Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3320
Peer-review started: October 13, 2020
First decision: February 12, 2021
Revised: February 26, 2021
Accepted: March 23, 2021
Article in press: March 23, 2021
Published online: May 16, 2021
Processing time: 198 Days and 4.7 Hours
Glomus tumors (GTs), defined by modified smooth cells and normal glomus body cells, usually present with a small mass occurring in the soft tissue or dermis of an extremity, especially in the subungual region. However, other unusual sites, such as the respiratory tract, have also been reported. They are usually sporadic. Their imaging findings are usually nonspecific and likely to appear as a well-delineated round mass that usually lacks calcification. To our knowledge, we report the first case of bronchial GTs with calcification, reminding clinicians and radiologists that GT is one of the differential diagnoses when a calcified nodular mass is found.
We report a case of a 33-yr-old Chinese man with cough and sputum for 11 d and hemoptysis for 5 d. Chest computed tomography revealed a calcified nodular lesion on the compressed posterior wall of the lower left main bronchus and bronchiectasis in the lower lobe of the left lung. To confirm the characteristics of calcified nodules, we performed fiberoptic bronchoscopy. The tumor tissue from the biopsy of bronchial mucosal lesions established the diagnosis of GT. Because the patient had no life-threatening symptoms, he was not treated with surgery. Clinical follow-up for 25 mo showed that the patient survived well without any discomfort.
Bronchial GTs are usually not accompanied by calcification on computed tomography scans. To our knowledge, we report the first calcified bronchial GT. We recommend that clinicians consider GT as a possible differential diagnosis when a calcified mass of the bronchi is found.
Core Tip: Bronchial glomus tumors (GTs) are rare soft tissue neoplasms. The imaging features of GTs are well-defined masses or nodules without calcification on plain computed tomography scans. On dynamic contrast-enhanced computed tomography images, the tumors were enhanced significantly. In this report, we present a case of bronchial GT with calcification, reminding clinicians that they should consider GT as a differential diagnosis when finding calcified masses in the bronchus.
- Citation: Zhang Y, Zhang QP, Ji YQ, Xu J. Bronchial glomus tumor with calcification: A case report. World J Clin Cases 2021; 9(14): 3320-3326
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3320.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3320
The World Health Organization defines glomus tumors (GTs) as benign tumors with perivascular cells[1]. GT is a distinctive neoplasm originating from modified smooth muscle cells, i.e., glomocytes of the neuromyoarterial glomus or the normal glomus body, which is a specialized structure involving temperature regulation[2]. They are usually located in the subcutis of the subungual region or dermis[3]. Several unusual sites have also been reported including the gastrointestinal tract, bone, cervix, mediastinum, stomach and respiratory tract[3]. Treatment options for tracheobronchial GTs include thoracotomy, bronchoscopic electrocautery, Nd: YAG laser and cryotherapy[3]. In terms of imaging, radiological imaging of GTs of visceral organs is limited due to its rarity, and no direct imaging technique is routinely used for the diagnosis of GTs[2]. On chest computed tomography (CT), GT is likely to appear as a well-delineated round mass without calcification[4]. To date, no calcified bronchial GT has been reported. We searched PubMed with the keywords “glomus tumor and calcified tumor” or “glomus tumor and calcification” and found only six cases of GT with calcification; none of which occurred in the respiratory system. Here, we present a case of a 33-yr-old patient with calcified bronchial GT.
A Chinese man aged 33 yrs was admitted to our hospital in January 2019 due to cough and sputum for 11 d and hemoptysis for 5 d.
The patient had no obvious cause of cough and yellow sputum or fever 11 d ago. The highest body temperature was 39.4°C. There was no other obvious discomfort. After taking cephalosporin, his body temperature returned to normal. However, 5 d ago, he had hemoptysis.
The patient had no history of prior illness.
No personal or family history of benign or malignant tumors exist.
His temperature was 36.8°C, resting respiratory rate 15 breaths/min, heart rate 78 bpm and blood pressure 130/90 mmHg. Physical examinations were normal except for vesicular breath sounds.
Laboratory examination showed a white blood cell count of 6.04×109/L, with 66% neutrophils, 165 g/L hemoglobin, 275×109/L platelets, erythrocyte sedimentation rate 2 mm/h and normal range of routine urine tests, routine fecal tests and occult blood test, electrolyte profile and blood biochemistry. In addition, the human immunodefici
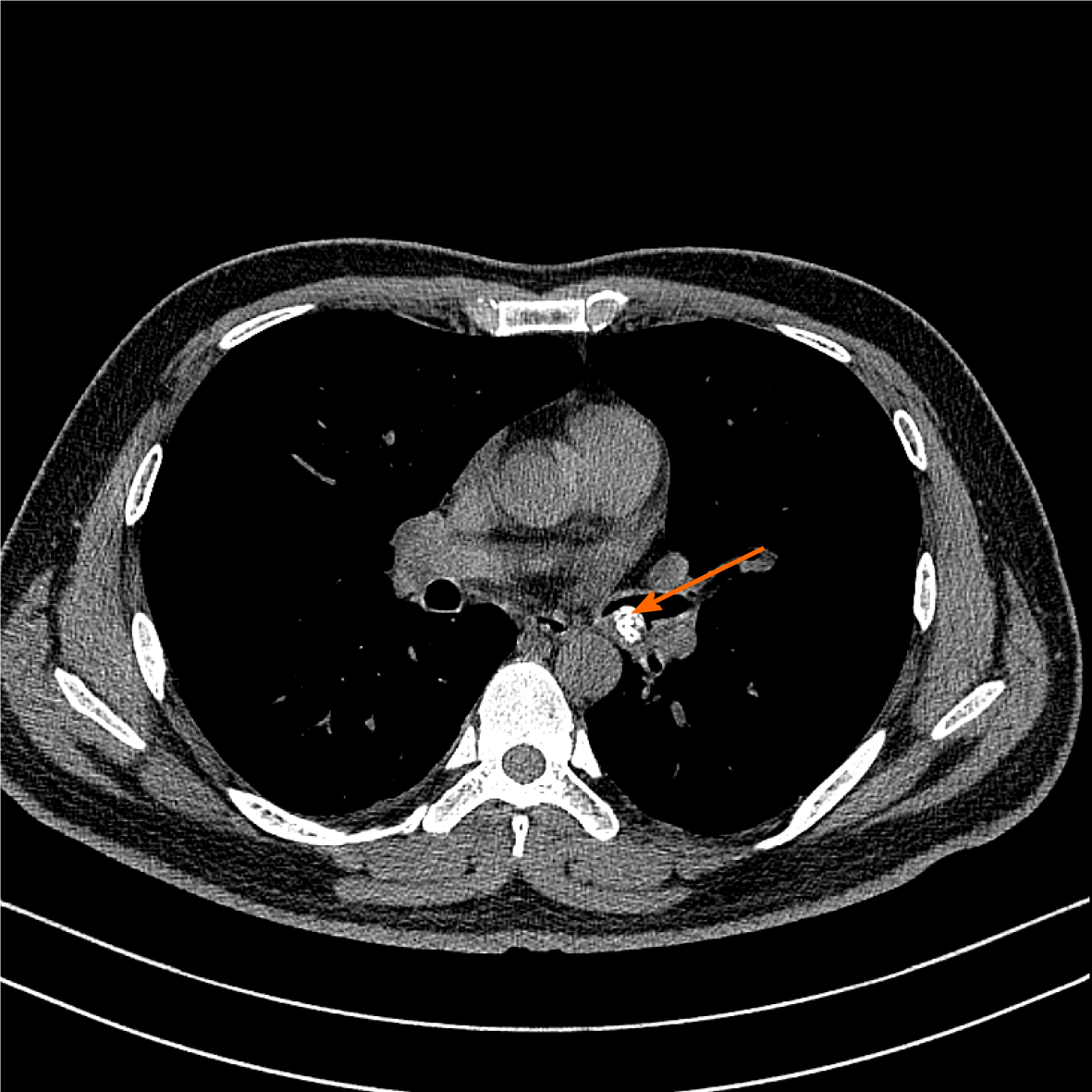
Chest CT revealed the presence of a 1.20 cm × 0.88 cm calcified nodular lesion on the compressed posterior wall of the lower left main bronchus (Figure 1). CT also showed bronchiectasis in the lower lobe of the left lung. Bronchial GT and carcinoid carcinoma were considered as possible diagnoses. However, it was difficult to distinguish one from another on the radiographic findings alone because they often have similar imaging features. GTs could be differentiated from carcinoids by tumor biopsy and immunohistochemistry.
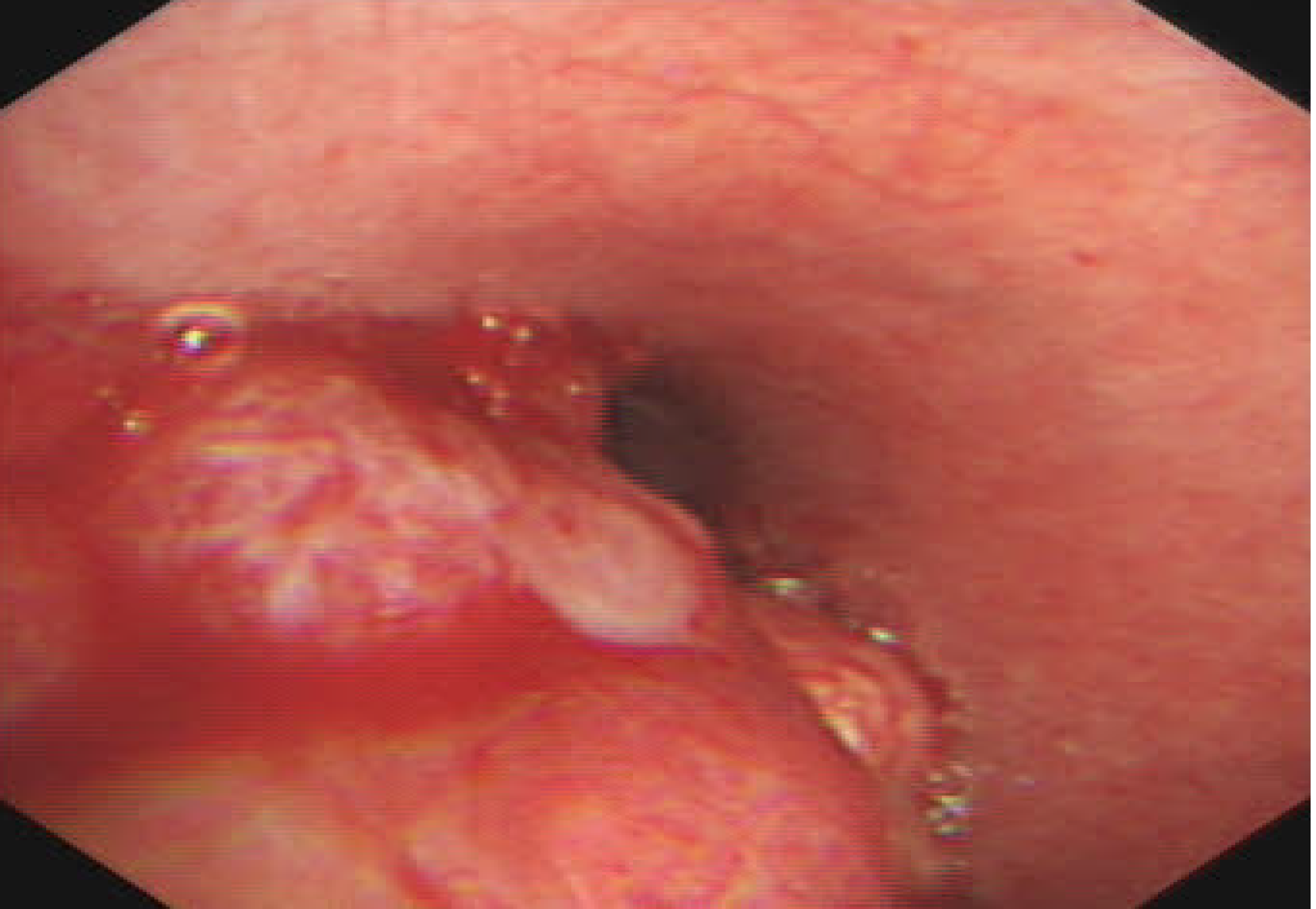
To confirm the characteristics of calcified nodules in the lower left main bronchus, fiberoptic bronchoscopy was performed, which showed a yellow–white, hard mass obstructing the entrance to the basal segment of the lower left lobe (Figure 2). Lateral to the entrance of the basal segment of the lower left lobe, neoplasms with multiple nodular ridges and superficial hyperemia were observed (Figure 2). Mucosal biopsy using a fiberoptic bronchoscopy was performed. The neoplasm was prone to bleeding.
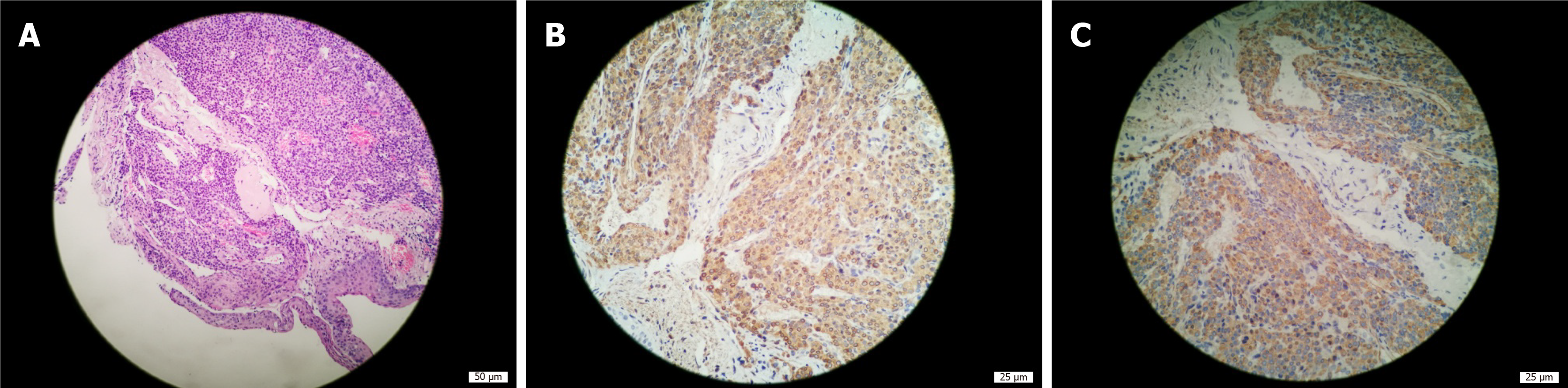
Microscopically, the tumor cells were uniformly round with smooth nuclear contours, fine chromatin and a modest amount of pink cytoplasm. They were arranged in sheet-like patterns between small blood vessels (Figure 3A). Smooth muscle actin (Figure 3B) and actin (Figure 3C) immunohistochemical staining were positive, and synaptophysin immunohistochemical staining was weakly positive. In contrast, CD56 (NK-1), chromogranin A, cytokeratins 5/6, cytokeratin 7, napsin-A, P40, thyroid transcription factor-1, cytokeratin, neuron specific enolase, S-100 and Ki-67 (<1%) immunohistochemical staining were negative. The histological characteristics and immunohistochemical staining patterns of the tumor were consistent with the diagnosis of GT. The clinical and pathological data of the patient are presented in Table 1.
| Project | Content |
| Biographical data | 33-yr-old man |
| Family history | No |
| Personal or family history | No |
| Chief complaint | Cough and sputum for 11 d and hemoptysis for 5 d |
| Physical examination of the lung | Normal except for vesicular breath sounds |
| Chest computed tomography | 1.20 cm × 0.88 cm calcified nodular lesion was found on the compressed posterior wall of the lower left main bronchus (Figure 1). Bronchiectasis in the lower lobe of the left lung was found. |
| Fiberoptic bronchoscopy | Yellow–white, slightly hard mass was found obstructing the entrance to the basal segment of the lower left lobe (Figure 2). Neoplasms with multiple nodular ridges and superficial hyperemia were observed in the lateral to the entrance of the basal segment of the lower left lobe (Figure 2). Mucosal biopsy using a fiberoptic bronchoscopy is prone to bleeding. |
| Pathology | Microscopically, the tumor cells were uniformly round with smooth nuclear contours, fine chromatin and a modest amount of pink cytoplasm. They were arranged in sheet-like patterns between small blood vessels (Figure 3A). Left main bronchial glomus tumor with immunohistochemistry results of SMA(+) (Figure 3B) and actin(+) (Figure 3C), CD56 (NK-1)(-), CgA(-), CK5/6(-), CK7(-), napsin-A(-), P40(-), TTF-1(-), CK(-), NSE(-), S-100(-) and Ki-67 (< 1%), and Syn immunohistochemical staining was weakly positive. |
| Final diagnosis | Bronchial glomus tumor and bronchiectasis |
| Treatment | Conservative treatment. Piperacillin–tazobactam 4.5 g twice daily and erdosteine 0.3 g twice daily for 9 d. Intravenous administration of Agkistrodon 2 U once. |
| Follow-up | Clinical follow-up for 25 mo showed that the patient had no symptoms. |
Therefore, this patient was diagnosed with bronchial GT and bronchiectasis.
Most bronchial GTs have a good prognosis because they are benign. Only a small proportion are malignant. However, if hemoptysis and asphyxia are caused by bronchial GTs, surgical resection or tracheoscopic resection is still recommended, even for benign GTs. Because our patient was diagnosed with benign bronchial GT without life-threatening symptoms, such as massive hemoptysis, he opted for conservative treatment. The hemoptysis was quickly relieved after intravenous administration of 2 U agkistrodon, which was then discontinued. At the same time, piperacillin– tazobactam 4.5g twice daily and erdosteine 0.3g twice daily were used for bronchiec
The patient exhibited no symptoms during long-term follow-up of 25 mo. As the patient was asymptomatic, he was not followed up with chest CT or fiberoptic bronchoscopy.
GTs are rare neoplasms that originate from the glomus apparatus, which is a specialized form of arteriovenous shunt involved in thermoregulation[5]. GTs show no gender predilection, but respiratory tract lesions are more prevalent in men, while subungual lesions are more prevalent in women[6]. The most common discomforts in respiratory symptomatic GTs are dyspnea (52.86%), cough (51.43%), hemoptysis (45.71%), chest pain (8.57%), fever and hoarseness (7.14%)[1].
CT and magnetic resonance imaging (MRI) findings of thoracic GTs are generally nonspecific. One study maintained that CT is the best modality to diagnose GTs arising from the chest wall, mediastinum, lungs or respiratory tract[7]. On chest CT, both malignant and benign GTs are likely to appear as well-delineated round masses[8]. Chest CT has revealed that the size of published pulmonary GTs ranges from 1.0 to 9.7 cm, with an average of 3.6 cm[8]. The tumors are described as either pulmonary nodules or masses, occasionally with the term coin lesion, as noted in some cases[8]. There is usually no calcification or fat decay[4]. On dynamic contrast-enhanced CT images, the tumors were enhanced significantly due to their rich vasculature. The chest X-rays might be normal[7].
MRI has proven to be a sensitive diagnostic method for GTs of the hand. GTs of the hand show a variety of MRI findings, and the appearances of GTs on MRI are decreased signal intensity, isointensity, increased signal intensity or inhomogeneous signal intensity in T1-weighted images and increased signal intensity in T2-weighted images[9]. After injection of gadolinium contrast agent, T1-weighted images are significantly enhanced[9]. These signals depend on the main cells of the tumor, including the following three types: Vascular, myxoid and solid[9]. However, to date, MRI manifestations of thoracic GTs have rarely been reported. In some cases, the mass center shows high intensity on both T1- and T2-weighted images[10]. With dynamic contrast-enhanced MRI, the mass might show strong, early-phase peripheral enhancement that expands in a centripetal direction with time[10]. The abundant vasculature of tumors is considered the most potent contributor to strong enhancement, which can provide additional information to isolate the nature of pulmonary nodules[10]. Because the MRI data of thoracic GTs are too limited, the MRI performance, sensitivity and specificity of thoracic GTs require more data analysis.
It is worth noting that none of the previous bronchial GTs reported internal calcification at imaging. Through a literature search, we found only six cases of GT with calcification, which were in the thigh[11], shoulder[12], musculotendinous junction of the rotator cuff[13], intracranial portion of a glomus jugular tumor[10], stomach[14] and thyroid gland[2]. To our knowledge, our case is the first reported GT with calcification in the respiratory tract.
The ideal management of tracheobronchial GT remains unclear[3]. In general, surgery may be the first choice for treating GTs that cause life-threatening airway obstruction and bleeding[15]. When the tumor arises from a lobar bronchus involving the origin or the main bronchus intraoperatively, a bronchoplastic procedure is needed[16]. When the patient is too weak to tolerate surgery or refuses to accept it, we prefer bronchoscopy. High-frequency electrocoagulation, laser resection and argon plasma coagulation are common endobronchial therapy techniques, but biopsy should be avoided due to the rich blood supply of GTs[1]. The advantages of conservative treatment for the patient in this case report were no surgical risk, avoidance of potential surgical complications and no financial pressure. The limitations of conservative treatment are that the GT may rupture, hemorrhage, transform malignantly, enlarge or even obstruct the airway. Therefore, it is necessary to closely monitor blood pressure and hemoptysis with follow-up chest CT and fiberoptic bronchoscopy.
According to the reported literature, bronchial GTs are described as either pulmonary nodules or masses, usually not accompanied by calcification on CT scans. We found only six cases of GT with calcification. To our knowledge, our case is the seventh case of GT with calcification and the first reported GT of the respiratory tract with calcification. We recommend that clinicians consider GT as a possible differential diagnosis when a calcified mass of the bronchi is found.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Spartalis M S-Editor: Zhang H L-Editor:Filipodia P-Editor: Li JH
| 1. | Wang C, Ma Y, Zhao X, Sun PL, Zhang YM, Huang M, Zhu Y, Jin SX. Glomus tumors of the trachea: 2 case reports and a review of the literature. J Thorac Dis. 2017;9:E815-E826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Chung DH, Kim NR, Kim T, Ahn J, Lee S, Lee YD, Cho HY. Malignant glomus tumor of the thyroid gland where is heretofore an unreported organ: a case report and literature review. Endocr Pathol. 2015;26:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Guo L, Wang K, Zhu H, Liu N, Zhu D. Treatment of primary tracheal glomus tumors: Two case reports and a literature review. Medicine (Baltimore). 2018;97:e0374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Ueno M, Nakashima O, Mishima M, Yamada M, Kikuno M, Nasu K, Kudo S. Pulmonary glomus tumor: CT and MRI findings. J Thorac Imaging. 2004;19:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Haro GJ, Seeley EJ, Jablons DM, Kratz JR. Central Airway Obstruction Due to Tracheal Glomus Tumor. Thorac Cardiovasc Surg Rep. 2018;7:e43-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Yun JS, Song SY, Na KJ, Kim S, Choi YD. Synchronous primary glomus tumor in a patient with adenocarcinoma of the ipsilateral lung. Thorac Cancer. 2019;10:1280-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Aryan Z, Zaki KS, Machuzak M, Mehta AC. Glomus tumor of the trachea. Clin Respir J. 2016;10:537-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Cunningham JD, Plodkowski AJ, Giri DD, Hwang S. Case report of malignant pulmonary parenchymal glomus tumor: imaging features and review of the literature. Clin Imaging. 2016;40:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Yijun S, Jianming H, Qi M, Hu X. [Application of MRI in the diagnosis of glomus tumor]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2015;31:259-262. [PubMed] |
| 10. | Moddy DM, Ghatak NR, Kelly DL Jr. Extensive calcification in a tumor of the glomus jugulare. Neuroradiology. 1976;12:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Dabadie A, Fernandez C, Gorincour G, Panuel M, Petit P. A rare case of a calcified glomus tumour in the thigh of an adolescent. Pediatr Radiol. 2013;43:1045-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Boretto JG, Lazerges C, Coulet B, Baldet P, Chammas M. Calcified glomus tumor of the shoulder. A case report. Chir Main. 2008;27:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Yoshikawa G, Murakami M, Ishizawa M, Matsumoto K, Hukuda S. Glomus tumor of the musculotendinous junction of the rotator cuff. A case report. Clin Orthop Relat Res. 1996;250-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Park SH, Han JK, Kim TK, Lee JW, Kim SH, Kim YI, Choi BI, Yeon KM, Han MC. Unusual gastric tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1435-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Obata T, Miyazaki T, Yamasaki N, Tsuchiya T, Matsumoto K, Hatachi G, Kitamura Y, Tabata K, Nagayasu T. Successful Resection of locally infiltrative Glomus Tumor without pulmonary resection. Int J Surg Case Rep. 2017;41:191-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Singh V, Kumar V, Singh H, Kakkar N. Primary pulmonary glomus tumour: a diagnostic challenge. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |