Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3342
Peer-review started: October 4, 2020
First decision: December 30, 2020
Revised: January 22, 2021
Accepted: March 3, 2021
Article in press: March 3, 2021
Published online: May 16, 2021
Processing time: 207 Days and 3.5 Hours
Bone cement implantation syndrome (BCIS) is characterized by hypotension, arrhythmia, diffuse pulmonary microvascular embolism, shock, cardiac arrest, any combination of these factors, or even death following bone cement implanta
An 80-year-old patient with pemphigus and Parkinson’s disease underwent total hip replacement under spinal subarachnoid block and developed acute pulmonary embolism after bone cement implantation. The patient received mask mechanical ventilation with a continuous intravenous infusion of adrenaline (2 μg/mL) at a rate of 30 mL/h. Subsequently, the symptoms of BCIS were markedly alleviated, and the infusion rate of adrenaline was gradually reduced until the infusion was completely stopped 45 min later. The patient was then transferred to the Department of Orthopedics, and anticoagulation therapy began at 12 h postoperatively. No other complications were observed.
This is a rare case of BCIS in a high-risk patient with pemphigus and Parkinson’s disease.
Core Tip: Bone cement implantation syndrome (BCIS) is an important cause of death and disability during the perioperative period of total hip replacement. The present patient with pemphigus and Parkinson's disease had a higher risk of BCIS than the general population due to pathophysiological changes and current medications. Therefore, the choice of anesthetic method for such patients must take full account of their current conditions. In addition, vital signs must be closely monitored intraopera
- Citation: Zhou W, Zhang WJ, Zhao GQ, Li K. Bone cement implantation syndrome during hip replacement in a patient with pemphigus and Parkinson’s disease: A case report. World J Clin Cases 2021; 9(14): 3342-3349
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3342.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3342
Pemphigus is an autoimmune blistering disease in which autoantibodies attack the non-chromosomal group of epithelial cells, which leads to damage of the cell-cell adhesion; the first-line treatment for pemphigus is still immunosuppressant drugs such as corticosteroids[1]. There is no anesthetic protocol established for patients with pemphigus. However, for older adults with concurrent diseases affecting multiple organs, the anesthetic protocol should be minimally invasive, simple, effective, and easy to control.
Bone cement implantation syndrome (BCIS) is a common medical condition in which the bone marrow cavity is seriously injured by compression and fixation with bone cement, and a chemical reaction takes place in the closed space filled with bone cement. Under high temperature and pressure, the bone marrow enters the blood circulation. The presence of lipid droplets larger than 20 μm is sufficient to cause pulmonary embolism[2]. Proposed severity classification of BCIS is classified as following: Grade 1, moderate hypoxia (SpO2 < 94%) or hypotension [fall in systolic blood pressure (SBP) > 20%]; grade 2, severe hypoxia (SpO2 < 88%) or hypotension (fall in SBP > 40%) or unexpected loss of consciousness; and grade 3, cardiovascular collapse requiring cardiopulmonary resuscitation. Because it is impossible to draw each meaningful hypotension and oxygen desaturation, the true incidence of BCIS, especially the lesser degrees, is not systematically collected or published, probably under-estimated[3].
Herein, we report the case of a high-risk older adult patient with a rare combination of pemphigus and Parkinson’s disease who had suspected pulmonary embolism due to BCIS during total hip replacement.
An 80-year-old man was hospitalized due to pain in the right hip joint with restricted mobility.
The patient’s symptoms started after trauma 3 d ago.
The patient had a history of pemphigus for 3 years, for which he was taking oral prednisone acetate (one 5 mg tablet daily). He also had a history of Parkinson’s disease for over 10 years, for which he had not received any specific treatment.
