavioral factors and greater longevity in these regions[5]. When diagnosing adenocarcinoma, CT scan and MRI is highly sensitive (96% and 93.5%, respectively), with CT scan being the most appropriate for assessing tumor resectability[6]. Currently, there is no established screening method for this type of cancer, which decreases the chances of early detection and increases the mortality rates of victims in developed and developing countries, since most tumors are shown to be unresect
Pancreatic ductal adenocarcinoma is an aggressive tumor associated with low rates of resectability at diagnosis (15%-20%)[9] and unfavorable prognosis, being considered a systemic disease even without evidence of metastases by imaging. Its staging is done through the Tumor, Node, Metastasis system of the American Joint Committee on Cancer, and with the use of imaging tests (CT and MRI) it can be classified as resectable, borderline, locally advanced and metastatic depending on its involvement with neighboring vascular structures[5].
In borderline and locally advanced pancreatic tumors (30%-40% of pancreatic tumors)[10], neoadjuvant chemotherapy has been considered a viable option to downstaging tumor, maximizing the potential for an R0 resection, treating micrometastatic disease early and increasing overall survival, since the only possible curative treatment of the disease involves tumor resection with negative margins[11-13]. Retrospective data suggest that neoadjuvant treatment may lead to results that are superior to those with a upfront surgery for pancreatic adenocarcinoma. Prospective, randomized trials ought to evaluate this further.
In the reported case, we had a borderline pancreatic tumor due to partial occlusion of the lumen of the superior mesenteric vein[14]. The normality of the tested markers, correlated to imaging tests, suggested the absence of a systemically advanced disease and good chances of resectability[15,16], mainly in association with neoadjuvant treatment. However, liver metastasis was not found on imaging, being detected only at the time of surgery. Such a peculiarity is in line with the literature regarding the establishment of magnetic resonance imaging as the gold standard for the detection of liver metastases greater than 3 mm, since the method presents high sensitivity (91%-97%) when compared to others, such as CT scan (71%-73.5%)[17]. Thus, it is possible that liver metastasis developed during chemotherapy, which indicates a possible failure of treatment with FOLFIRINOX.
Despite being considered equivalent to gemcitabine plus nab-paclitaxel as a first-line choice in guidelines of the National Comprehensive Cancer Network and American Society of Clinical Oncology, the FOLFIRINOX regimen has been chosen as the first treatment option in patients with good performance due to its greater response and evidence of increased survival[18-19]. In view of the failure of FOLFIRINOX, there is little evidence regarding the efficacy of gemcitabine plus nab-paclitaxel as a subsequent treatment in borderline or locally advanced pancreatic tumors, with Vreeland et al[20] identifying the benefit of the exchange mainly when done before the disease becomes metastatic. In this case, the switching of the schemes resulted in success, without the appearance of other liver metastases and with complete remission of the tumor as demonstrated in the anatomopathological examination, suggesting that gemcitabine plus nab-paclitaxel may be a good choice in the face of FOLFIRINOX failure, presenting a surprising result, rarely commented in the literature.
There is no doubt that pancreatic cancer patients need more tailored perioperative therapy than is currently available. Multimodal therapy for pancreatic cancer has never been more challenging in theory and practice than it is today.
There is still little evidence on the best chemotherapy choice for treating borderline and locally advanced pancreatic tumors. The reported case demonstrates that gemcitabine plus nab-paclitaxel may be effective when FOLFIRINOX fails as the first line of treatment, suggesting the need for further studies to identify which patients would benefit from each type of therapeutic approach.
The authors are thankful to Axel-Berg J for English corrections.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American College of Surgeons, No. 932068; Society of American Gastrointestinal and Endoscopic Surgeons, No. 201515986; European Association for Endoscopic Surgery, No. 4664; and Société Française de Chirurgie Endoscopique, No. 01224.
Specialty type: Surgery
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ding JX, Gupta N, Tsujinaka S S-Editor: Liu M L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20504] [Article Influence: 2050.4] [Reference Citation Analysis (20)] |
| 2. | Instituto Nacional de Cancer. Estatísticas de cancer. [cited 24 March 2020]. Available from: https://www.inca.gov.br/numeros-de-cancer. |
| 3. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1259] [Article Influence: 179.9] [Reference Citation Analysis (39)] |
| 4. | Napolitano F, Formisano L, Giardino A, Girelli R, Servetto A, Santaniello A, Foschini F, Marciano R, Mozzillo E, Carratù AC, Cascetta P, De Placido P, De Placido S, Bianco R. Neoadjuvant Treatment in Locally Advanced Pancreatic Cancer (LAPC) Patients with FOLFIRINOX or Gemcitabine NabPaclitaxel: A Single-Center Experience and a Literature Review. Cancers (Basel). 2019;11:981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694-9705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1003] [Cited by in RCA: 956] [Article Influence: 106.2] [Reference Citation Analysis (24)] |
| 6. | Chu LC, Goggins MG, Fishman EK. Diagnosis and Detection of Pancreatic Cancer. Cancer J. 2017;23:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 209] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 7. | Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JW. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 552] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 8. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2113] [Article Influence: 150.9] [Reference Citation Analysis (3)] |
| 9. | Klaiber U, Hackert T. Conversion Surgery for Pancreatic Cancer-The Impact of Neoadjuvant Treatment. Front Oncol. 2019;9:1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1168] [Article Influence: 77.9] [Reference Citation Analysis (1)] |
| 11. | Assifi MM, Lu X, Eibl G, Reber HA, Li G, Hines OJ. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery. 2011;150:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Xu JZ, Wang WQ, Zhang SR, Xu HX, Wu CT, Qi ZH, Gao HL, Li S, Ni QX, Yu XJ, Liu L. Neoadjuvant Therapy is Essential for Resectable Pancreatic Cancer. Curr Med Chem. 2019;26:7196-7211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Scheufele F, Hartmann D, Friess H. Treatment of pancreatic cancer-neoadjuvant treatment in borderline resectable/Locally advanced pancreatic cancer. Transl Gastroenterol Hepatol. 2019;4:32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol. 2014;20:10740-10751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 15. | Kim YC, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI, Shin JH. Can preoperative CA19-9 and CEA levels predict the resectability of patients with pancreatic adenocarcinoma? J Gastroenterol Hepatol. 2009;24:1869-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | van Manen L, Groen JV, Putter H, Vahrmeijer AL, Swijnenburg RJ, Bonsing BA, Mieog JSD. Elevated CEA and CA19-9 serum levels independently predict advanced pancreatic cancer at diagnosis. Biomarkers. 2020;25:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 17. | Karaosmanoglu AD, Onur MR, Ozmen MN, Akata D, Karcaaltincaba M. Magnetic Resonance Imaging of Liver Metastasis. Semin Ultrasound CT MR. 2016;37:533-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5629] [Article Influence: 402.1] [Reference Citation Analysis (1)] |
| 19. | Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, El-Rayes BF, Wang-Gillam A, Lacy J, Hosein PJ, Moorcraft SY, Conroy T, Hohla F, Allen P, Taieb J, Hong TS, Shridhar R, Chau I, van Eijck CH, Koerkamp BG. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 738] [Cited by in RCA: 689] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 20. | Vreeland TJ, McAllister F, Javadi S, Prakash LR, Fogelman DR, Ho L, Varadhachary G, Aloia TA, Vauthey JN, Lee JE, Kim MP, Katz MHG, Tzeng CD. Benefit of Gemcitabine/Nab-Paclitaxel Rescue of Patients With Borderline Resectable or Locally Advanced Pancreatic Adenocarcinoma After Early Failure of FOLFIRINOX. Pancreas. 2019;48:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |