Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3365
Peer-review started: November 6, 2020
First decision: January 17, 2021
Revised: February 3, 2021
Accepted: March 10, 2021
Article in press: March 10, 2021
Published online: May 16, 2021
Processing time: 173 Days and 20.5 Hours
Endocardial fibroelastosis (EFE) is a rare heart disease characterized by thickening of the endocardium caused by massive proliferation of collagenous and elastic tissue, usually leading to impaired cardiac function. Multimodality cardiovascular imaging for the evaluation of EFE with thrombi is even rarer.
We report a rare case of EFE associated with multiple cardiovascular thrombi. Three-dimensional (3D) and contrast echocardiography (CE) were used to assess ventricular thrombi. Anticoagulant therapy was administered to eliminate the thrombi. The peripheral contrast-enhanced thrombi with the highest risk were dissolved with anticoagulant therapy at the time of reexamination, which was consistent with the presumption of fresh loose thrombi.
This new echocardiography technique has a great advantage in the diagnosis and treatment of EFE. On the basis of conventional echocardiography, 3D echocardio
Core Tip: We report a rare case of endocardial fibroelastosis with multiple thrombi of the left ventricle, abdominal aorta, common iliac artery, and renal artery occlusion diagnosed using multimodality cardiovascular imaging. The incremental value of three-dimensional and contrast echocardiography in clinical diagnosis of thrombi is explained.
- Citation: Sun LJ, Li Y, Qiao W, Yu JH, Ren WD. Incremental value of three-dimensional and contrast echocardiography in the evaluation of endocardial fibroelastosis and multiple cardiovascular thrombi: A case report. World J Clin Cases 2021; 9(14): 3365-3371
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3365.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3365
Endocardial fibroelastosis (EFE) is a rare heart disease that frequently occurs in infants and children. It is characterized by diffuse thickening of the endocardium due to the proliferation of collagen and elastic fibers, presenting as unexplained heart failure. Patients with EFE are prone to endocardial thrombosis, which may be due to enlarged cardiac cavity, systolic dysfunction, and altered endothelium lining[1,2]. Previous studies have also reported that a thrombus can easily form in sites of akinetic, dyskinetic, or aneurysmal segments that were potentially at risk for thrombo-embolism[3]. If the embolus is detached from the mural thrombus, complications arise and other organ changes occur. Herein, we report a case of EFE with multiple thrombi of the left ventricle, abdominal aorta, renal artery, and common iliac artery diagnosed by multimodality cardiovascular imaging. The incremental role of three-dimensional (3D) and contrast echocardiography (CE) in the clinical diagnosis of thrombi is also explained.
An 11-year-old girl was admitted to Shengjing Hospital with nausea and vomiting for 4 d.
The patient developed nausea and vomiting 4 d ago and has vomited 1-2 times a day. Vomiting included the stomach content and was non-ejective. In order to achieve standardized treatment, the patient was admitted to the department of Pediatrics at Shengjing Hospital of China Medical University on June 1, 2020 with "endocardial elastic fiber hyperplasia and vomiting pending investigation".
Her medical history was significant for EFE diagnosed 10 years ago. She has been undergoing regular examinations and treatments for a decade, and her condition was relatively stable.
Physical examination showed that the liver margin was palpable 4 cm below the costal margin and 6 cm below the xiphoid process. Bilateral dorsal foot pulse was not detected.
The positive results from laboratory tests were as follows: erythrocyte 5.0 × 1012/L, hemoglobin 118 g/L, erythrocyte sedimentation rate 38 mm/h, urine protein quantification 1.41 g/L, 24 h urine protein quantification 1.41 g/d, uric acid 537 µmol/L, total protein 52.8 g/L, albumin 25.4 g/L, serum cystatin C 1.28 mg/L, interleukin-6 8.70 pg/mL, prothrombin time 17.1 s, prothrombin time activity 55%, activated partial thromboplastin time 38 s, D-dimer 1258 µg/L, and fibrinogen degradation product 12.4 mg/L. Blood gas analysis revealed that the oxygen partial pressure was reduced to 43.5 mmHg.
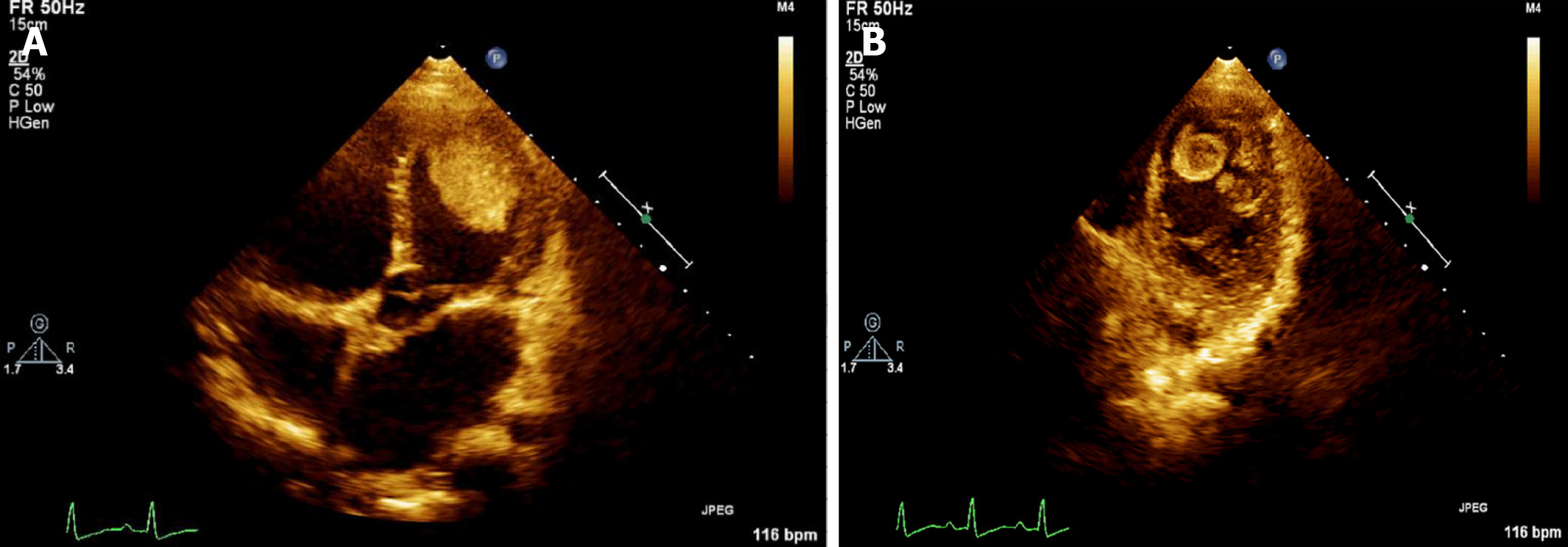
Two-dimensional (2D) echocardiography revealed that the whole heart was significantly enlarged (left ventricular end-diastolic dimension of 67 mm), and left ventricular wall movement was generally significantly reduced with uncoordinated movement. The endocardium was thicker, about 2.4 mm at the thickest, and the echo was enhanced. Multiple mass images were observed in the left ventricle. There was a high-low mixed echo mass about 50 mm × 33 mm in size near the apex of the left ventricular lateral wall that was slightly deformed (Figure 1A). A mixed strong and weak echogenic mass 40 mm × 15 mm in size was also detected at the apex of the left ventricle with obvious activity and deformation. Its base was narrow and thin at about 1-2 mm (Figure 1B). Another 8 mm × 10 mm medium-high echo mass with obvious activity was detected at the apex of the heart (Figure 1B). Multiple trabeculations were detected at the apex of the left ventricle. Small amounts of regurgitation were detected in the mitral valve. The left ventricular systolic function was significantly reduced at rest with an ejection fraction of 28%.
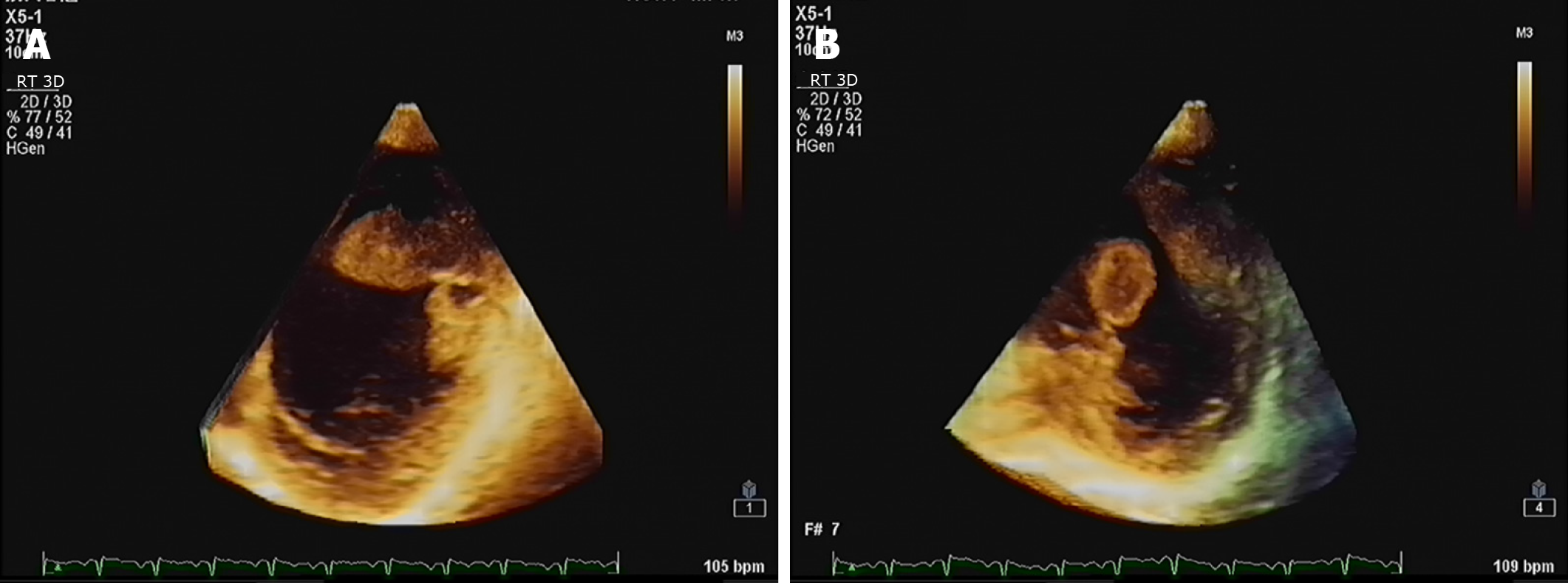
3D echocardiography showed that a thrombus with a wide base and low mobility protruded from the side wall of the left ventricle (Figure 2A). A mobile thrombus component with a thin pedicle was protruding from the left ventricular apex (Figure 2B).
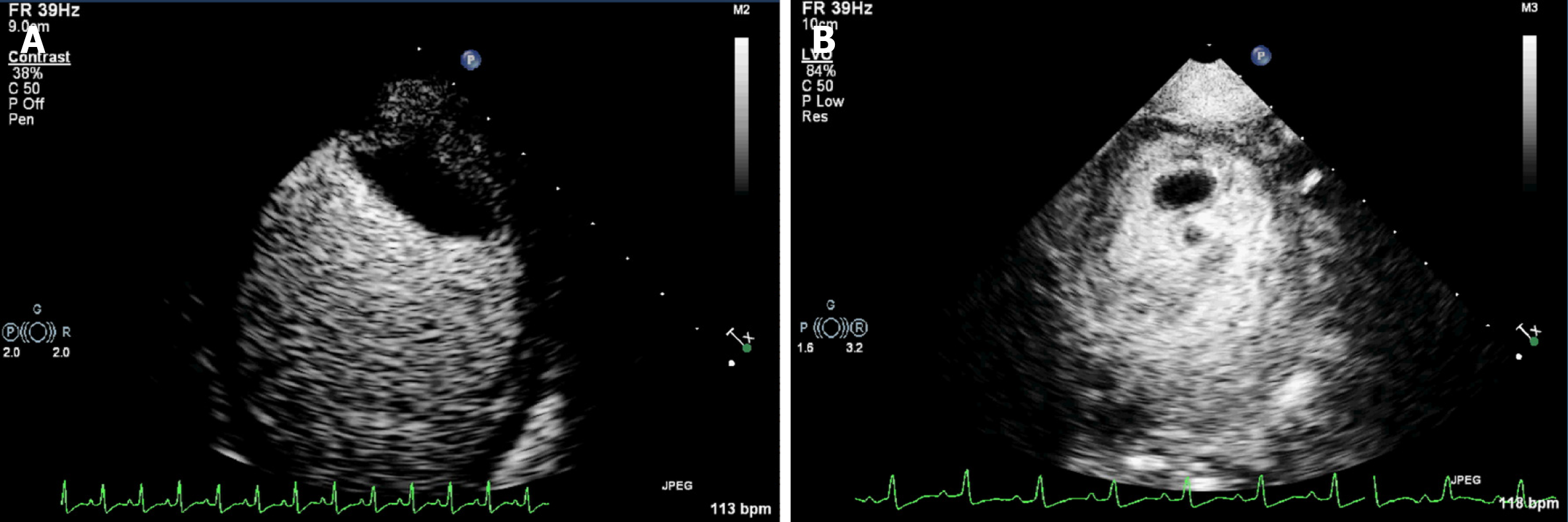
The CE results showed that the mass on the left ventricular lateral wall near the apex was relatively fixed and had slight activity and deformation, but there was contrast agent entering at a part of the junction between the base of the mass and the left ventricular wall. The other part of the base was tightly connected to the left ventricular wall. Its inner portion showed no contrast enhancement (Figure 3A). The masses in the left ventricular apex had great mobility and deformation. Contrast agent was detected in the peripheral part of these masses, while most of their center portions were not contrast-enhanced (Figure 3B). This mass was determined to be of the highest risk according to CE results. These masses were considered to be thrombi due to their echocardiographic morphological characteristics and the patient's primary disease.
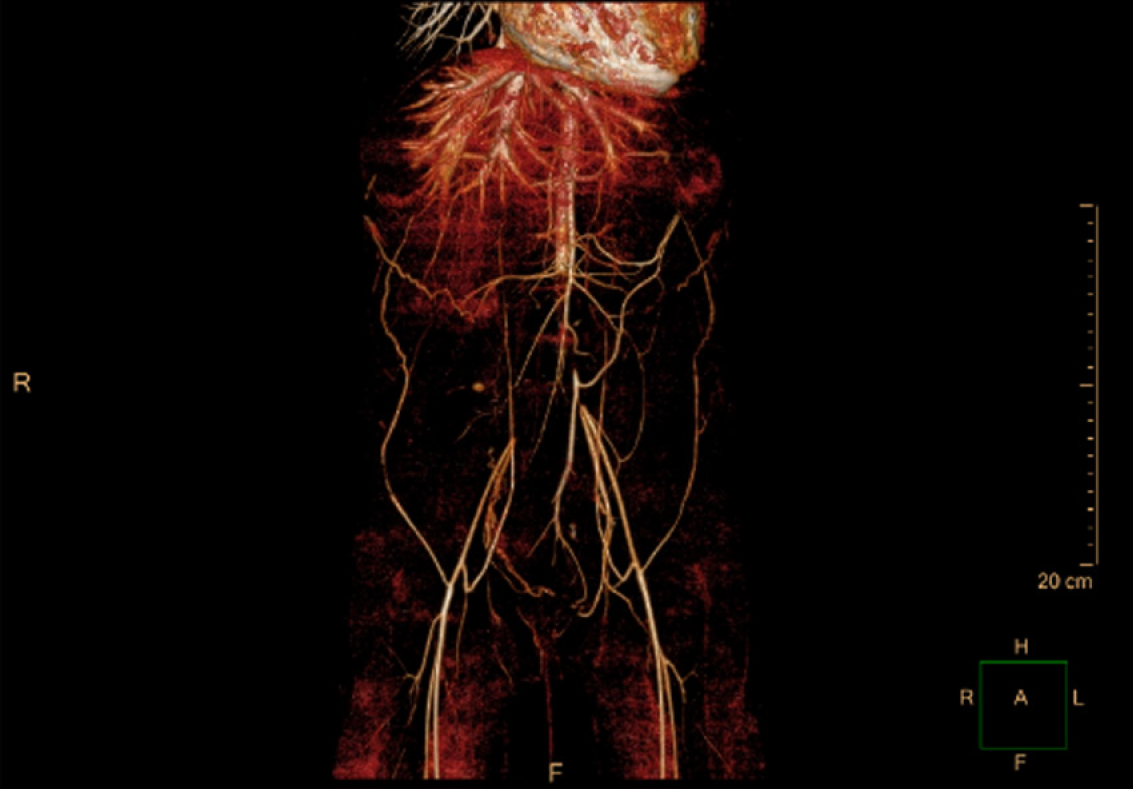
The 3D computed tomography angiography demonstrated thromboembolism from the abdominal aorta (level of the right accessory renal artery) to the bilateral common iliac artery (Figure 4). Thrombosis was also detected in the proximal left renal artery and the opening of the right renal artery, with multiple ischemic foci in both kidneys.
Color Doppler ultrasonography revealed ischemic changes in the arterial spectrum of both lower limbs.
EFE with multiple thrombi of the left ventricle, abdominal aorta, renal artery, and common iliac artery.
Anticoagulation and other symptomatic treatments were administered to eliminate the thrombus and relieve the patient's other symptoms.
After 5 d of anticoagulant treatment, the smallest thrombus at the apex of the left ventricle disappeared and the other thrombus became significantly smaller (from 40 mm × 15 mm to 3 mm × 4.5 mm). The change in the largest thrombus in the left ventricular lateral wall near the apex was not significant (from 50 mm × 33 mm × 17 mm to 50 mm × 22 mm × 18 mm). After 12 d of anticoagulation treatment, the active thrombus disappeared, and only the thrombus located in the left ventricular lateral wall near the apex was detected, the size of which was about 49 mm × 19 mm. Despite adequate medical measures, congestive heart failure did not improve completely, and after two months the patient eventually developed cardiogenic shock, respiratory failure, sepsis, and severe pneumonia. Finally, the patient and her parents abandoned treatment and asked to be discharged. The timeline of information related to this case report is shown in Table 1.
| Time | Information |
| June 1, 2020 | Admitted to hospital |
| Laboratory examinations | |
| June 2, 2020 | Color Doppler ultrasonography of the abdominal aorta, renal artery, left and right lower limb artery |
| June 3, 2020 | Dynamic electrocardiogram |
| June 4, 2020 | 2D and 3D echocardiography |
| June 5, 2020 | 3D abdominal enhanced computed tomography angiography |
| Contrast echocardiography | |
| June 9, 2020 | 2D echocardiography |
| June 16, 2020 | 2D echocardiography |
| June 23, 2020 | 2D echocardiography |
| Contrast echocardiography | |
| July 9, 2020 | 2D echocardiography |
| Chest computed tomography | |
| July 28, 2020 | Her parents abandoned treatment and asked for her to be discharged |
EFE is a rare heart disease characterized by thickening of the endocardium, cardiac enlargement, and myocardial dysfunction. The exact etiology of EFE is unknown and may be related to many factors, including infection[4], congenital developmental malformation[5], autoimmune diseases[6], chromosomal abnormalities and gene mutations[7], and myocardial ischemia and hypoxia[8].
Traditional 2D echocardiography is the most convenient imaging method to evaluate the morphology and mobility of the mass due to its availability, versatility, and low cost. However, the accuracy of 2D echocardiography is limited because the mass calculations are based on geometric assumptions. Real-time 3D echocardio-graphy provides a novel echocardiographic method to measure masses by directly observing the myocardial boundaries of the entire left ventricle. On the basis of 2D echocardiography diagnosis, 3D echocardiography comprehensively shows the position, shape, and narrow base of the mass. The 3D echocardiography is an accurate method for quantifying left ventricular mass. It is in better agreement with the reference value of cardiac magnetic resonance imaging (cMRI), which is considered to be the gold standard for quantifying left ventricular masses[9]. It also has advantages over cMRI in its availability, rapid image acquisition, and processing, and in cases where the patient cannot access a cMRI scanner.
Using the combination of 3D echocardiography and CE, the mass can be displayed more accurately and vividly. We further performed CE to detect the nature, density, and connection to the left ventricular wall of the mass in order to make an accurate clinical diagnosis. CE is widely used in cardiovascular diseases. It can be used to evaluate left ventricular structure and function to improve image quality, reader confidence, and reproducibility[10]. Compared to computed tomography (CT), MRI, positron emission tomography (PET), and PET-CT, CE is a fast, effective, well-tolerated, and inexpensive technology[11]. The image of the thrombus needs to be distinguished from the tumors. Previous literature has reported that CE has a high diagnostic accuracy in differentiating thrombi from benign or malignant tumors[12]. A mass with no contrast enhancement was confirmed as a thrombus. Incomplete or complete enhancement of a mass might be considered a benign or malignant tumor[12,13]. The present study found that CE plays a great role in delineating the outline of the thrombus, clarifying its loose and dense parts, and determining the tightness of the connection between the base and the left ventricular wall. CE does not only help to confirm the diagnosis of the thrombus, but also determines its risk. Contrast enhancement was observed in the peripheral part of the two active thrombi. There was no contrast enhancement in their central portion. The results suggested that the peripheral structure of the mass might be loose, like a gap structure, while the central part was dense. These thrombi were considered to have a very high risk of emboli shedding based on their activity and deformation. The newly formed thrombus was usually highly mobile and protruded into the ventricular cavity, while the old thrombus often had a smooth surface and was usually relatively static. This case had both fresh active and old thrombi, which might have been the source of multiple celiac artery thromboses. At the time of reexamination, the peripheral contrast-enhanced thrombi were first dissolved with anticoagulant therapy, which was consistent with the presumption of fresh loose thrombi.
Left ventricular thrombosis has been identified as a marker of adverse cardiovas
The new echocardiography technique has a great advantage in the diagnosis and treatment of EFE. On the basis of conventional echocardiography, 3D echocardio-graphy is used to display the position, shape, and narrow base of the thrombus. CE does not only help to confirm the diagnosis of the thrombus, but also determines its risk. The prognosis of EFE with left ventricular thrombus is generally poor.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Or T S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LL
| 1. | Ozdemir D, Cortopassi IO, McNamara RL. An illustrative case of endocardial fibroelastosis and recalcitrant intracardiac thrombosis: a case report. Thromb J. 2019;17:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Lane KL, Herzberg AJ, Reimer KA, Bradford WD, Schall SA. Endocardial fibroelastosis with coronary artery thromboembolus and myocardial infarction. Clin Pediatr (Phila). 1991;30:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Fazlinezhad A, Narayanasamy H, Wilansky S, Naqvi TZ. Detection of LV apical thrombus by three-dimensional transesophageal echocardiography. Echocardiography. 2020;37:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Lurie PR. Changing concepts of endocardial fibroelastosis. Cardiol Young. 2010;20:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Ursell PC, Neill CA, Anderson RH, Ho SY, Becker AE, Gerlis LM. Endocardial fibroelastosis and hypoplasia of the left ventricle in neonates without significant aortic stenosis. Br Heart J. 1984;51:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Wainwright B, Bhan R, Trad C, Cohen R, Saxena A, Buyon J, Izmirly P. Autoimmune-mediated congenital heart block. Best Pract Res Clin Obstet Gynaecol. 2020;64:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Hodgson S, Child A, Dyson M. Endocardial fibroelastosis: possible X linked inheritance. J Med Genet. 1987;24:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Ma F, Zhou K, Shi X, Wang X, Zhang Y, Li Y, Hua Y, Wang C. Misdiagnosed anomalous left coronary artery from the pulmonary artery as endocardial fibroelastosis in infancy: A case series. Medicine (Baltimore). 2017;96:e7199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Avegliano GP, Costabel JP, Asch FM, Sciancalepore A, Kuschnir P, Huguet M, Tobon-Gomez C, Frangi AF, Ronderos R. Utility of Real Time 3D Echocardiography for the Assessment of Left Ventricular Mass in Patients with Hypertrophic Cardiomyopathy: Comparison with Cardiac Magnetic Resonance. Echocardiography. 2016;33:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, Nihoyannopoulos P, Edvardsen T, Lancellotti P; EACVI Scientific Documents Committee for 2014–16 and 2016–18. Clinical practice of contrast echocardiography: recommendation by the European Association of Cardiovascular Imaging (EACVI) 2017. Eur Heart J Cardiovasc Imaging. 2017;18:1205-1205af. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 11. | Tarantino L, Ambrosino P, Di Minno MN. Contrast-enhanced ultrasound in differentiating malignant from benign portal vein thrombosis in hepatocellular carcinoma. World J Gastroenterol. 2015;21:9457-9460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Tang QY, Guo LD, Wang WX, Zhou W, Liu YN, Liu HY, Li L, Deng YB. Usefulness of contrast perfusion echocardiography for differential diagnosis of cardiac masses. Ultrasound Med Biol. 2015;41:2382-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Diebold B. Contrast echocardiography, tumors and thrombus: a new episode in a 50-year history. Arch Cardiovasc Dis. 2009;102:163-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Saric M, Armour AC, Arnaout MS, Chaudhry FA, Grimm RA, Kronzon I, Landeck BF, Maganti K, Michelena HI, Tolstrup K. Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism. J Am Soc Echocardiogr. 2016;29:1-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 257] [Article Influence: 28.6] [Reference Citation Analysis (0)] |