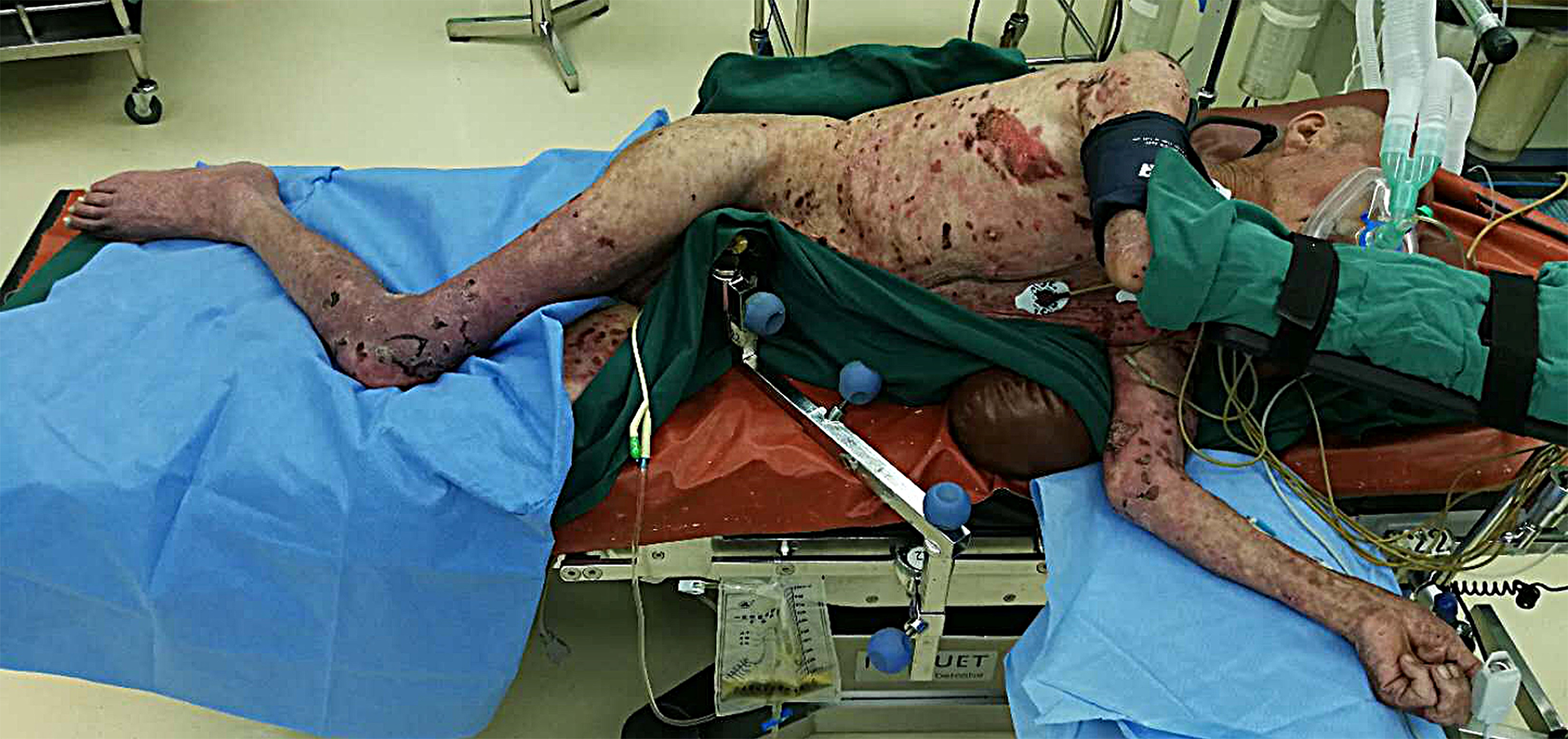
Physical examination findings were: height 170 cm and weight 42 kg; severe tremoring of both hands; extensive erosion and purulent scabs on the chest, abdomen, and all four limbs, but with a better integrity of the skin on the back; and scattered moist rales in both lungs. The degree of mouth opening was two fingers wide, with erosion and bloody scabs in the mouth and oral mucosa, and a Mallampati grade of III. There was neither pain or stiffness nor swelling or redness below the knees.
Auxiliary examination showed that the coagulation function was normal. Biochemical liver test showed that aspartate aminotransferase was 77.73 IU/L, alanine aminotransferase was 66.21 IU/L, and albumin was 26.88 g/L. Arterial blood gas analysis findings were: pH 7.52, partial pressure of oxygen 51 mmHg, and partial pressure of carbon dioxide 42 mmHg.
Cerebral computed tomography revealed bilateral multiple lacunar infarcts, leukoara
The patient was diagnosed with a fracture of the right femoral neck, intramuscular venous thrombosis in the right calf (acute stage), Parkinson’s disease, pemphigus, type I respiratory failure, grade 1 hypertension (high risk), and cachexia syndrome.
The patient was scheduled for total hip replacement under combined epidural/spinal anesthesia; no preoperative anticoagulation therapy was given to reduce the risk of intraspinal hematoma.
Figure 1 shows an image of the patient during treatment. The patient received mask oxygen therapy and non-invasive blood pressure (BP) monitoring, which showed a BP of 118/76 mmHg, heart rate (HR) of 88 bpm, and SpO2 of 96%. Venous access was established only in the left elbow due to extensive skin ulceration, and hydroxyethyl starch was rapidly infused at a dose of 8 mL/kg. The patient was placed in the supine position and underwent urethral catheterization. Puncture was performed at the L2-L3 intervertebral space in the left lateral position, and 16 mg of 0.5% isobaric ropivacaine was injected into the subarachnoid space for spinal anesthesia. An indwelling epidural catheter was placed with a length of 3 cm on the cephalic side. The patient was cooperative during the above-mentioned procedures and made no complaint of discomfort.
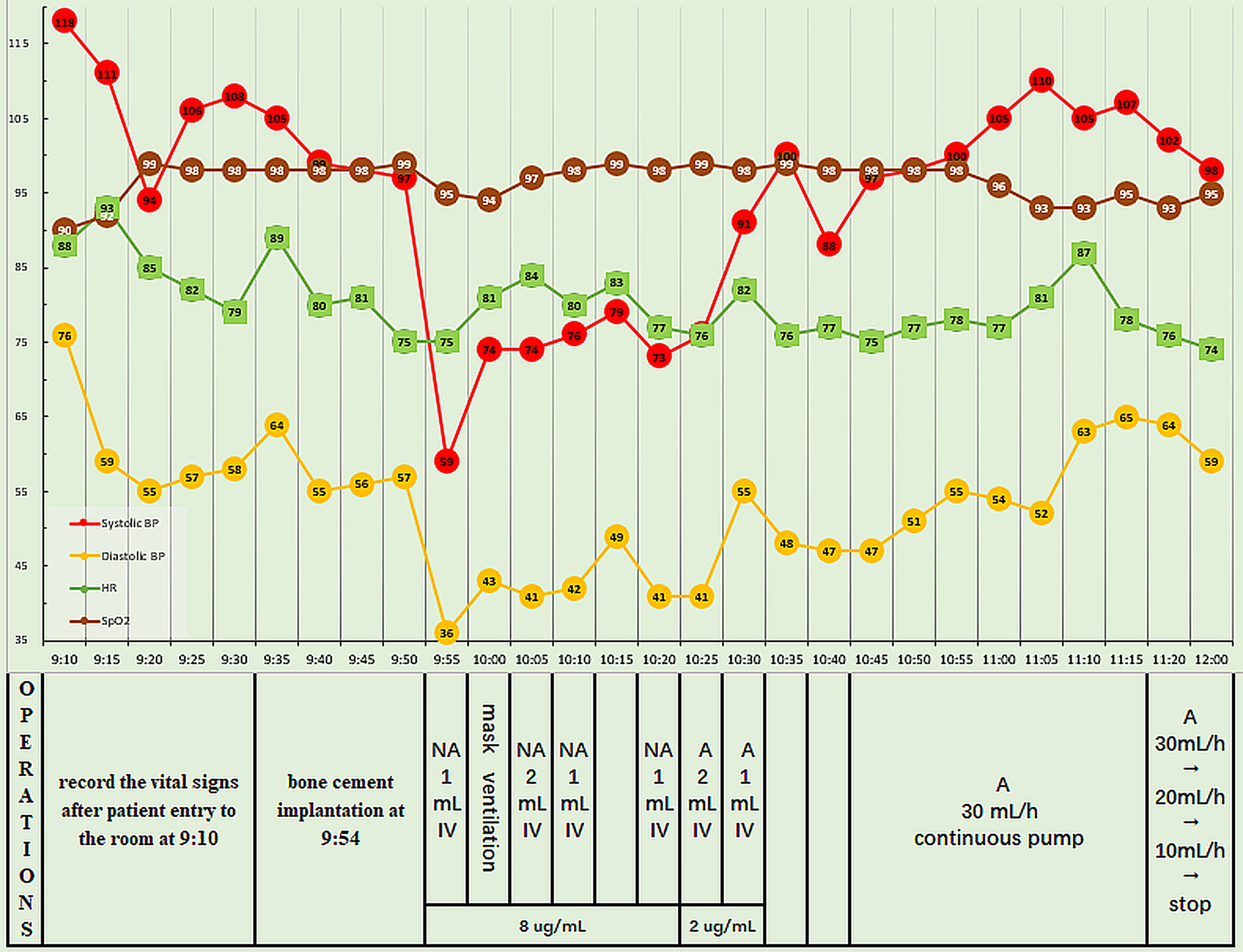
Five minutes after the administration of spinal anesthesia, analgesia was confirmed at the T10 level and the surgery began. The vital signs were stable initially, and the bone cement was implanted 30 min later, shortly after precautionary pulse lavage of the medullary cavity. However, at 1 min post-implantation, the patient experienced chest tightness and agitation, and had a BP of 59/36 mmHg, HR of 75 bpm, and SpO2 of 94%. Fluid replacement and intravenous injection of noradrenaline (NA) 8 μg were performed immediately; at this time, the BP was 74/43 mmHg, HR was 85 bpm, and SpO2 was 94%. With mask mechanical ventilation, duplicated 1 mL of NA (8 μg/mL) was intravenously injected four times, resulting in a BP of 74-79/41-49 mmHg, HR of 80–83 bpm, and SpO2 of 95%-97%. However, the patient still reported chest tightness and agitation. Pulmonary embolism was suspected, and so 2 mL of adrenaline (2 μg/mL) was injected intravenously, resulting in a BP of 91/55 mmHg, HR of 82 bpm, and SpO2 of 99%. After another intravenous injection of 1 mL of adrenaline, the BP was 100/48 mmHg. Adrenaline (2 μg/mL) was continuously infused at 30 mL/h, and the infusion rate was gradually decreased. The vital signs stabilized and the patient reported no further discomfort. The surgery was completed 30 min later, the epidural catheter was removed, and the patient was transferred to the resuscitation room. The adrenaline infusion was completely stopped 45 min later, at which time the BP was 98/59 mmHg, HR was 74 bpm, and SpO2 was 95%. Figure 2 shows the intraoperative vital signs and the timeline of interventions performed.
The patient was then transferred to the regular nursing floor of Orthopedics Department, and anticoagulation therapy was initiated 12 h postoperatively. No major complications occurred during 2 d of postoperative follow-up, and the patient was discharged 5 d postoperatively.
BCIS is characterized by hypotension, arrhythmia, diffuse pulmonary microvascular embolism, shock, cardiac arrest, any combination of these conditions, or even death after implantation. The incidence of BCIS is about 65%, and those with BCIS have an incidence of cardiac arrest of 0.5%-10%, and a mortality rate of 0.6%-1.0%[4]. There are several mechanisms proposed for the development of BCIS. The serious mechanical injury in the bone marrow cavity associated with compression and fixation, together with a series of chemical reactions occurring in the confined space caused by bone cement, is the possible explanations. Under high temperature and high pressure, the bone marrow enters the circulation in a retrograde fashion, and pulmonary embolism can result from a lipid droplet with a diameter greater than 20 μm[2]. The bone cement monomers that have entered the bloodstream act on the calcium channels in the vascular smooth muscle, dilating peripheral vessels and producing myocardial toxicity[5]. The cells damaged by the bone cement monomers release proteolytic enzymes that dissolve the cells and tissues; other consequences include increased platelet activity, thrombosis development, and aggravation of the inflammatory response[6]. The main lipid mediators of BCIS are endocannabinoids, including anandamide and 2-arachidonoylglycerol; these neurotransmitters are extremely strong vasodilators, which can cause hypotension[7]. The physiological consequences of embolization are considered to be the result of both a mechanical effect and mediator release, which provokes increased pulmonary vascular tone[4].
The diagnosis of BCIS is made based on the following criteria: (1) During the process of bone cement implantation, the patient presents with signs of sudden pulmonary embolism syndrome, including hypoxemia, pulmonary edema, transient hypotension or a marked reduction in BP, transient bradycardia, and even severe cardiac arrest; (2) Hemodynamic monitoring shows increased pulmonary artery pressure and pulmonary vessel resistance; and (3) Thrombi may be seen on transesophageal echocardiography, but these are not always detected. Therefore, the key to BCIS diagnosis is a high degree of clinical suspicion and vigilance[8].
The clinical manifestations of BCIS are as follows[2]: (1) An early, rapid, and large decrease in transient BP. The most marked decrease in BP occurs 3-5 min after implantation, mainly due to a decrease in diastolic BP. The BP can generally be recovered after 10 min. Some patients show a marked drop in BP, while the HR increases substantially after the decrease in BP. Some patients with BCIS experience bradycardia of a longer duration; (2) Electrocardiography shows an arrhythmia characterized by premature beats and ST-T changes; (3) Most patients have varying symptoms such as nausea, vomiting, dizziness, headache, and loss of consciousness; (4) SpO2 and SaO2 decrease, causing shortness of breath; and (5) When acute pul
Pemphigus is an autoimmune blistering disease that usually results in multiple oral and general erosions with flaccid bullae and positivity for Nikolsky's sign. However, the pathogenesis of pemphigus remains unclear, and immunosuppressant drugs such as corticosteroids still represent the first-line treatment, despite the potential for serious adverse effects[1]. A previous review found no recommended anesthetic protocol for pemphigus[10]. Local anesthesia is the first choice, and should be performed under strictly sterile conditions to avoid secondary infection arising from lesion exfoliation. However, local puncture and infiltration anesthesia cannot be performed for bullous skin lesions. Opioids used during general anesthesia may lead to pruritus and aggravate the progression of pemphigus. Moreover, it is best to avoid tracheal intubation to prevent damage to the vulnerable larynx[11]. A case report of anesthetic management for emergency laparotomy in a patient with pemphigus emphasized that touch or injury of the patient must be minimized during monitoring equipment installation, intravenous injection, and airway intervention, as the performance of these procedures may cause further blistering[12]. Moreover, local anesthesia should be avoided if possible before deep vein puncture and arterial intubation. After the preparatory work is done, no tapes should be used to fix the tubes, which should instead be sutured to the skin to avoid creating blisters. Clinicians should avoid using monitoring equipment that causes skin friction, such as electrocardiogram electrodes or BP cuffs. If tracheal intubation cannot be avoided, small-sized tubes should be used. Furthermore, the laryngoscope lens and tracheal catheter should be lubricated, and adrenaline in normal saline (1:500000) should be placed in the throat to reduce the bleeding and stimulation caused by tube withdrawal. An appropriate amount of supplemental corticosteroids can help patients with pemphigus to survive the perioperative period without markedly increasing the occurrence of infections and poor wound healing. This is considered a safe and reliable perioperative management strategy for patients with pemphigus who are on long-term oral hormone replacement therapy. In the present patient who underwent total joint replacement (considered moderate surgical stress), cortisol production rates suggest that the glucocorticoid target is about 50 to 75 mg per day of a hydrocortisone equivalent for 1 to 2 d. Thus, the present patient should have received 5 mg of oral prednisone acetate preoperatively, and 50 mg of intravenous hydrocortisone intraoperatively. We suggest the intravenous administration of 60 mg of hydro
In Parkinson’s disease, autonomic neuropathy can lead to a resting tremor, cough, and swallowing difficulty, which increases the risk of respiratory tract infection[14]. Although the incidence of aspiration pneumonia is low in those with Parkinson’s disease, aspiration pneumonia is still the leading cause of death in this population[15]. In Parkinson’s disease, general anesthesia may be associated with a greater risk of postoperative pneumonia, delirium, and cognitive impact than combined epidural-spinal anesthesia[16]. Furthermore, fasting from food and water may interfere with the effect of oral L-dopa, while residual neuromuscular-blocking drugs and antagonists may mask or aggravate respiratory muscle rigidity, bradykinesia, polysialia, atelectasis, aspiration, and respiratory tract infection. However, few local anesthetics are available for use. It is important to achieve minimal disturbance to brain neurotransmitters, and oral drugs should not be withdrawn. Adrenaline can enhance the β-adrenergic effect of peripheral dopamine, while ephedrine can promote dopamine release; thus, both of these drugs should be avoided in patients with Parkinson’s disease.
The present patient was an older adult, had concomitant diseases in multiple systems, as well as pemphigus, Parkinson’s disease, an ASA grade of III, and acute intramuscular venous thrombosis, and was on long-term oral medications. He also had difficulty in opening his mouth and oral mucosal ulceration, and was considered high risk for general anesthesia and associated complications; thus, we performed spinal anesthesia rather than general anesthesia. Although the circulation is usually stable during nerve block, the procedures are made more challenging by the use of larger anesthetic doses, multiple puncture sites, and a longer anesthetic duration. A nerve block may be used in combination with shallow general anesthesia. Combined epidural-spinal anesthesia involves fewer puncture sites and easy manipulation, and has a proven effect, adjustability, longer maintenance of anesthetic effect, and little disturbance to the routine oral medications.
In patients with suspected BCIS, the inspired oxygen concentration should be increased to 100%, and supplementary oxygen should be continued postopera
We have reported a case of suspected pulmonary embolism during total hip replacement in a patient with pemphigus and Parkinson’s disease. Pemphigus with concomitant Parkinson’s disease is rare, especially in an older adult patient with multisystem disorders. The anesthetic protocol used in such patients should be minimally invasive, simple, effective, and easy to control. These patients are more likely to develop BCIS, and so active invasive arterial monitoring and other comprehensive preventive measures should be adopted. When vasoactive drugs such as ephedrine and dopamine are ineffective, the immediate use of adrenaline may be the best option.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Labusca L S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Vodo D, Sarig O, Sprecher E. The Genetics of Pemphigus Vulgaris. Front Med (Lausanne). 2018;5:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Gu Y, Li L, Zhao F, Li K. [Diagnosis and treatment of acute pulmonary fat embolism induced cardiac arrest: an analysis of 1 case]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:759-760. [PubMed] |
| 3. | Dradjat RS, Pradana AS, Putra DP, Hexa Pandiangan RA, Cendikiawan F, Mustamsir E. Successful management of severe manifestation bone cemented implantation syndrome during hemiarthroplasty surgery in patient with multiple comorbidities: A case report. Int J Surg Case Rep. 2021;78:331-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Donaldson AJ, Thomson HE, Harper NJ, Kenny NW. Bone cement implantation syndrome. Br J Anaesth. 2009;102:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 5. | Govil P, Kakar PN, Arora D, Das S, Gupta N, Govil D, Gupta S, Malohtra A. Bone cement implantation syndrome: a report of four cases. Indian J Anaesth. 2009;53:214-218. [PubMed] |
| 6. | Blinc A, Bozic M, Vengust R, Stegnar M. Methyl-methacrylate bone cement surface does not promote platelet aggregation or plasma coagulation in vitro. Thromb Res. 2004;114:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Motobe T, Hashiguchi T, Uchimura T, Yamakuchi M, Taniguchi N, Komiya S, Maruyama I. Endogenous cannabinoids are candidates for lipid mediators of bone cement implantation syndrome. Shock. 2004;21:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Singh V, Bhakta P, Zietak E, Hussain A. Bone cement implantation syndrome: a delayed postoperative presentation. J Clin Anesth. 2016;31:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Olsen F, Kotyra M, Houltz E, Ricksten SE. Bone cement implantation syndrome in cemented hemiarthroplasty for femoral neck fracture: incidence, risk factors, and effect on outcome. Br J Anaesth. 2014;113:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Bansal A, Tewari A, Garg S, Kanwal A. Anesthetic considerations in pemphigus vulgaris: Case series and review of literature. Saudi J Anaesth. 2012;6:165-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Vasiliou A, Nikolopoulos TP, Manolopoulos L, Yiotakis J. Laryngeal pemphigus without skin manifestations and review of the literature. Eur Arch Otorhinolaryngol. 2007;264:509-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Mahalingam TG, Kathirvel S, Sodhi P. Anaesthetic management of a patient with pemphigus vulgaris for emergency laparotomy. Anaesthesia. 2000;55:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 208] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Shaikh SI, Verma H. Parkinson's disease and anaesthesia. Indian J Anaesth. 2011;55:228-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Lee JH, Lee KW, Kim SB, Lee SJ, Chun SM, Jung SM. The Functional Dysphagia Scale Is a Useful Tool for Predicting Aspiration Pneumonia in Patients With Parkinson Disease. Ann Rehabil Med. 2016;40:440-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Shanthanna H. Stiff man syndrome and anaesthetic considerations: successful management using combined spinal epidural anaesthesia. J Anaesthesiol Clin Pharmacol. 2010;26:547-548. [PubMed] |
| 17. | Schwarzkopf E, Sachdev R, Flynn J, Boddapati V, Padilla RE, Prince DE. Occurrence, risk factors, and outcomes of bone cement implantation syndrome after hemi and total hip arthroplasty in cancer patients. J Surg Oncol. 2019;120:1008-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |