Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3385
Peer-review started: November 17, 2020
First decision: December 28, 2020
Revised: December 31, 2020
Accepted: January 25, 2021
Article in press: January 25, 2021
Published online: May 16, 2021
Processing time: 163 Days and 8.6 Hours
Several reports with clinical, histological and imaging data have observed the involvement of lung vascular function to explain the severe hypoxemia in coronavirus disease 2019 (COVID-19) patients. It has been hypothesized that an increased pulmonary blood flow associated with an impairment of hypoxic pulmonary vasoconstriction is responsible for an intrapulmonary shunt. COVID-19 may lead to refractory hypoxemia (PaO2/FiO2 ratio below 100 mmHg) despite mechanical ventilation and prone positioning. We hypothesized that the use of a pulmonary vasoconstrictor may help decrease the shunt and thus enhance oxygenation.
We report our experience with three patients with refractory hypoxemia treated with almitrine to enhance oxygenation. Low dose almitrine (Vectarion®; Servier, Suresnes, France) was started at an infusion rate of 4 μg × kg/min on a central line. The PaO2/FiO2 ratio and total respiratory system compliance during almitrine infusion were measured. For the three patients, the PaO2/FiO2 ratio time-course showed a dramatic increase whereas total respiratory system compliance was unchanged. The three patients were discharged from the intensive care unit. The intensive care unit length of stay for patient 1, patient 2 and patient 3 was 30 d, 32 d and 31 d, respectively. Weaning from mechanical ventilation was performed 13 d, 18 d and 15 d after almitrine infusion for patient 1, 2 and 3, respectively. We found no deleterious effects on the right ventricular function, which was similar to previous studies on almitrine safety.
Almitrine may be effective and safe to enhance oxygenation in coronavirus disease 2019 patients. Further controlled studies are required.
Core Tip: It has been hypothesized that increased pulmonary blood flow associated with impaired hypoxic vasoconstriction may lead to significant intrapulmonary shunt that plays a major role in coronavirus disease 2019 related severe hypoxia. In this report, three coronavirus disease 2019 patients with refractory hypoxemia were treated with continuous infusion of almitrine, a specific pulmonary vasoconstrictor. Almitrine infusion enhanced oxygenation of patients with severe coronavirus disease 2019 related acute respiratory distress syndrome without any change in pulmonary artery pressures and right ventricular function.
- Citation: Huette P, Abou Arab O, Jounieaux V, Guilbart M, Belhout M, Haye G, Dupont H, Beyls C, Mahjoub Y. Almitrine for COVID-19 critically ill patients – a vascular therapy for a pulmonary vascular disease: Three case reports. World J Clin Cases 2021; 9(14): 3385-3393
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3385.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3385
Several reports with clinical, histological and imaging data in coronavirus disease 2019 (COVID-19) have observed that the involvement of lung vascular function may explain the severe hypoxemia[1-5]. It has been hypothesized that an increased pulmonary blood flow is responsible for an intrapulmonary shunt with a decrease of ventilation/perfusion ratio[2,3]. Recently, three retrospective case series reported the use of almitrine, a pulmonary vasoconstrictor, in COVID-19 patients with inconsistent effects on oxygenation and outcome[6-8]. However, no data on key safety factors, such as right ventricular function or pulmonary artery pressure, were reported in these series. We report our experience with three patients with refractory hypoxemia treated with almitrine to enhance oxygenation. Complete hemodynamic assessment with transesophageal echocardiography and pulmonary artery catheter was performed.
Patients were admitted at Amiens University hospital intensive care unit for severe acute respiratory distress syndrome (ARDS) related to COVID-19. Severe acute respiratory syndrome coronavirus-2 infection was confirmed by real-time reverse transcription polymerase chain reaction on a sample from nasopharyngeal swab. Patient condition deteriorated rapidly leading to mechanical ventilation and refractory hypoxemia despite medical management.
Case 1: The patient was admitted to the hospital 7 d after onset of symptoms (dyspnea and headache). Initial treatment included lopinavir/ritonavir and high flow nasal oxygen for 2 d. Respiratory worsening lead to mechanical ventilation 2 d after hospital admission.
Case 2: The patient was admitted to the hospital 11 d after onset of symptoms (dyspnea and cough). Initial treatment included lopinavir/ritonavir. Respiratory worsening required mechanical ventilation 5 d after intensive care unit (ICU) admission.
Case 3: The patient was admitted to the hospital 5 d after symptoms onset (anosmia, dyspnea and cough). He was admitted to the ICU because of respiratory worsening 6 d after hospital admission. After 5 d with high flow nasal oxygen, respiratory worsening required mechanical ventilation. After 2 d of mechanical ventilation, the patient developed severe ARDS.
The patients’ medical histories is presented in Table 1. The first patient was a 57-year-old female with a medical history of smoking and hypertension. The second patient was a 56-year-old female with a medical history of dyslipidemia and obesity (body mass index (BMI) = 39 kg/m2). The third patient was a 53-year-old male with a history of obesity (BMI = 40 kg/m2) and rheumatoid arthritis treated with steroids.
| Variables | Case 1 | Case 2 | Case 3 |
| Age in yr | 57 | 56 | 53 |
| BMI in kg/m2 | 38.9 | 39.0 | 40.1 |
| Gender | Female | Female | Male |
| Medical history | Smoking, hypertension, obesity | Dyslipidemia, obesity | Rheumatoid arthritis, obesity |
| Medication use | None | Statin | Steroids |
| Days from symptom onset to hospital admission in d | 7 | 11 | 5 |
| Days from hospital admission to ICU admission in d | 2 | 6 | 6 |
| High flow or low flow oxygen support prior to mechanical ventilation in d | 2 | 5 | 5 |
| Mechanical ventilation prior to almitrine infusion in d | 2 | 1 | 2 |
| Chest CT scan | GGO, crazy paving and severe lobar involvement (> 75%), no pulmonary embolism | GGO, crazy paving and moderate lobar involvement (50%-75%), no pulmonary embolism | GGO, bilateral crazy paving, consolidation, limited lobar involvement (25%), no pulmonary embolism |
| Respiratory management | Two sessions | No | Two sessions |
| Prone positioning before almitrine infusion/Position during almitrine infusion | Supine | Supine | Supine |
| Hemodynamic parameters ICU admission | |||
| HR as /min | 66 | 115 | 61 |
| SAP in mmHg/DAP in mmHg MAP in mmHg | 115/50 (71) | 119/69 (85) | 124/61 (82) |
| SpO2, % | 90 | 90 | |
| Lactate in mmol/L | 1.800 | 1.900 | 1.493 |
| Norepinephrine infusion as μg × kg × min-1 | 0.09 | - | 0.35 |
| SAPS II | 68 | 40 | 56 |
| SOFA | 13 | 7 | 8 |
| Respiratory parameters | |||
| Baseline | |||
| PaO2/FiO2 | 77 | 91 | 95 |
| PEEP in cmH2O | 14 | 14 | 15 |
| Plateau pressure in cmH2O | 28 | 27 | 29 |
| Compliance in mL/cmH2O | 32 | 33 | 31 |
| H1 | |||
| PaO2/FiO2 | 82 | 150 | 121 |
| PEEP in cmH2O | 14 | 14 | 15 |
| Plateau pressure in cmH2O | 30 | 29 | 28 |
| Compliance in mL/cmH2O | 29 | 28 | 33 |
| H2 | |||
| PaO2/FiO2 | 163 | 330 | 135 |
| PEEP in cmH2O | 14 | 14 | 15 |
| Plateau pressure in cmH2O | 30 | 29 | 28 |
| Compliance in mL/cmH2O | 28 | 29 | 33 |
| H12 | |||
| PaO2/FiO2 | 233 | 160 | 214 |
| PEEP in cmH2O | 14 | 14 | 15 |
| Plateau pressure in cmH2O | 30 | 27 | 28 |
| Compliance in mL/cmH2O | 27 | 32 | 34 |
| Pulmonary artery catheter | |||
| Baseline | |||
| Mean PAP in mmHg | 42 | 32 | 34 |
| Systolic PAP in mmHg | 57 | 42 | 44 |
| PAOP in mmHg | 7 | 9 | 11 |
| CI in L/min/m2 | 2.6 | 2.9 | 2.9 |
| PVR in Wood Units | 5.9 | 4.1 | 3.3 |
| H1 | |||
| Mean PAP in mmHg | 42 | 30 | 35 |
| Systolic PAP in mmHg | 65 | 39 | 41 |
| PAOP in mmHg | 8 | 9 | 13 |
| CI in L/min/m2 | 2.6 | 2.9 | 2.6 |
| PVR in Wood Units | 5.9 | 4.2 | 3.5 |
| H2 | |||
| Mean PAP in mmHg | 37 | 30 | 39 |
| Systolic PAP in mmHg | 52 | 33 | 45 |
| PAOP in mmHg | 8 | 11 | 14 |
| CI in L/min/m2 | 3.9 | 3.1 | 2.6 |
| PVR in Wood Units | 3.4 | 3.2 | 3.9 |
| H12 | |||
| Mean PAP in mmHg | 36 | 29 | 35 |
| Systolic PAP in mmHg | 50 | 49 | 40 |
| PAOP in mmHg | 6 | 11 | 10 |
| CI in L/min/m2 | 3.0 | 2.6 | 2.5 |
| PVR in Wood Units | 4.5 | 3.6 | 4.2 |
| TEE-Right ventricular parameters | |||
| Baseline | |||
| RV FAC, % | 45 | 42 | 58 |
| RV outflow tract VTI in cm | 19 | 13 | 19 |
| H2 | |||
| RV FAC, % | 44 | 42 | 34 |
| RV outflow tract VTI in cm | 16 | 15 | 24 |
| H12 | |||
| RV FAC, % | 48 | 34 | 42 |
| RV outflow tract VTI in cm | 15 | 16 | 19 |
| TEE 2D-STE parameters | |||
| Baseline | |||
| RVGLS, % | 13.5 | 25.3 | 14.8 |
| TMAD septal in mm | 13.0 | 29.7 | 23.5 |
| H2 | |||
| RVGLS, % | 13.3 | 24.7 | 22.7 |
| TMAD septal in mm | 9.6 | 27.6 | 25.0 |
| H12 | |||
| RVGLS, % | 16.9 | 25.0 | 18.7 |
| TMAD septal in mm | 16 | 32 | 23 |
| ICU course | |||
| Length of stay in d | 30 | 32 | 31 |
| Mechanical ventilation duration in d | 18 | 22 | 21 |
| Outcomes | Discharged from ICU | Discharged from ICU | Discharged from ICU |
The patients had no personal or family history.
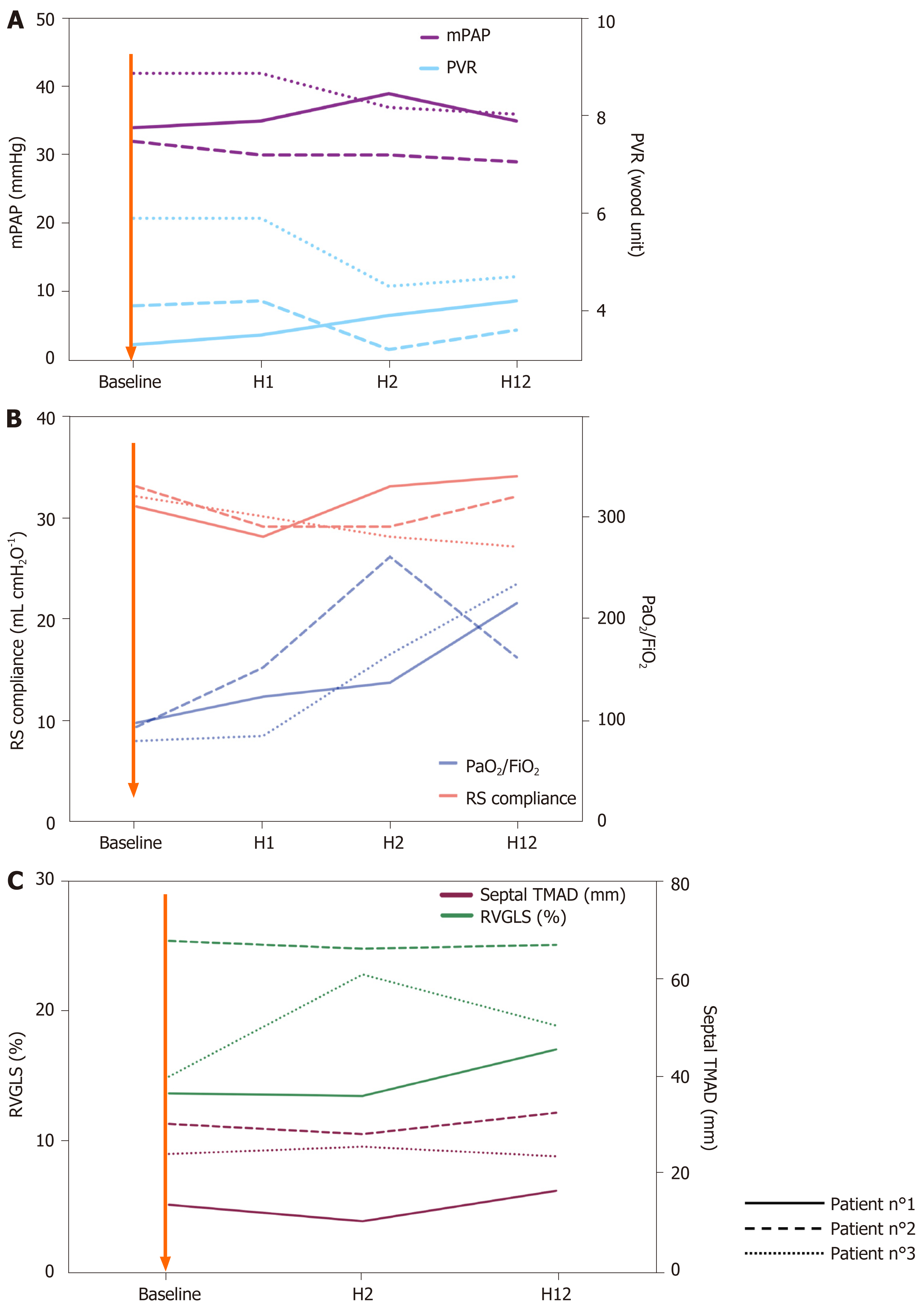
Case 1: Prior to almitrine infusion, the plateau pressure was 28 cmH2O and positive end expiratory pressure (PEEP) at 14 cmH2O. Baseline pulmonary compliance was 32 mL/cmH2O. Pulmonary artery catheter showed pulmonary vascular resistance at 5.9 Wood units and pulmonary artery occlusion pressure of 7 mmHg. Cardiac index (CI) was 2.6 L/min/m2. Right ventricular (RV) fractional area change (FAC) was 45%. Speckle tracking based right ventricular two-dimensional strain was performed (Table 1 and Figure 1).
Case 2: Prior to almitrine infusion, the plateau pressure was 27 cmH2O and PEEP at 14 cmH2O. Baseline pulmonary compliance was 33 mL/cmH2O. Pulmonary artery catheter showed pulmonary vascular resistance at 4.1 Wood units and pulmonary artery occlusion pressure of 9 mmHg. CI was 2.9 L/min/m2. RV FAC was 42%.
Case 3: Prior to almitrine infusion, the plateau pressure was 29 cmH2O and PEEP at 15 cmH2O. Baseline pulmonary compliance was 31 mL/cmH2O. Pulmonary artery catheter showed pulmonary vascular resistance at 3.3 Wood units and pulmonary artery occlusion pressure of 11 mmHg. CI was 2.9 L/min/m2. RV FAC was 58%.
Case 1: Prior to almitrine infusion, the PaO2/FiO2 ratio was 77 mmHg. Arterial blood gas sample showed PaCO2 of 46.2 mmHg, pH at 7.4 and lactate at 1.8 mmol/L. Laboratory investigations revealed lymphopenia at 550/mm3 and high C reactive protein level at 65 mg/L.
Case 2: Prior to almitrine infusion, the PaO2/FiO2 ratio was 91 mmHg. Arterial blood gas sample showed PaCO2 of 39.5 mmHg, pH at 7.4 and lactate at 1.9 mmol/L. Laboratory investigations revealed lymphopenia at 370/mm3 and high C reactive protein level at 95 mg/L.
Case 3: Prior to almitrine infusion, the PaO2/FiO2 ratio was 95 mmHg. Arterial blood gas sample showed PaCO2 of 49 mmHg, pH at 7.3 and lactate at 1.4 mmol/L. Laboratory investigations did not reveal any other specificities.
Case 1: Chest computed tomography (CT) angiography showed ground-glass opacity with crazy paving and severe lobar involvement (> 75%). There was no pulmonary embolism.
Case 2: Chest CT angiography showed ground-glass opacity with crazy paving and moderate lobar involvement (50%-75%). There was no pulmonary embolism.
Case 3: Chest CT angiography showed ground-glass opacity with bilateral crazy paving, consolidation and limited lobar involvement (25%). There was no pulmonary embolism.
All three patients were admitted to the ICU for severe ARDS according to the Berlin definition with a PaO2/FiO2 ratio < 150. They were treated with protective ventilation (low tidal volume 6-7 mL/kg, PEEP for plateau pressure ≤ 30 cmH2O), neuromuscular blocking agents and inhaled nitric oxide (at 10 ppm). Despite medical management they presented persistent severe hypoxemia.
The local institutional review board waived the need for written informed consent; data storage was authorized by national licensing authority (CNIL PI2020_843_0026). Patients and/or next of kin were informed of the study. We prospectively collected data from three consecutive patients with COVID-19 and severe ARDS who received almitrine infusion.
Low dose of almitrine (Vectarion®; Servier, Suresnes, France) was started at an infusion rate of 4 μg × kg × min-1on a central line. The effect of almitrine infusion on respiratory parameters (blood gases, PaO2/FiO2 ratio, total respiratory system compliance) was evaluated. Pulmonary artery pressure (PAP), cardiac output and pulmonary vascular resistance (PVR) were monitored via a pulmonary artery catheter. RV FAC, RV global longitudinal strain and tricuspid longitudinal annular displacement were monitored via transesophageal echocardiography. Data were collected before and 1 h, 2 h and 12 h after almitrine infusion start.
Case 1: Almitrine infusion was started after 2 d of mechanical ventilation and sustained for 3 d.
Case 2: Almitrine was started at day 6 after ICU admission (5 d with high flow oxygen and 1 d under mechanical ventilation). Almitrine infusion was given for 3 d.
Case 3: Almitrine infusion was started after 2 d of mechanical ventilation. Almitrine infusion was given for 4 d.
The PaO2/FiO2 ratio and total respiratory system compliance during almitrine infusion are presented in Figure 1 and Table 1. For the three patients, PaO2/FiO2 ratio time-course showed a dramatic increase whereas total respiratory system compliance was unchanged. The three patients were discharged from the ICU. ICU length of stay for patient 1, patient 2 and patient 3 was 30 d, 32 d and 31 d, respectively. Weaning from mechanical ventilation was performed 13 d, 18 d and 15 d after almitrine infusion for patient 1, 2 and 3, respectively.
Time-course of pulmonary artery catheter parameters and echocardiographic parameters during almitrine infusion are presented in Figure 1. PAP and PVR remained unchanged during almitrine infusion. Regarding RV function, RV FAC and RV global longitudinal strain remained unchanged for patient 1 and patient 2 and improved for patient 3. Tricuspid longitudinal annular displacement remained unchanged for the three patients. None of the patients needed extracorporeal membrane oxygenation therapy. Liver function test remained unchanged.
In this report, we found that almitrine infusion enhanced oxygenation of patients with severe COVID-19 related ARDS without any change in PAP and RV function. At baseline, the patients showed an increased PAP without pulmonary embolism and left ventricular dysfunction (normal pulmonary artery occlusion pressures and CI). Hence, we cannot discard the occurrence of at least a certain level of hypoxic pulmonary vasoconstriction that has been suggested by some authors[3].
In our patients, almitrine led to increased oxygenation without any changes in ventilatory parameters or right-side circulation. Hence, almitrine improved the ventilation/perfusion ratio by decreasing pulmonary flow. The lack of increase in PVR with almitrine is not in favor of an increase in hypoxic pulmonary vasoconstriction as encountered in typical ARDS. Recently, Ackermann et al[4] observed that the pulmonary histological pattern that distinguishes COVID-19 infected lungs from influenzae infected lung is the amount of new vessel growth in the COVID-19 lung. Moreover, Lang et al[1] studied lung perfusion by dual-energy chest CT scan for COVID-19 patients and found an increased perfusion of the lungs especially proximal to lung opacities. Increase of pulmonary vessels number is probably responsible of an intrapulmonary shunt with an increased ventilation/perfusion mismatch.
Almitrine acts as a selective pulmonary vessel vasoconstrictor that is able to decrease and redistribute pulmonary blood flow from shunt areas to pulmonary units with normal ventilation/pulmonary flow ratios[9]. We believe that the improvement in oxygenation with almitrine for our patients was related to a decrease in intrapulmonary shunt in both aerated and non-aerated lung regions[2]. Inhaled nitric oxide is a selective pulmonary vasodilator, and intravenously administered almitrine is a selective pulmonary vasoconstrictor. The resulting effects of combining almitrine and inhaled nitric oxide are likely related to the respective vascular effect of each drug on aerated and non-aerated lung compartments and explain why additive respiratory effects were observed. Previous studies confirmed that when reinforcing hypoxic pulmonary vasoconstriction by small doses of almitrine, the nitric oxide-induced increase in arterial oxygenation can be markedly enhanced[10,11].
The major risk with short term use of almitrine is the increase of RV afterload by excessive vasoconstriction. Hence, we evaluated RV function with very sensitive and reproducible parameters: RV global longitudinal strain and tricuspid longitudinal annular displacement[12,13]. We found no deleterious effects on RV function, which was similar to previous studies on almitrine safety[14]. Recently, Barthélémy et al[6] tested almitrine for COVID-19 patients, but crucial data on RV function was not reported.
Despite a small sample size, almitrine seems to be effective and safe to enhance oxygenation for COVID-19 critically ill patients. Further controlled studies are required.
Manuscript source: Unsolicited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shahini E S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Lang M, Som A, Mendoza DP, Flores EJ, Reid N, Carey D, Li MD, Witkin A, Rodriguez-Lopez JM, Shepard JO, Little BP. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020;20:1365-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 2. | Mahjoub Y, Rodenstein DO, Jounieaux V. Severe Covid-19 disease: rather AVDS than ARDS? Crit Care. 2020;24:327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Archer SL, Sharp WW, Weir EK. Differentiating COVID-19 Pneumonia From Acute Respiratory Distress Syndrome and High Altitude Pulmonary Edema: Therapeutic Implications. Circulation. 2020;142:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 4. | Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4313] [Cited by in RCA: 4058] [Article Influence: 811.6] [Reference Citation Analysis (0)] |
| 5. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4576] [Article Influence: 915.2] [Reference Citation Analysis (0)] |
| 6. | Barthélémy R, Blot PL, Tiepolo A, Le Gall A, Mayeur C, Gaugain S, Morisson L, Gayat E, Mebazaa A, Chousterman BG. Efficacy of Almitrine in the Treatment of Hypoxemia in Sars-Cov-2 Acute Respiratory Distress Syndrome. Chest. 2020;158:2003-2006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Cardinale M, Esnault P, Cotte J, Cungi PJ, Goutorbe P. Effect of almitrine bismesylate and inhaled nitric oxide on oxygenation in COVID-19 acute respiratory distress syndrome. Anaesth Crit Care Pain Med. 2020;39:471-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Losser MR, Lapoix C, Delannoy M, Champigneulle B, Payen D. Almitrine as a non-ventilatory strategy to improve intrapulmonary shunt in COVID-19 patients. Anaesth Crit Care Pain Med. 2020;39:467-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Reyes A, Roca J, Rodriguez-Roisin R, Torres A, Ussetti P, Wagner PD. Effect of almitrine on ventilation-perfusion distribution in adult respiratory distress syndrome. Am Rev Respir Dis. 1988;137:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 70] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Naeije R, Lejeune P, Vachiéry JL, Leeman M, Mélot C, Hallemans R, Delcroix M, Brimioulle S. Restored hypoxic pulmonary vasoconstriction by peripheral chemoreceptor agonists in dogs. Am Rev Respir Dis. 1990;142:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Gallart L, Lu Q, Puybasset L, Umamaheswara Rao GS, Coriat P, Rouby JJ. Intravenous almitrine combined with inhaled nitric oxide for acute respiratory distress syndrome. The NO Almitrine Study Group. Am J Respir Crit Care Med. 1998;158:1770-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Li Y, Li H, Zhu S, Xie Y, Wang B, He L, Zhang D, Zhang Y, Yuan H, Wu C, Sun W, Li M, Cui L, Cai Y, Wang J, Yang Y, Lv Q, Zhang L, Xie M. Prognostic Value of Right Ventricular Longitudinal Strain in Patients With COVID-19. JACC Cardiovasc Imaging. 2020;13:2287-2299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 326] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 13. | Beyls C, Bohbot Y, Huette P, Abou-Arab O, Mahjoub Y. Tricuspid Longitudinal Annular Displacement for the Assessment of Right Ventricular Systolic Dysfunction during Prone Positioning in Patients with COVID-19. J Am Soc Echocardiogr. 2020;33:1055-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Esnault P, Hraiech S, Bordes J, Forel JM, Adda M, Rambaud R, Lehingue S, Roch A, Papazian L, Guervilly C. Evaluation of Almitrine Infusion During Veno-Venous Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome in Adults. Anesth Analg. 2019;129:e48-e51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |