Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3327
Peer-review started: October 7, 2020
First decision: January 17, 2021
Revised: January 24, 2021
Accepted: March 4, 2021
Article in press: March 4, 2021
Published online: May 16, 2021
Processing time: 188 Days and 17.2 Hours
Acute flaccid paralysis (AFP) and neurogenic respiratory failure rarely occur in children. At the end of 2018, some children with such symptoms were admitted to our hospital. In this study, we aimed to assess two children with AFP and neurogenic respiratory failure associated with enterovirus D68 (EV-D68).
Two children admitted to our hospital presented with symptoms and imaging results different from those of acute disseminated encephalomyelitis and hand, foot, and mouth disease. Their main symptoms were AFP and neurogenic respiratory failure. Magnetic resonance imaging showed severe inflammatory injury mainly to the anterior horn cells of the spinal cord. Blood and cerebrospinal fluid samples were collected to assess for pathogens, including bacteria, tuberculosis, cryptococcus, herpes virus, and coxsackie virus, and the results were negative. At the beginning, the two cases were not assessed for EV-D68 in the nasopharyngeal, blood, and cerebrospinal fluid specimens. About 2 mo later, EV-D68 was detected in the stool sample of one of the cases. The symptom of AFP was caused by injury to the anterior horn cells at levels C5-L5 of the spinal cord, while neurogenic respiratory failure was at levels C3-C5.
We should pay attention to the detection and diagnosis of EV-D68 and make efforts to develop antivirus drugs and vaccines.
Core Tip: This paper reports two pediatric cases of acute flaccid paralysis (AFP) and neurogenic respiratory failure associated with enterovirus D68 (EV-D68) infection which rarely occur. We found that EV-D68 might have caused inflammatory injury to the anterior horn cells of the spinal cord. AFP was caused at levels C5-L5, while neurogenic respiratory failure was caused at levels C3-C5. Our findings indicate that EV-D68 should be carefully monitored in children and that further research must be carried out to develop special antivirus drugs and vaccines. We believe that our research is of particular interest and use to clinicians and experts in infectious diseases.
- Citation: Zhang Y, Wang SY, Guo DZ, Pan SY, Lv Y. Acute flaccid paralysis and neurogenic respiratory failure associated with enterovirus D68 infection in children: Report of two cases. World J Clin Cases 2021; 9(14): 3327-3333
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3327.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3327
The incidence of acute flaccid paralysis (AFP) has been on the rise in recent years. The symptom of AFP is thought to be caused by enterovirus infections such as polio virus and enterovirus A71 (EV-A71). Recently, research attention has focused on enterovirus D68 (EV-D68) as a causative agent, which has been prevalent intermittently in various parts of the world. Most clinical reports have described respiratory symptoms caused by EV-D68. In 2015, EV-D68 caused severe acute respiratory tract infection, AFP, and cranial nervous system injury among children in North America, which attracted the attention of the international community[1]. This paper reports two cases of encephalo
Case 1: A 6-year-old boy presented to our hospital with a 2 mo history of upper limb weakness and respiratory failure.
Case 2: A 3-year-old girl presented to our hospital with a 4 mo history of all limb weakness and respiratory failure.
Case 1: The boy began to experience a productive cough with no obvious inducement on September 22, 2018. On September 25, 2018, he had a fever (39 °C peak temperature) with headache and dizziness. On September 26, 2018, he experienced weakness of both upper limbs, with the left side being more severe. He was unable to lift and hold objects using the left upper limb. He was admitted to the hospital. On September 30, 2018, asthmatic suffocation and respiratory failure occurred.
Case 2: The girl experienced a fever (38 °C peak temperature) on September 15, 2018, accompanied by a seizure manifested as limb shaking and epileptic nystagmus, which lasted for approximately 1 min. The girl was able to recover from the seizure by herself. On September 19, 2018, sighing breathing was observed, and mechanical ventilation was performed through endotracheal intubation.
Case 1: Results of the physical examination were as follows: Temperature 38.3 °C, heart rate 106 times/min, respiratory rate 22 times/min, blood pressure 114/87 mmHg, soft neck, shortness of breath, thick breath sounds in both lungs, phlegm, few wheezes, regular heart rhythm, powerful heart sounds, clear mind, normal light reflex of both pupils, normal eye movements in both sides, low muscle tension in both upper limbs, level 1 muscle strength of the left upper limb, level 2 muscle strength of the right upper limb, and normal muscle tension and strength of both lower limbs.
Case 2: The physical examination showed the following results: Oxygen saturation (measured via skin) 88%, no spontaneous breathing, pressure and oxygen supply via tracheal intubation and resuscitation capsule, light coma, soft neck, equal circles of pupils, 3 mm pupil diameter, slow pupillary reflection of light, no cyanosis, thick breath sounds in both lungs, audible and moderately thick moist rales, powerful heart sounds, heart rate 160 times/min, regular heart rhythm, and no murmur in the auscultation area of each heart valve. The blood pressure was 150/100 mmHg, and limb muscle tension was low. Bilateral biceps brachii tendon reflex, triceps brachii tendon reflex, knee tendon reflex, and Achilles tendon reflex were not elicited. The muscle strength was level 0.
Case 1: Laboratory tests were conducted on September 28, 2018, and the results were as follows. Complete blood counts were: White blood cells 8.91 × 109/L, lymphocytes 33.3%, neutrophils 55.4%, red blood cells 4.94 × 1012/L, hemoglobin 119 g/L, and platelets 345 × 109/L. The C-reactive protein (CRP) level was 1.94 mg/L, and the erythrocyte sedimentation rate was 47 mm/h. Arterial blood gas analysis showed partial pressure of oxygen 58 mmHg, partial pressure of carbon dioxide 41 mmHg, blood pH 7.40, lactate 0.8 mmol/L, base excess 2.6 mmol/L, and SO2 91%. Blood biochemistry tests revealed procalcitonin 0.04 ng/mL, alkaline phosphatase 268 U/L, pre-albumin 157.00 mg/mL, total protein 95.2 g/L, albumin 47.2 g/L, globulin 48.0 g/L, urea nitrogen 4.1 mmol/L, and creatinine 33.00 μmol/L. Coagulation tests showed prothrombin time 13.7 s and activated partial thrombo plastin time 25.9 s; other indexes were normal. Routine cerebrospinal fluid (CSF) test revealed nucleated cells 259 × 106/L, mononuclear cells 93%, multiple nuclear cells 7.0%, and normal biochemistry. Common culture of venous blood revealed no bacterial growth. The CSF, throat swabs, and venous blood were all negative for common pathogens. In November 2018, stool samples were collected and sent to the CDC for etiological examination and were found to be positive for EV-D68.
Case 2: The laboratory test results were as follows. Routine blood and CRP tests (September 18, 2018) showed leukocytes 11.11 × 109/L, neutrophils 57.1%, lymphocytes 37.5%, red blood cells 4.68 × 1012/L, hemoglobin 135 g/L, hematocrit 40%, platelets 314 × 109/L, and CRP 4mg/L. Routine CSF and biochemical tests (September 18, 2018) revealed colorless, transparent, and clear CSF; white blood cells 97 × 106/L; monocytes 95.2%; and multiple nuclear cells 4.8%. The glucose level was 4.78 mmol/L, chlorine 118.2 mmol/L, and protein 450 mg/L. Respiratory pathogen tests (September 30, 2018) revealed positivity for syncytial virus and negativity for others.
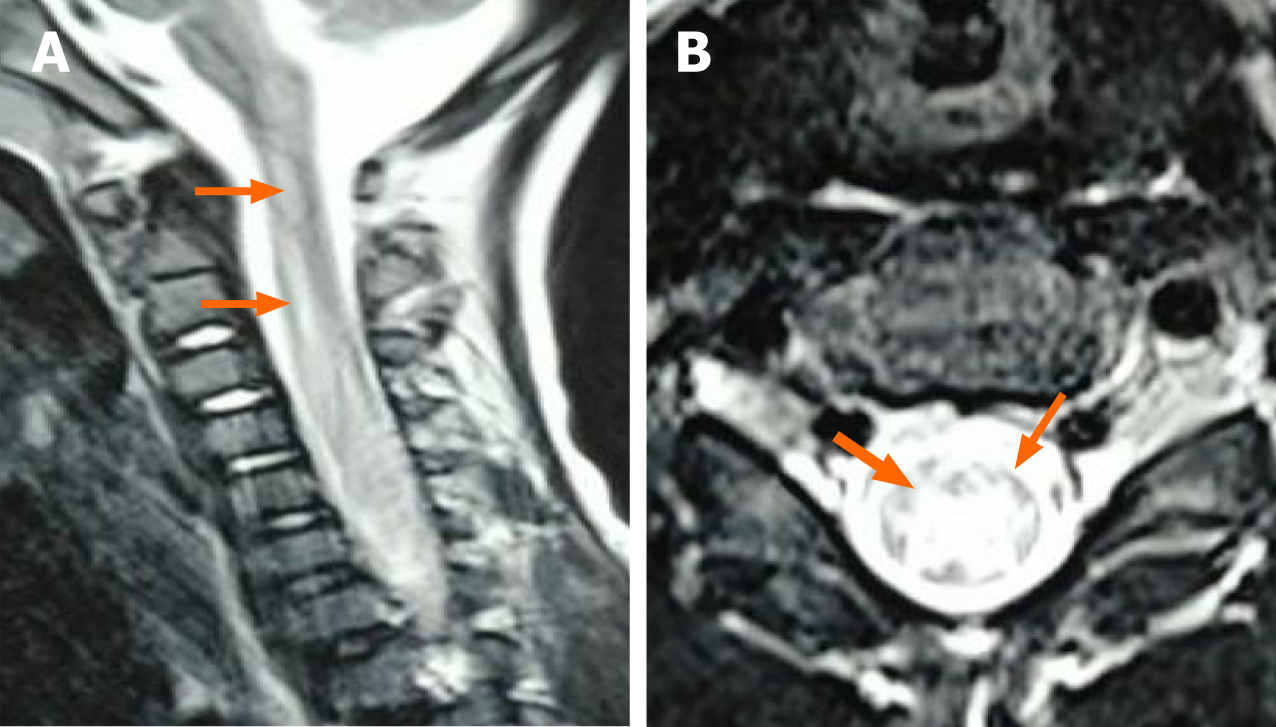
Case 1: Chest radiograph (September 27, 2018) revealed that bilateral lung markings slightly increased. Magnetic resonance imaging (MRI) of the brain and spinal cord (September 29, 2018) showed: (1) Abnormal signals in the left frontal lobe, with a high possibility of cerebral softening; and (2) abnormal signals in the brainstem and cervical and thoracic spinal cord, possibly indicating inflammatory lesions (the patient was recommended for reexamination after treatment) (Figure 1). Electromyography (November 1, 2018) revealed neurogenic injury.
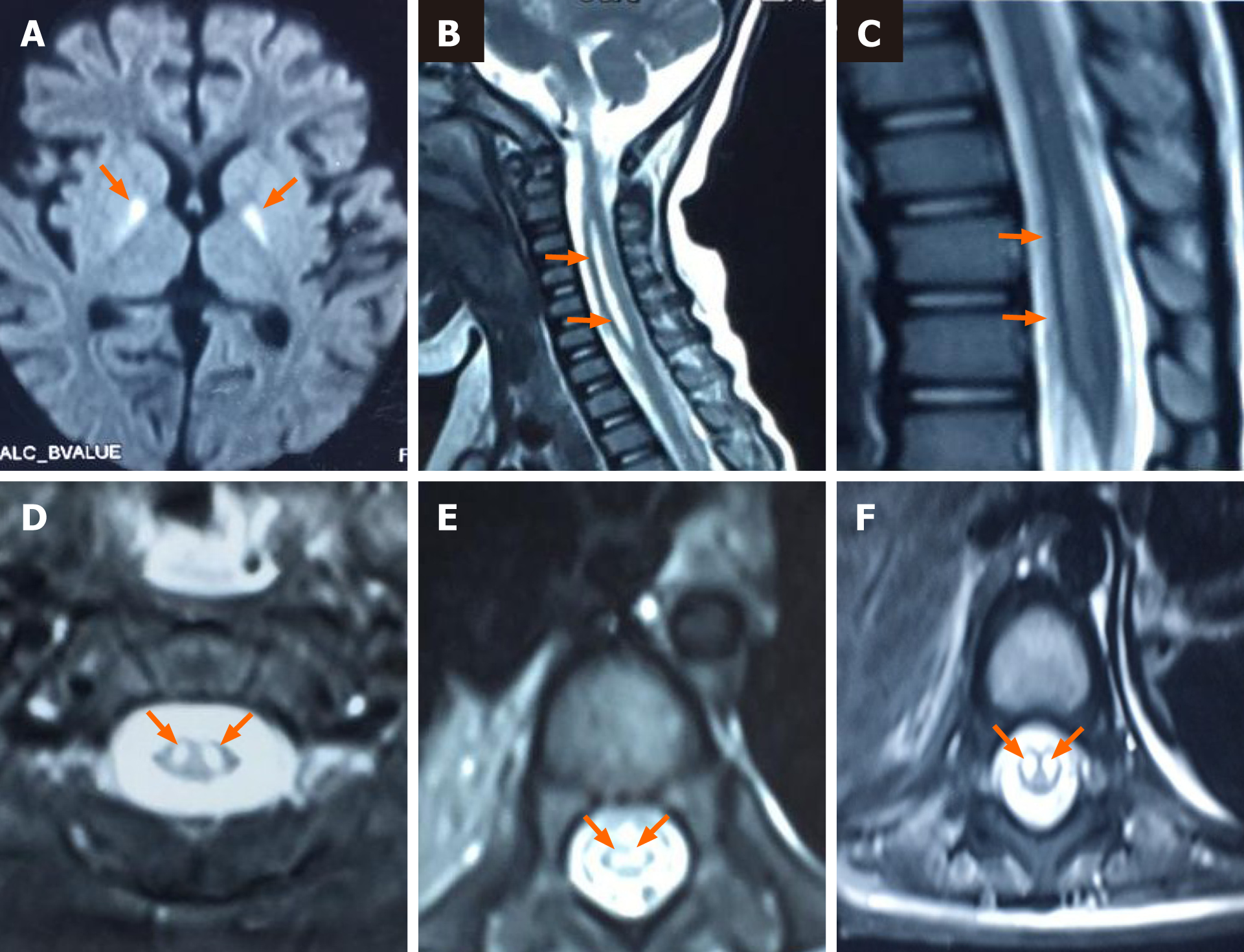
Case 2: Cranial and spinal MRI (October 3, 2018) showed: (1) Widened sulci in the bilateral cerebral hemispheres; (2) abnormal signals in the bilateral basal ganglia, hippocampal area, and mesencephalon; and (3) high signals in the spinal cord at levels C1-T1 and T8-L1, especially in the anterior horn (Figure 2).
Case 1: Intracranial infection was suspected as the reason for the weakness of both upper limbs and respiratory failure in case 1. Complete blood counts were: White blood cells 8.91 × 109/L, lymphocytes 33.3%, neutrophils 55.4%, red blood cells 4.94 × 1012/L, hemoglobin 119 g/L, and platelets 345 × 109/L. The CRP level was 1.94 mg/L, and the erythrocyte sedimentation rate was 47 mm/h. Routine CSF test revealed nucleated cells 259 × 106/L, mononuclear cells 93%, multiple nuclear cells 7.0%, and normal biochemistry. Common culture of venous blood showed no bacterial growth. The CSF, throat swabs, and venous blood were all negative for common pathogens.
Case 2: The original diagnoses of case 2 were viral brainstem encephalitis, sepsis, Guillain-Barre syndrome, and acute disseminated encephalomyelitis (ADEM). The routine blood and CRP tests (September 18, 2018) showed leukocytes 11.11 × 109/L, neutrophils 57.1%, lymphocytes 37.5%, red blood cells 4.68 × 1012/L, hemoglobin 135 g/L, hematocrit 40%, platelets 314 × 109/L, and CRP 4 mg/L. Routine CSF and biochemical tests (September 18, 2018) showed colorless, transparent, and clear CSF; white blood cells 97 × 106/L; monocytes 95.2%; and multiple nuclear cells 4.8%. The glucose level was 4.78 mmol/L, chlorine 118.2 mmol/L, and protein 450 mg/L. Respiratory pathogen tests (September 30, 2018) revealed positivity for syncytial virus and negativity for others.
Case 1: The stool samples were collected and sent to the CDC for etiological examination and were found to be positive for EV-D68 about 2 mo after the onset of the disease.
Case 2: The stool samples were sent for examination nearly half a year after the onset of the disease. However, EV-D68 was negative.
Case 1: The final diagnosis of the case 1 was encephalomyelitis due to EV-D68.
Case 2: The latest diagnosis of the case 2 was encephalomyelitis likely due to EV-D68.
Case 1: The patient received antivirals, anti-dehydration measures, hormones, gamma globulins, ventilator-assisted respiration, etc. After treatment, the muscle strength of both upper limbs recovered to level 2, tracheotomy was still performed, and intermittent ventilator support was still used to assist breathing.
Case 2: The patient received plasma exchange therapy, methylprednisolone sodium succinate shock therapy, oral prednisone, human immunoglobulin, ganciclovir treatment, mechanical ventilation, etc.
Case 1: After treatment, the muscle strength of both upper limbs recovered to level 2, tracheotomy was still performed, and intermittent ventilator support was still used to assist breathing.
Case 2: After treatment, the child demonstrated a normal body temperature, with no further convulsions, clear consciousness, low limb muscle tension, level 0 muscle strength of both upper limbs, and level 1 muscle strength of both lower limbs. She then underwent tracheotomy, wore a plastic sleeve, and had continuous ventilator-assisted breathing support (alternate modes of synchronized intermittent mandatory ventilation and autonomous breathing).
The two cases in this study demonstrated AFP and neurogenic respiratory failure caused by severe inflammatory injury mainly to the anterior horn cells of the spinal cord. These conditions are different from ADEM, which mainly involves the white matter of the nervous system. Therefore, the diagnosis of ADEM was ruled out.
Among the pathogens that can affect both the respiratory and nervous systems, enteroviruses have always been the focus of research. Among these enteroviruses, coxsackievirus A (CA16) and EV-A71 are the main causative agents of hand, foot, and mouth disease (HFMD), which is an acute infectious disease with a high prevalence in children. Most cases of HFMD in children present as a mild self-limiting disease and mainly manifest as herpes and maculopapular rashes in the mouth, hands, and feet, accompanied by fever. A few cases can rapidly develop into fatal central nervous system complications, especially those caused by EV-A71[2,3]. Some children may have brainstem encephalitis, aseptic meningitis, or encephalomyelitis in severe cases, which may result in life-threatening or severe neurological sequelae[4]. In this study, the two cases did not show any typical manifestations of herpes and maculopapular rashes in the mouth, hands, and feet; hence, HFMD was ruled out. However, one of the cases was later found to be positive for EV-D68. At present, EV-D68 has been considered the most common pathogen for respiratory and nervous system diseases reported in the literature.
Most medical institutions in China are not capable of carrying out the detection of EV-D68 in patients. With increasing awareness of EV-D68, disease control centers at different levels have gradually increased the monitoring of the virus in nasopharyn
Enteroviruses belong to the orders Micrornaviridae and Enteroviridae, with 106 serotypes, and are divided into nine groups (enteroviruses A-J), among which groups A-D consist of human enteroviruses[5]. Except for EV-A71 and CA16, most types of enteroviruses are not currently receiving much attention because they have not caused any large epidemic. In 1962, EV-D68 was first isolated from four children with acute respiratory infection and pneumonia in the United States[6]. It was included in the National Enterovirus Surveillance System in 1987[7]. From 2004 to 2005, eight cases of enterovirus infection were detected in patients with respiratory disease and fever in the United States, seven of which were EV-D68, and this was the first report of an epidemic of EV-D68[8]. In Asia, the first case of EV-D68 was reported in Japan in 2005[9]. The first EV-D68 cases reported in China were in 2006[10]. After that, there have been few reports of the virus for a long time. However, in mid-August 2014 toward mid-January 2015, 1153 cases of EV-D68 respiratory tract infection were confirmed in the United States, including 14 deaths, which was rated as one of the top 10 public health challenges of the year in the country, attracting global attention[11].
The clinical manifestations of EV-D68 infection are diverse and have not been fully elucidated so far. Some reports[12] summarized the common symptoms of the virus as consisting of runny nose, sneezing, cough, muscle pain, asthma, dyspnea, and other serious symptoms[13]. However, as a non-polio myelitis enterovirus, EV-D68 has been found to cause relaxant myasthenia, neurological dysfunction, and other related diseases with polio-like symptoms. This disease is known as acute flaccid myelitis[1], which suggests that EV-D68 may also cause nervous system diseases. The two cases in our study not only showed limb paralysis but also showed neurogenic myasthenia of respiratory muscles and peripheral respiratory failure. Case 1 showed cough, expectoration, and hoarseness at the onset of illness, followed by fever, headache, and poor spirit after 3 d, upper limb weakness after 4 d, severe dyspnea after 8 d, and tracheotomy and invasive mechanical ventilation thereafter. After treatment, he recovered from respiratory and brain symptoms, but symptoms of respiratory muscle weakness and bilateral upper limb weakness persisted. Permanent damage to the anterior horn cells of the cervical spinal cord was therefore suspected. Meanwhile, the symptoms of upper respiratory tract infection in case 2 were not severe. Respiratory failure occurred after 4 d, after which tracheotomy and mechanical ventilation were performed. Because the child had convulsions at the onset of the disease, sedative and antiepileptic drugs were used. After discontinuing sedative treatment for 6 d, weakness of the limbs was observed. After treatment, the patient recovered from respiratory and brain symptoms; however, respiratory muscle weakness and peripheral respiratory failure remained. These persistent symptoms were thought to be related to permanent injury to the anterior horn cells of the cervical and thoracolumbar spinal cord. In both cases, respiratory muscles and neurons in the arm were obviously involved. This is in sharp contrast to poliovirus, which prefers to infect neurons that dominate the legs rather than neurons in the arms or respiratory muscles. Moreover, EV-A71 is different from both cases as it can cause brainstem encephalitis.
The mechanism of nervous system injury caused by EV-D68 has not been clarified, and there is currently no specific antiviral treatment. The usual treatment methods are immunoglobulin, hormone, plasma exchange, and broad-spectrum antiviral drug therapies[1].
In summary, our two cases showed AFP and neurogenic respiratory failure caused by severe inflammatory injury mainly to the anterior horn cells of the spinal cord-thought to be caused by EV-D68. It has to be admitted that the injuries caused by EV-D68 were more serious every time its epidemic came back again in the world. Thus, in cases of AFP and respiratory failure, EV-D68 should be monitored in nasopharyngeal swabs and sputum, CSF, blood, and stool specimens, and MRI should be performed on the brain and the whole spinal cord, to help for the diagnosis. Because of the potential epidemic and severe injury that EV-D68 can cause, further research on specific antiviral drugs and vaccines should be carried out.
We would like to thank Ren AR for imaging technical support and to thank Yang H for pediatric medicine support.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kenar T, Spasojevic SD S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Messacar K, Schreiner TL, Maloney JA, Wallace A, Ludke J, Oberste MS, Nix WA, Robinson CC, Glodé MP, Abzug MJ, Dominguez SR. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet. 2015;385:1662-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 2. | Chen Z, Sun H, Yan Y, Wang Y, Zhu C, Zhou W, Huang L, Wang M, Mize M, Tian J, Ji W. Epidemiological profiles of hand, foot, and mouth disease, including meteorological factors, in Suzhou, China. Arch Virol. 2015;160:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Li L, Yin H, An Z, Feng Z. Considerations for developing an immunization strategy with enterovirus 71 vaccine. Vaccine. 2015;33:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Puenpa J, Mauleekoonphairoj J, Linsuwanon P, Suwannakarn K, Chieochansin T, Korkong S, Theamboonlers A, Poovorawan Y. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PLoS One. 2014;9:e98888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | King AMQ, Lefkowitz EJ, Mushegian AR, Adams MJ, Dutilh BE, Gorbalenya AE, Harrach B, Harrison RL, Junglen S, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Nibert ML, Rubino L, Sabanadzovic S, Sanfaçon H, Siddell SG, Simmonds P, Varsani A, Zerbini FM, Davison AJ. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2018). Arch Virol. 2018;163:2601-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 6. | Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol. 1967;85:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 269] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA; Centers for Disease Control and Prevention. Enterovirus surveillance--United States, 1970-2005. MMWR Surveill Summ. 2006;55:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Wang Z, Malanoski AP, Lin B, Long NC, Leski TA, Blaney KM, Hansen CJ, Brown J, Broderick M, Stenger DA, Tibbetts C, Russell KL, Metzgar D. Broad spectrum respiratory pathogen analysis of throat swabs from military recruits reveals interference between rhinoviruses and adenoviruses. Microb Ecol. 2010;59:623-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Ikeda T, Mizuta K, Abiko C, Aoki Y, Itagaki T, Katsushima F, Katsushima Y, Matsuzaki Y, Fuji N, Imamura T, Oshitani H, Noda M, Kimura H, Ahiko T. Acute respiratory infections due to enterovirus 68 in Yamagata, Japan between 2005 and 2010. Microbiol Immunol. 2012;56:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Xiang Z, Gonzalez R, Wang Z, Ren L, Xiao Y, Li J, Li Y, Vernet G, Paranhos-Baccalà G, Jin Q, Wang J. Coxsackievirus A21, enterovirus 68, and acute respiratory tract infection, China. Emerg Infect Dis. 2012;18:821-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Centers for Disease Control and Prevention. EnterovirusD68 [EB/OL] 2016. [cited 1 May 2020]. Available from: http://www.cdc.gov/non-polio-enterovirus/about/ev-d68.html. |
| 12. | Oberste MS, Maher K, Schnurr D, Flemister MR, Lovchik JC, Peters H, Sessions W, Kirk C, Chatterjee N, Fuller S, Hanauer JM, Pallansch MA. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol. 2004;85:2577-2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Steele MT, Walsh I. Commentary. Severe Respiratory Illness Associated With Enterovirus D68—Missouri and Illinois, 2014. Ann Emerg Med. 2015;65:335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |