Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3334
Peer-review started: September 28, 2020
First decision: December 14, 2020
Revised: December 24, 2020
Accepted: January 7, 2021
Article in press: January 7, 2021
Published online: May 16, 2021
Processing time: 212 Days and 20.3 Hours
The metastasis of liver cancer to skeletal muscle is extremely rare compared to other sites. We herein report a case of rapidly developing skeletal metastases following liver transplantation due to primary liver cancer.
A 70-year-old male with underlying chronic hepatitis B virus infection was diagnosed with hepatocellular carcinoma (HCC), for which he underwent liver transplantation in 2014. Six years after receiving the transplant, pathological examination confirmed the presence of HCC without vascular invasion. He was admitted to the hospital with a rapidly growing mass on his right thigh. Ultrasound examination revealed a mixed echo mass in the lateral soft tissue of the middle part of the right femur. Magnetic resonance imaging showed heterogeneous iso-signal intensity on T1-weighted images and heterogeneous hyper-intensity on T2-weighted images compared to the surrounding muscles. Pathological examination of the ultrasound-guided needle biopsy specimen revealed that it was similar to the previously detected liver cancer; the diagnosis was metastasis of HCC. Surgical excision was performed. There were no other sites of metastasis, and the patient recovered well after surgery.
This report presents a rare case of skeletal metastasis following liver transplant
Core Tip: Skeletal muscle metastasis from hepatocellular carcinoma is extremely rare and is often accompanied by metastasis to other organs. We herein report a case of rapidly developing skeletal metastases after liver transplantation due to primary liver cancer, without any other organ metastases. There is a dearth of literature on the detailed prognosis of patients with skeletal muscle metastasis. Therefore, the correct interpretation of the molecular and pathophysiological mechanisms of skeletal muscle metastasis may help with the development of novel therapies to combat the progression of the disease.
- Citation: Song Q, Sun XF, Wu XL, Dong Y, Wang L. Skeletal muscle metastases of hepatocellular carcinoma: A case report and literature review . World J Clin Cases 2021; 9(14): 3334-3341
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3334.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3334
More than half a million new cases of hepatocellular carcinoma (HCC) are diagnosed annually worldwide. In China, HCC is the fourth most common cancer in males after pulmonary, stomach, and esophagus cancers, and the sixth most common in females[1]. Extrahepatic HCC can occur in one of three ways: direct extension, hematogenous spread, or lymphatic invasion. Rupture of HCC may result in intraperitoneal implantation of tumor cells on peritoneal or omental surfaces. Reported HCC metastatic sites include lungs, lymph nodes, bone, and brain. Rare sites of metastasis include the rectum, spleen, diaphragm, duodenum, esophagus, pancreas, seminal vesicle, and the urinary bladder. Treatment is often based on the stage of a tumor[2]. In addition to chemotherapy, liver transplantation offers the advantage of radical treatment for HCC because it can remove the tumor and restore normal liver function. A rare but possible outcome of this modality is skeletal muscle metastasis (SMM), a malignant tumor.
In this study, we report a case of a 70-year-old patient with long-term SMM of primary liver cancer in the muscularis of the right thigh, detected 6 years after liver transplantation without any other organ metastases.
A 70-year-old male had a rapidly growing mass on his right thigh, with no pain, redness, and swelling observed on the surface of the mass.
The patient had underlying chronic hepatitis B virus infection, and was diagnosed with HCC. He had received a liver transplant for primary liver cancer 6 years prior. He was admitted to the hospital after finding a rapidly growing mass on his right thigh.
The patient underwent a liver transplant for primary liver cancer 6 years prior.
The patient was retired, living with his son, and had no family history of hereditary disease.
The patient was hospitalized and his physical parameters were examined. The vital signs were as follows: Body temperature 36 °C, heart rate 85 beats/min, respiratory rate 16 breaths/min, blood pressure 130/85 mmHg, and oxygen saturation in room air 95%. There were no apparent signs of cranial nerve dysfunction. Muscle tone, strength, and deep tendon reflexes were normal in the upper extremities, while low in the inferior extremities with more on the right side. Abdominal cutaneous reflexes were normal. The remaining physical examination was unremarkable.
After re-sectioning the right lateral femoral soft tissue mass, the patient was examined intraoperatively (right thigh mass) with a circumscribed mass of 5 cm × 3 cm × 2 cm. The findings revealed that small muscle tissue was attached to the surrounding mass, and a section of the mass was light brown, solid, and multinodular.
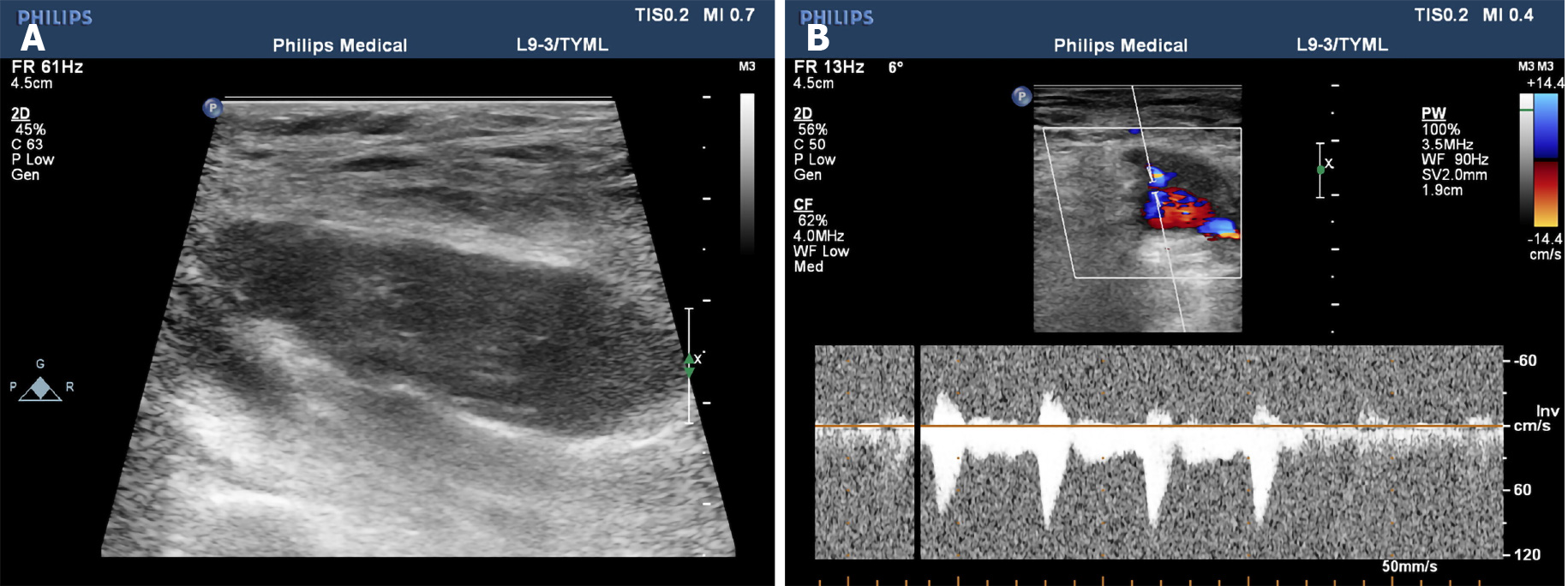
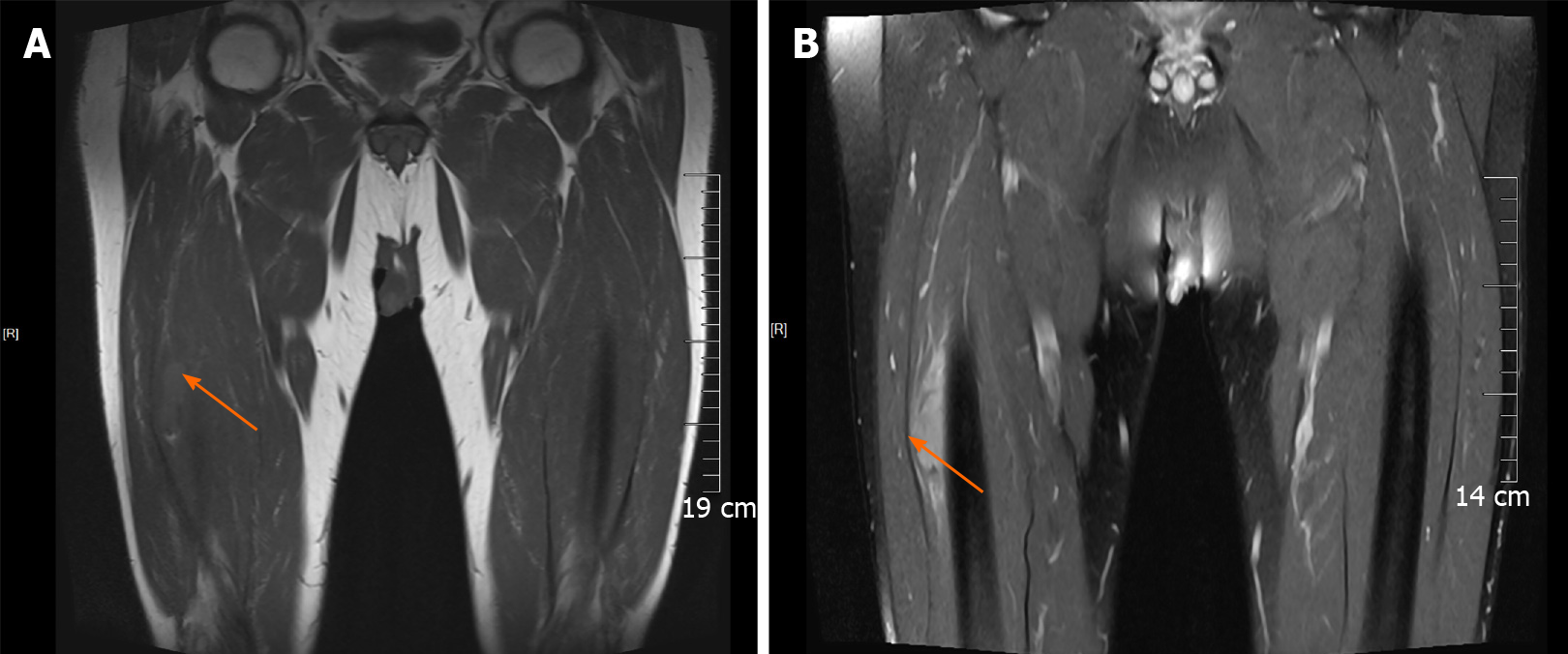
During admission, physical examination showed an oval-shaped mass with a diameter of about 5 cm on the anterolateral part of the right thigh. It was hard to touch, had poor mobility, and was attached to the surrounding soft tissue. He had normal blood flow from the right lower limb with adequate movement; the skin temperature was normal, no abnormality was found in the sensory nerve examination, the physiological reflex was normal, and no abnormality was found in the rest of the review. The ultrasound examination revealed a mixed echo mass in the lateral soft tissue of the middle part of the right femur, with a size of approximately 5 cm × 2 cm × 3 cm, which had a clear boundary and regular shape. Figure 1A presents an ultrasound image of the muscularis in the right thigh in which the blood flow is indicated with undefined boundaries. Figure 1B depicts the arterial spectrum taken by color Doppler flow imaging. The ultrasound image showed that soft tissues covered the right side of the femur, the nature of which remains to be determined. Figure 2 presents circular abnormal signals in the middle part of the right femur near the lateral soft tissue, accompanied by surrounding soft tissue edema by magnetic resonance imaging (MRI). The images showed heterogeneous iso-signal intensity on T1WI and heterogeneous hyper-intensity on T2WI compared to the surrounding muscles, which had signal inhomogeneity. The size was about 4.5 cm × 1.4 cm × 3.1 cm; the boundary was slightly blurred, and there were a few pieces of long T2 signal in the adjacent soft tissue (Figure 2A and B).
The final diagnosis of skeletal metastases was made based on pathological results and clinical history, where the patient had liver transplantation for liver cancer without affecting any other organ metastases.
The patient underwent mass and peripheral musculature resection of the right vastus lateralis.
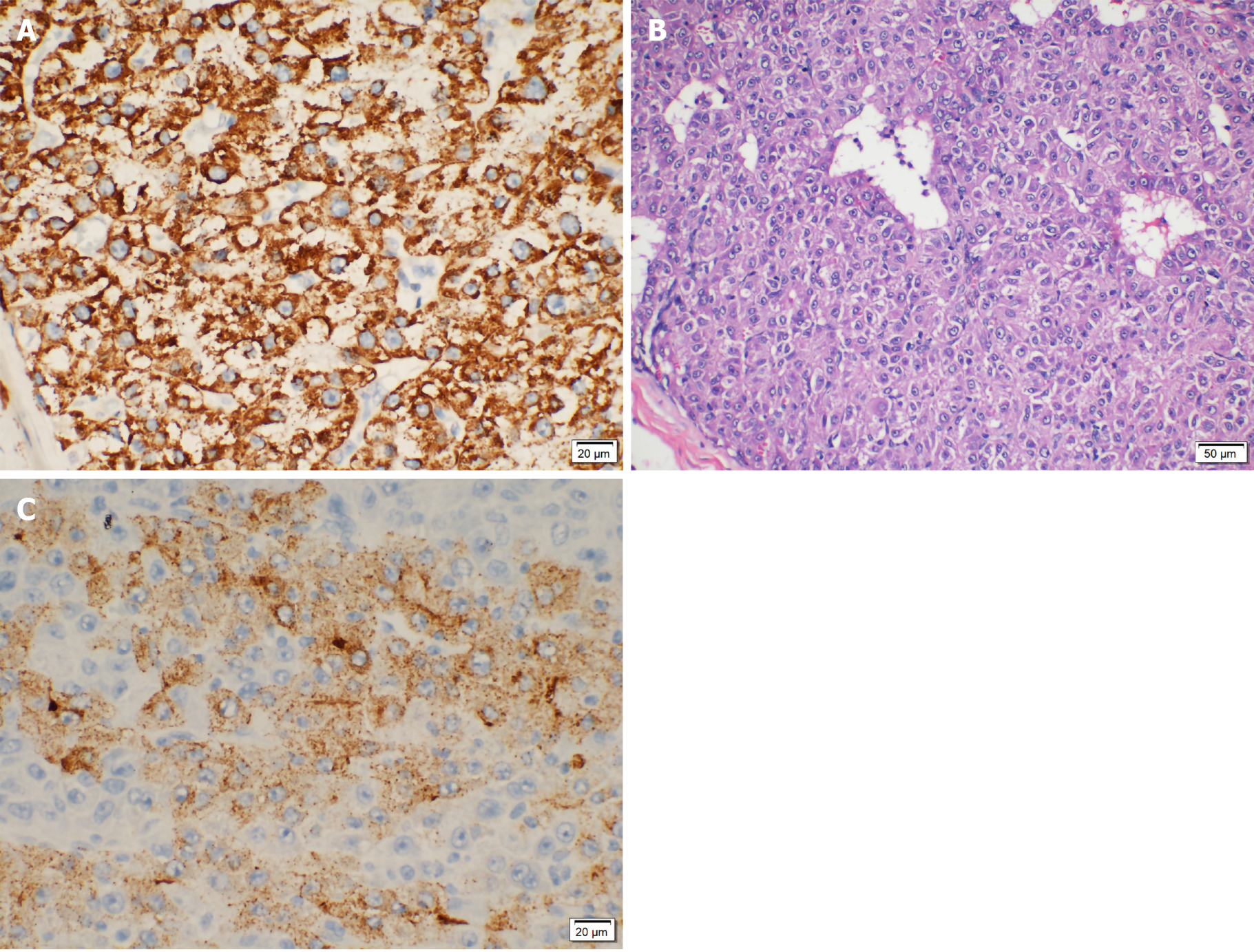
After resectioning the right lateral femoral soft tissue mass, the patient was examined intraoperatively (right thigh mass) with a circumscribed mass of 5 cm × 3 cm × 2 cm. Small muscle tissue was attached to the surrounding mass, and a section of the mass was light brown, solid, and multinodular. Figure 3 shows the immunohistochemical results: cluster of differentiation 68 (-), GATA (-), Inhibin A (-), Ki-67 (+40%), prostate-specific antigen (-), vimentin (-) cytokeratin 20 (CK20) (-), CK7 (-), CK-pan (+), giant cell fibroma-3 (lesions+), hepatocyte paraffin 1 (-), S-100 (-), thyroid transcription factor-1 (-), villin (weak + in local parts). The pathological testing confirmed the diagnosis of a malignant tumor based on medical history and immunohistochemical analysis, suggesting metastatic HCC with tumor thrombus visible in the vessel. After 1 mo, the patient was discharged, and he had no other symptoms.
HCC is ranked sixth in incidence of malignancies and fourth in mortality rate. The World Health Organization has predicted a global mortality of more than 1 million from HCC by 2030[1]. In China, liver cancer is the second leading cause of cancer-associated deaths[2]. Liver transplantation offers the advantage of radical treatment for HCC, as it can remove the tumor and restore normal liver function. The occurrence SMM, a malignant tumor that often occurs in patients with advanced cancer and appears to be a sign of systemic hematogenous metastases, is rare after liver transpl
Here we report a case of an elderly male with SMM to his right thigh, 6 years after liver transplantation for liver cancer without metastases to other organs. HCC metastasis to the skeletal muscle is rare, not previously reported, and recognized as a possibility in patients with HCC.
SMMs have a poor prognosis that could cause functional impairment and increased short-term postoperative morbidity in patients with and without malignant diseases[34]. However, 1 mo later, this patient was discharged with no radiation or chemotherapy; we will continue to follow up for further analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Frena A, Paramesh AS, Rao V S-Editor: Zhang L L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3161] [Article Influence: 526.8] [Reference Citation Analysis (37)] |
| 2. | Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, Mullany EC, Redford SB, Liu H, Naghavi M, Hay SI, Murray CJL, Liang X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2386] [Article Influence: 397.7] [Reference Citation Analysis (1)] |
| 3. | Crombé A, Lintingre PF, Le Loarer F, Lachatre D, Dallaudière B. Multiple skeletal muscle metastases revealing a cardiac intimal sarcoma. Skeletal Radiol. 2018;47:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Basile D, Parnofiello A, Vitale MG, Cortiula F, Gerratana L, Fanotto V, Lisanti C, Pelizzari G, Ongaro E, Bartoletti M, Garattini SK, Andreotti VJ, Bacco A, Iacono D, Bonotto M, Casagrande M, Ermacora P, Puglisi F, Pella N, Fasola G, Aprile G, Cardellino GG. The IMPACT study: early loss of skeletal muscle mass in advanced pancreatic cancer patients. J Cachexia Sarcopenia Muscle. 2019;10:368-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Almusarhed M, Eldeeb H. Solitary biceps muscle metastasis from breast cancer. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Emmering J, Vogel WV, Stokkel MP. Intramuscular metastases on FDG PET-CT: a review of the literature. Nucl Med Commun. 2012;33:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Leitner J, Pelster S, Schöpf V, Berghoff AS, Woitek R, Asenbaum U, Nenning KH, Widhalm G, Kiesel B, Gatterbauer B, Dieckmann K, Birner P, Prayer D, Preusser M, Furtner J. High correlation of temporal muscle thickness with lumbar skeletal muscle cross-sectional area in patients with brain metastases. PLoS One. 2018;13:e0207849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Martínez Mullor C, de Aspe de la Iglesia E, Cordido Carro M. [Skeletal muscle metastases as the initial manifestation of an unknown primary lung cancer]. Semergen. 2017;43:261-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Dohzono S, Sasaoka R, Takamatsu K, Hoshino M, Nakamura H. Low paravertebral muscle mass in patients with bone metastases from lung cancer is associated with poor prognosis. Support Care Cancer. 2020;28:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Wright LE, Harhash AA, Kozlow WM, Waning DL, Regan JN, She Y, John SK, Murthy S, Niewolna M, Marks AR, Mohammad KS, Guise TA. Aromatase inhibitor-induced bone loss increases the progression of estrogen receptor-negative breast cancer in bone and exacerbates muscle weakness in vivo. Oncotarget. 2017;8:8406-8419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Carey K, Bestic J, Attia S, Cortese C, Jain M. Diffuse skeletal muscle metastases from sacral chordoma. Skeletal Radiol. 2014;43:985-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Cincibuch J, Mysliveček M, Melichar B, Neoral C, Metelková I, Zezulová M, Procházková-Študentová H, Flodr P, Zlevorová M, Aujeský R, Cwiertka K. Metastases of esophageal carcinoma to skeletal muscle: single center experience. World J Gastroenterol. 2012;18:4962-4966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Salman R, Sebaaly MG, Asmar K, Nasserdine M, Bannoura S, Khoury NJ. Rare skeletal muscle metastasis from renal cell carcinoma: case report and review of the literature. CEN Case Rep. 2018;7:316-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Guidi M, Fusetti C, Lucchina S. Skeletal Muscle Metastases to the Flexor Digitorum Superficialis and Profundus from Urothelial Cell Carcinoma and Review of the Literature. Case Rep Urol. 2016;2016:2387501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Katafigiotis I, Athanasiou A, Levis PK, Fragkiadis E, Sfoungaristos S, Ploumidis A, Michalinos A, Alamanis C, Felekouras E, Constantinides CA. Metastasis to sartorius muscle from a muscle invasive bladder cancer. Case Rep Med. 2014;2014:524757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Pergolini I, Crippa S, Santinelli A, Marmorale C. Skeletal muscle metastases as initial presentation of gastric carcinoma. Am J Case Rep. 2014;15:580-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Luo C, Jiang Y, Liu Y, Li X. Experimental study on mechanism and rarity of metastases in skeletal muscle. Chin Med J (Engl). 2002;115:1645-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Magee T, Rosenthal H. Skeletal muscle metastases at sites of documented trauma. AJR Am J Roentgenol. 2002;178:985-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Gómez-León N, Pacheco-Barcia V, Ballesteros AI, Fraga J, Colomer R, Friera A. Skeletal muscle and solitary bone metastases from malignant melanoma: multimodality imaging and oncological outcome. Melanoma Res. 2018;28:562-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Eriksson S, Nilsson JH, Strandberg Holka P, Eberhard J, Keussen I, Sturesson C. The impact of neoadjuvant chemotherapy on skeletal muscle depletion and preoperative sarcopenia in patients with resectable colorectal liver metastases. HPB (Oxford). 2017;19:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Ong N, George M, Dutta R, Ng CH. CT imaging features of skeletal muscle metastasis. Clin Radiol. 2019;74:374-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Nocuń A, Chrapko B. Multiple and solitary skeletal muscle metastases on 18F-FDG PET/CT imaging. Nucl Med Commun. 2015;36:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 23. | Arpaci T, Ugurluer G, Akbas T, Arpaci RB, Serin M. Imaging of the skeletal muscle metastases. Eur Rev Med Pharmacol Sci. 2012;16:2057-2063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Li Q, Wang L, Pan S, Shu H, Ma Y, Lu Z, Fu X, Jiang B, Guo Q. Skeletal muscle metastases on magnetic resonance imaging: analysis of 31 cases. Contemp Oncol (Pozn). 2016;20:242-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Beşe NS, Ozgüroğlu M, Dervişoğlu S, Kanberoğlu K, Ober A. Skeletal muscle: an unusual site of distant metastasis in gastric carcinoma. Radiat Med. 2006;24:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Wang G, Biswas AK, Ma W, Kandpal M, Coker C, Grandgenett PM, Hollingsworth MA, Jain R, Tanji K, Lόpez-Pintado S, Borczuk A, Hebert D, Jenkitkasemwong S, Hojyo S, Davuluri RV, Knutson MD, Fukada T, Acharyya S. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat Med. 2018;24:770-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 27. | Bekir Hacioglu M, Kostek O, Kurt N, Kucukarda A, Gokyer A, Ustabasioglu FE, Karatas F, Tuncbilek N, Uzunoglu S, Bilici A, Cicin I, Erdogan B. Comparison of skeletal muscle mass loss in patients with metastatic colorectal cancer treated with regorafenib or TAS-102. J BUON. 2019;24:2198-2204. [PubMed] |
| 28. | van Vugt JLA, Gaspersz MP, Vugts J, Buettner S, Levolger S, de Bruin RWF, Polak WG, de Jonge J, Willemssen FEJA, Groot Koerkamp B, IJzermans JNM. Low Skeletal Muscle Density Is Associated with Early Death in Patients with Perihilar Cholangiocarcinoma Regardless of Subsequent Treatment. Dig Surg. 2019;36:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Kurk SA, Stellato RK, Peeters PHM, Dorresteijn B, Jourdan M, Oskam MJ, Punt CJA, Koopman M, May AM. Trajectory of body mass and skeletal muscle indices and disease progression in metastatic colorectal cancer patients. Am J Clin Nutr. 2019;110:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Weiss L. Biomechanical destruction of cancer cells in skeletal muscle: a rate-regulator for hematogenous metastasis. Clin Exp Metastasis. 1989;7:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Plaza JA, Perez-Montiel D, Mayerson J, Morrison C, Suster S. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer. 2008;112:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Kim YW, Seo KJ, Lee SL, Kwon KW, Hur J, An HJ, Ko YH, Kim JS, Won HS. Skeletal muscle metastases from breast cancer: two case reports. J Breast Cancer. 2013;16:117-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Purkayastha A, Singh S, Bisht N, Mishra PS, Husain A. Upfront Skeletal Muscle Metastases from Non-small Cell Lung Carcinoma: Report of an Extremely Rare Occurrence Detected by 18F-Fluorodeoxyglucose Positron Emission Computed Tomography Scan. Indian J Nucl Med. 2018;33:337-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 1489] [Article Influence: 99.3] [Reference Citation Analysis (0)] |