Published online May 16, 2021. doi: 10.12998/wjcc.v9.i14.3252
Peer-review started: January 7, 2021
First decision: January 18, 2021
Revised: February 6, 2021
Accepted: March 19, 2021
Article in press: March 19, 2021
Published online: May 16, 2021
Processing time: 111 Days and 17.3 Hours
Studies have suggested that atrial fibrillation (AF) in patients with rheumatic diseases (RD) may be due to inflammation.
To determine the highest association of AF among hospitalized RD patients and to determine morbidity and mortality associated with AF in hospitalized patients with RD.
The National inpatient sample database from October 2015 to December 2017 was analyzed to identify hospitalized patients with RD with and without AF. A subgroup analysis was performed comparing outcomes of AF among different RD.
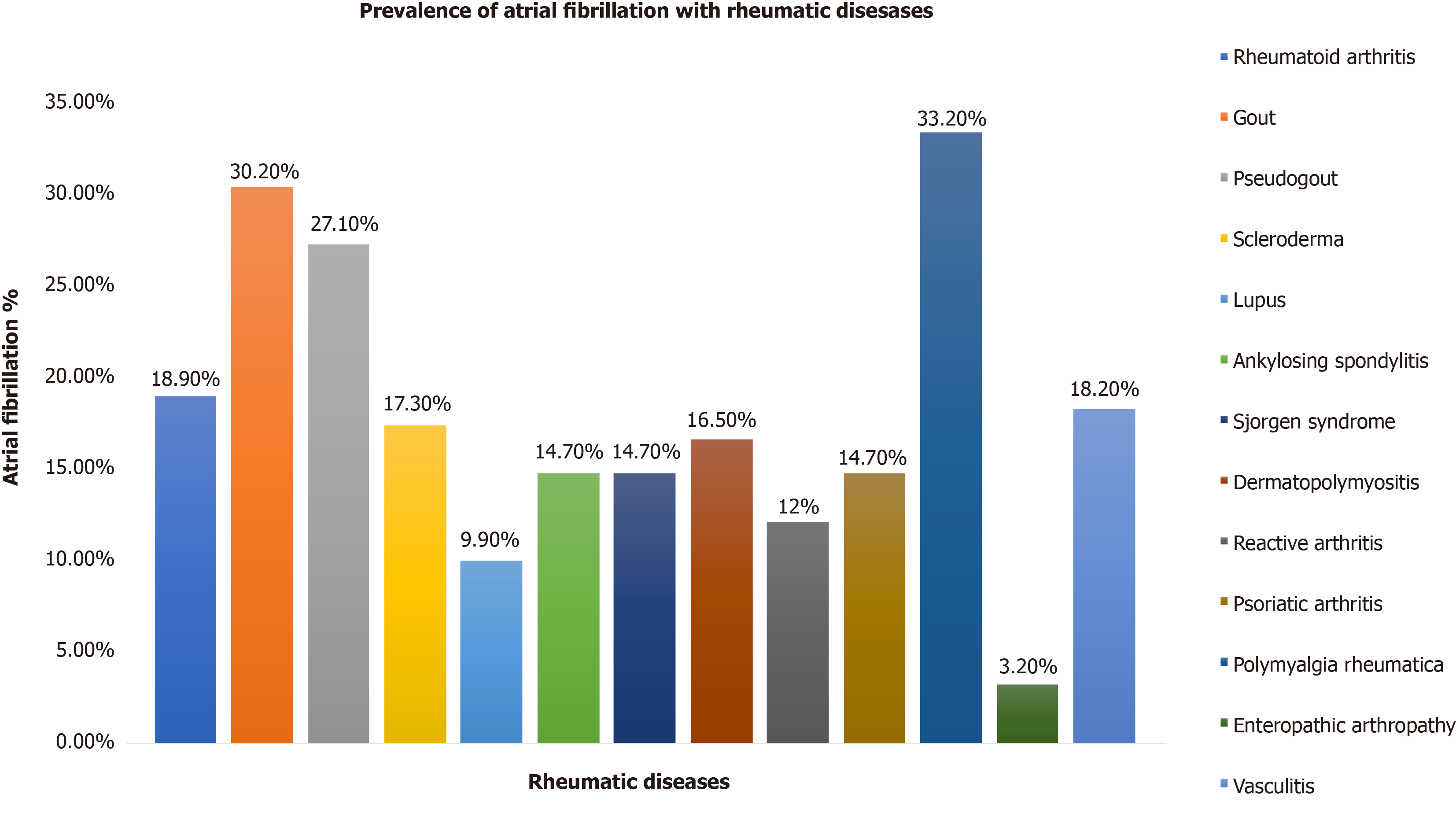
The prevalence of AF was 23.9% among all patients with RD (n = 3949203). Among the RD subgroup, the prevalence of AF was highest in polymyalgia rheumatica (33.2%), gout (30.2%), and pseudogout (27.1%). After adjusting for comorbidities, the odds of having AF were increased with gout (1.25), vasculitis (1.19), polymyalgia rheumatica (1.15), dermatopolymyositis (1.14), psoriatic arthropathy (1.12), lupus (1.09), rheumatoid arthritis (1.05) and pseudogout (1.04). In contrast, enteropathic arthropathy (0.44), scleroderma (0.96), ankylosing spondylitis (0.96), and Sjorgen’s syndrome (0.94) had a decreased association of AF. The mortality, length of stay, and hospitalization costs were higher in patients with RD having AF vs without AF. Among the RD subgroup, the highest mortality was found with scleroderma (4.8%), followed by vasculitis (4%) and dermatopolymyositis (3.5%).
A highest association of AF was found with gout followed by vasculitis, and polymyalgia rheumatica when compared to other RD. Mortality was two-fold higher in patients with RD with AF.
Core Tip: Current study showed an association of atrial fibrillation in rheumatic disease patients. We found that atrial fibrillation is significantly associated with gout followed by vasculitis and polymaylgia rheumatica. Overall mortality, length of stay and hospitalization cost was higher among rheumatic disease patients with atrial fibrill
- Citation: Khan MZ, Patel K, Patel KA, Doshi R, Shah V, Adalja D, Waqar Z, Franklin S, Gupta N, Gul MH, Jesani S, Kutalek S, Figueredo V. Burden of atrial fibrillation in patients with rheumatic diseases. World J Clin Cases 2021; 9(14): 3252-3264
- URL: https://www.wjgnet.com/2307-8960/full/v9/i14/3252.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i14.3252
Atrial fibrillation (AF) is the most common arrhythmia in the United States[1]. Several risk factors increase the risk of AF such as hypertension, diabetes, obesity, age, valvular heart disease, and cardiomyopathy[2]. Studies have shown that systemic inflammation can also induce AF[3,4]. Among the systemic inflammatory markers, tumor necrosis factor alpha, interleukin (IL)-2, IL-6, and C-reactive protein have been associated with the development of AF[3-5]. Many diseases are associated with systemic inflammation, including rheumatic diseases (RD). A more robust relationship between rheumatoid arthritis (RA) and AF was evaluated in the Danish cohort study[6]. They demonstrated an approximately 40% increased risk of AF in patients with RA compared to that of the general population (16%). In contrast, another study has shown a lack of relationship between RA and AF[7]. The association of gout with AF is also recognized[8]. Recently, Lim et al[9] found in their study that SLE had a significant association with AF. Some RD can be associated with higher levels of inflammatory markers and cause more systemic inflammation. Many diseases are associated with systemic inflammation, including RD. A more robust relationship between RA and AF was evaluated in the Danish cohort study. Additionally, we analyzed in-hospital mortality, length of stay, and associated cost in hospitalized RD patients with AF.
The present study was conducted using the National Inpatient Sample (NIS) database, the largest inpatient database in the United States. The NIS is a part of the Healthcare Cost and Utilization Project developed by the Agency for Healthcare Research and Quality[10]. The data was collected from 48 states. NIS represents more than 97% of the United States population, and the data has an average of 7-8 million discharges each year. NIS data are obtained from more than 7 million hospital stays each year, and it estimates more than 35 million hospitalizations nationally. Each admission contains information on patient characteristics, including demographics, comorbidity complications, as well as the primary and secondary discharge diagnoses. This has been explained in detail in previous studies[11-13]. The International Classification of Disease, 10th revision, Clinical Modification (ICD 10-CM) codes were used to identify diagnosis in the NIS database. Data included in this study were obtained between October 2015 and December 2017 as data prior to October 2015 included the use of ICD-9-CM codes. The study cohort was derived from a de-identified and publicly available database; hence, the study was considered exempt from formal approval of the Institutional review board.
The present study included the common RD such as systemic lupus erythematosus, ankylosing spondylitis, Sjogren's syndrome, dermatopolymyositis, polymyalgia rheumatica, vasculitis, RA, gout, pseudogout, scleroderma, reactive arthritis, psoriatic arthritis, and enteric arthropathy. All entries in the database carry information about patients’ demographics, age, gender, race, and comorbidities. For the calculation of total cost, we first merged the NIS data with cost-to-charge ratio files provided by the sponsor. These total charges were converted to cost estimates using the group average all-payer in hospital cost information from the reports provided by hospitals to the Centers for Medicare and Medicaid Services.
We extracted RD and AF hospitalizations using appropriate ICD-10 diagnosis codes in primary or secondary diagnosis (Supplementary Table 1). Furthermore, we documented the following comorbidities: hypertension, diabetes, congestive heart failure, pulmonary hypertension, chronic obstructive pulmonary disease, deficiency anemia, hypothyroidism, coronary artery disease, smoking, obesity, and chronic kidney disease (CKD) in our study cohort. This information was derived from the 29 comorbidities that are documented in the NIS using the Elixhauser method. The primary outcome of our study was to determine which RD had the highest association of AF. Secondary outcomes included in-hospital mortality, length of stay, and the total cost of hospitalization.
All the data extraction and analysis were done using statistical analysis system statistical software version 9.4. Analyses of survey responses were performed using SURVEY procedures. Each hospital admission is linked to a ‘discharge weight’ which was utilized to analyze projected national estimates for in-hospital outcomes after accounting for the hierarchical structure of the dataset as recommended by the sponsor. All continuous variables were compared using Student’s t-test, and categorical variables were analyzed using the Pearson Chi-square test. Categorical data were presented as a weighted frequency in percentages. Continuous data were presented as means and standard deviations for normally distributed variables, medians and interquartile ranges were used for non-Gaussian distributed variables. Continuous variables with non-Gaussian distribution were analyzed using Wilcoxon signed rank-test as appropriate. A P value < 0.05 was considered statistically significant. During a multivariable regression analysis, AF was the main outcome and enter method was used. In each model, the association of each rheumatic disease along with risk factors sex, age, heart failure, smoking, alcohol, hypertension, diabetes mellites, valvular heart disease, coronary heart disease, cardiomyopathy, Obstructive sleep apnea (OSA), and CKD were analyzed with AF. For the length of stay, mortality, and hospitalization cost we used the Student’s t-test as mentioned above.
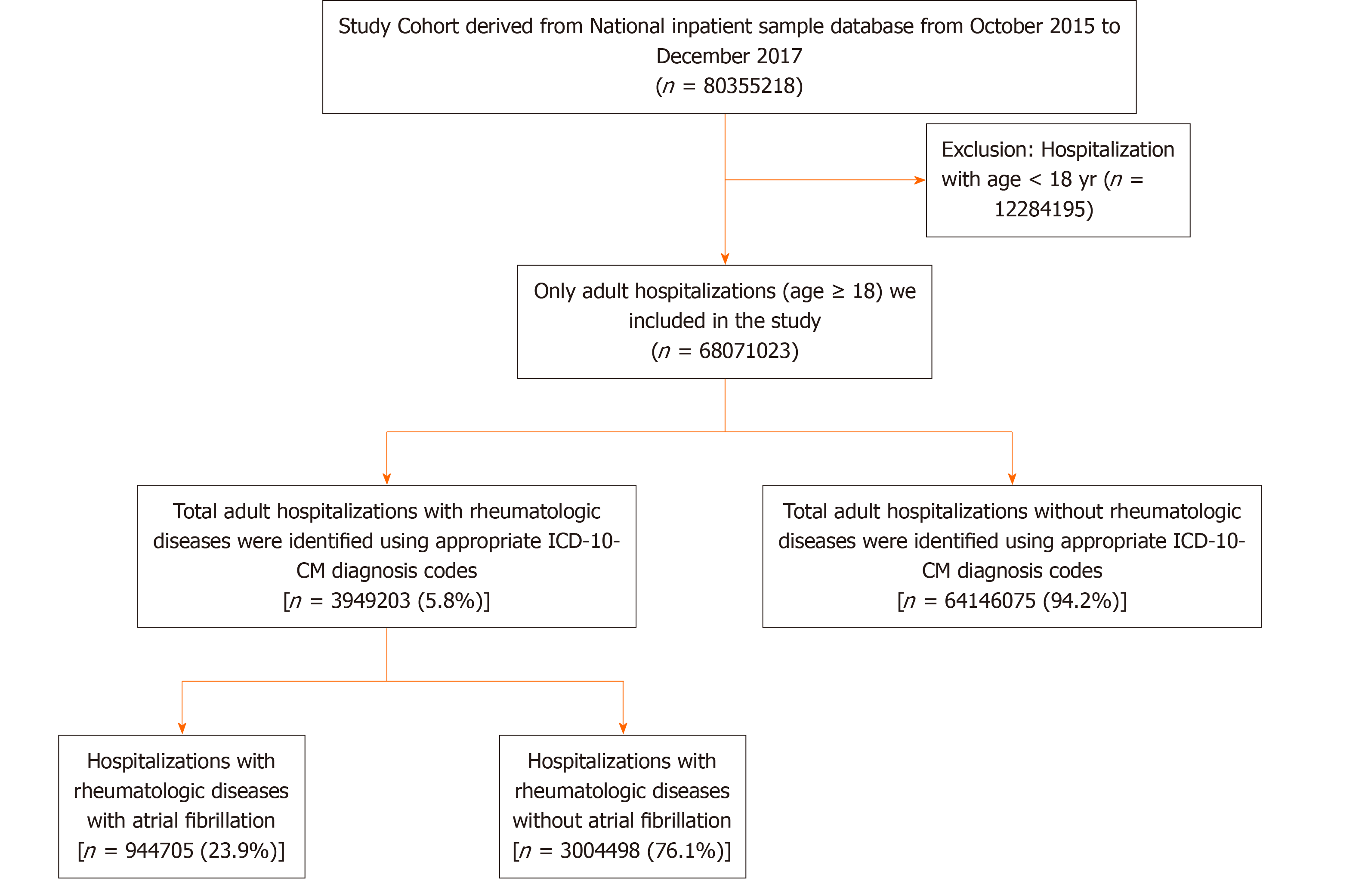
Data were obtained on 80355218 hospitalizations during the study period. Hospitalizations below 18 years of age were excluded from the analysis (n = 12284195). After exclusion, a total of 68071023 adult hospitalizations were analyzed. The total number of hospitalizations with AF was 9721440 (14.3%), and the total number of hospitalizations with the RD was 3947668 (5.8%). Among hospitalizations with a RD, 944705 (23.9%) had AF. The cohort selection flow chart is depicted in Figure 1. Baseline demographics are shown in Table 1. The mean age was 75 ± 11 years in RD and AF and 65 ± 15 years in RD without AF. When hospitalizations were stratified into RD with AF vs RD without AF, hospitalizations with RD and AF were more likely to be male (54.5%) than hospitalizations with RD without AF (43.2%). RD with AF group (76.4%) were more likely to be white than non-white compare to the RD without AF group (65%). Comorbidities such as congestive heart failure, coronary artery disease, valvular heart disease, renal failure, OSA, pulmonary hypertension, hypertension, diabetes mellitus, and hypothyroidism were significantly higher in the RD and AF cohort compared to the RD alone. Table 2 shows the baseline clinical characteristics and prevalence of AF according to the RD subtypes. Among the RD, the prevalence of AF was highest in polymyalgia rheumatica, gout, and pseudogout (Figure 2). During Univariate regression analysis, the odds of having AF were increased with gout, scleroderma, dermatopolymyositis, vasculitis, polymyalgia rheumatica, psoriatic arthropathy, ankylosing spondylitis, RA and pseudogout (Table 3). However, enteropathic arthropathy, reactive arthritis, and lupus had a decreased association of AF. After adjusting for sex, age, heart failure, smoking, alcohol, hypertension, diabetes mellites, valvular heart disease, coronary heart disease, OSA, and CKD disease by multivariate regression analysis gout, vasculitis, polymyalgia rheumatic, dermatopoly
| Characteristics | Total | RD with AF | RD without AF | P value |
| n = 3949203 | n = 944705 (23.9%) | n = 3004498 (76.1%) | ||
| Age | < 0.001 | |||
| Age, yr, mean ± SD | 67.6 ± 15 | 75.4 ± 10.8 | 65.1 ± 15.3 | |
| Gender | < 0.001 | |||
| Male | 1811949 (45.9) | 514495 (54.5) | 1297454 (43.2) | |
| Female | 2135719 (54.1) | 429900 (45.5) | 1705819 (56.8) | |
| Missing-1535 | ||||
| Race | < 0.001 | |||
| Caucasians | 2675194 (67.7) | 721595 (76.4) | 1953599 (65) | |
| African-Americans | 653520 (16.5) | 110355 (11.7) | 543165 (18.1) | |
| Others | 620455 (15.7) | 112750 (11.9) | 507705 (16.9) | |
| Comorbidities | ||||
| Congestive heart failure | 682755(17.3) | 290870 (30.8) | 391885 (13) | < 0.001 |
| Coronary arterial disease | 1236959 (31.3) | 444550 (47.1) | 792410 (26.4) | < 0.001 |
| Valvular disease | 188325 (4.8) | 73870 (7.8) | 114455 (3.8) | < 0.001 |
| Obstructive sleep apnea | 504430 (12.8) | 165060 (17.5) | 339370 (11.3) | < 0.001 |
| Chronic pulmonary disease | 954299 (24.2) | 259025 (27.4) | 695275 (23.1) | < 0.001 |
| Hypertension | 2536294 (64.2) | 609890 (64.6) | 1926404 (64.1) | < 0.001 |
| Diabetes w/o chronic complications | 545765 (13.8) | 129010 (13.7) | 416755 (13.9) | < 0.001 |
| Diabetes w/ chronic complications | 676450 (17.1) | 193330 (20.5) | 483120(16.1) | < 0.001 |
| Pulmonary Hypertension | 286580 (7.3) | 135975 (14.4) | 150605 (5) | < 0.001 |
| Hypothyroidism | 628090 (15.9) | 161685 (17.1) | 466405 (15.5) | < 0.001 |
| Renal failure | 1054424 (26.7) | 344655 (36.5) | 709770 (23.6) | < 0.001 |
| Obesity | 716165 (18.1) | 166220 (17.6) | 549945 (18.3) | < 0.001 |
| Alcohol abuse | 123780 (3.1) | 20585 (2.2) | 103195 (3.4) | < 0.001 |
| Drug abuse | 99110 (2.5) | 10800 (1.1) | 88310 (2.9) | < 0.001 |
| Outcomes | ||||
| In-hospital mortality, Missing-3435 | 94495 (2.4) | 37965 (4) | 56530 (1.9) | < 0.001 |
| Adjusted mortality ratio1: 1.46 (1.43-1.48) | ||||
| Length of stay, d, mean ± SD | 5.3 ± 5.9 | 6 ± 6.1 | 5.1 ± 5.9 | < 0.001 |
| Hospitalization cost, $, mean ± SD | 14419 ± 18731 | 15816 ± 20764 | 13981 ± 18023 | < 0.001 |
| Disposition | < 0.001 | |||
| Discharge to home | 2051184 (51.9) | 380575 (40.3) | 1670609 (55.6) | |
| Transfer (to skilled nursing facility, intermediate-care | 881490 (22.3) | 281705 (29.8) | 599785 (19.9) | |
| Home health care | 795225 (20.1) | 214655 (22.7) | 580570 (19.3) | |
| Against medical advice | 33465 (0.8) | 4295 (0.5) | 29170 (0.9) | |
| Missing-3435 | ||||
| Characteristics | Lupus (n = 397935) | Ankylosing Spondylitis (n = 39250) | Sjogren syndrome (n = 114885) | Dermato-polymyositis (n = 30400) | Scleroderma (n = 69865) | Rheumatoid arthritis (n = 1249979) | Gout (n = 1953389) | Reactive arthritis (n = 5690) | Psoriaticarthritis (n = 87110) | Pseudogout (n = 43375) | Polymyalgia rheumatica (n = 172655) | Enteropathic arthritis (n = 935) | Vasculitis (n = 47125) |
| Age, yr, mean ± SD | 52.1 ± 16.9 | 60.2 ± 16.2 | 64 ± 15.5 | 60.6 ± 16.5 | 62.6 ± 14.4 | 67.2 ± 14.2 | 70.5 ± 12.9 | 54.8 ± 17.9 | 60.8 ± 13.9 | 75.4 ± 12.1 | 79.1 ± 9.4 | 45.6 ± 17.4 | 60.9 ± 16.3 |
| Gender | |||||||||||||
| Male | 11.5% | 65.1% | 8.4% | 31.5% | 15.5% | 26% | 67.5% | 63.3% | 42.8% | 48.5% | 31.7% | 37.9% | 47.5% |
| Female | 88.5% | 34.9% | 91.5% | 68.5% | 84.5% | 74% | 32.5% | 36.7% | 57.2% | 51.4% | 68.3% | 62% | 52.5% |
| Elixhauser Comorbidities | |||||||||||||
| Afib. | 9.9% | 14.7% | 14.7% | 16.5% | 17.3% | 18.9% | 30.2% | 12% | 14.7% | 27.1% | 33.2% | 3.2% | 18.2% |
| Coronary Arterial Disease | 17.9% | 21.5% | 18.9% | 21.7% | 22.5% | 25.8% | 38.7% | 16.1% | 21.6% | 28.6% | 35.3% | 7.5% | 22.7% |
| Congestive heart failure | 12.8% | 10.1% | 11% | 14.2% | 17.7% | 14.1% | 21.1% | 8.6% | 8.8% | 15.5% | 18.5% | 5.3% | 15.7% |
| Obstructive sleep apnea | 7.4% | 11.1% | 10.2% | 9.4% | 7.8% | 9.7% | 16.4% | 8.6% | 14.7% | 7.8% | 11.2% | 6.4% | 9.4% |
| Valvular disease | 4% | 3.5% | 4.7% | 3.5% | 5.5% | 4.4% | 5% | 2.7% | 3.2% | 7.1% | 7.5% | 0.5% | 4.3% |
| Chronic pulmonary disease | 22.3% | 19.3% | 24.2% | 18.7% | 23.1% | 27.8% | 22.8% | 17.9% | 21.9% | 18.5% | 23.2% | 17.6% | 28.2% |
| Hypertension | 53.4% | 52.7% | 51.6% | 53.9% | 50.8% | 61.1% | 69.4% | 44.8% | 58.2% | 68.9% | 67.6% | 26.7% | 58.4% |
| Diabetes w/o chronic complications | 8.9% | 11.7% | 8.5% | 14,5% | 6.8% | 12.9% | 15.9% | 7.9% | 13.5% | 12.6% | 12.6% | 9.1% | 8.6% |
| Diabetes w/ chronic complications | 9.7% | 10.6% | 8% | 13.4% | 7% | 12.1% | 23% | 10.7% | 14.4% | 14.2% | 14.6% | 4.8% | 14.2% |
| Pulmonary Hypertension | 6.6% | 3.5% | 7.5% | 8.7% | 29.5% | 5.5% | 8.3% | 2.1% | 3.8% | 4.9% | 6.9% | 1.1% | 5.8% |
| Hypothyroidism | 15.3% | 10.1% | 23.9% | 14.7% | 20.5% | 17.7% | 14.2% | 8.2% | 15.6% | 16.5% | 22.5% | 6.4% | 12.6% |
| Renal failure | 23.5% | 11.5% | 13.3% | 10.9% | 17.4% | 14.7% | 37.1% | 11.1% | 11.6% | 20.6% | 23.6% | 4.8% | 39.1% |
| Obesity | 15.5% | 15.3% | 13.5% | 14.9% | 7.5% | 15.3% | 21.8% | 11.7% | 23.4% | 11.1% | 11.6% | 14.4% | 12.7% |
| Alcohol abuse | 1.7% | 3.5% | 1% | 1.4% | 1.4% | 2.1% | 4.2% | 4.3% | 4.4% | 2.9% | 1.1% | 0.5% | 2.4% |
| Drug abuse | 4.9% | 4.9% | 2.5% | 2.5% | 2.6% | 3.2% | 1.7% | 7.5% | 3.6% | 1.8% | 1.1% | 5.3% | 3.5% |
| Depression | 15.6% | 14.6% | 17.2% | 12.5% | 13.2% | 15.6% | 10.4% | 10.5% | 17.6% | 10.7% | 13.9% | 14.9% | 11.1% |
| Outcomes | |||||||||||||
| In-hospital mortality | 1.9% | 2.4% | 2% | 3.5% | 4.8% | 2.3% | 2.4% | 1.3% | 1.4% | 1.1% | 2.6% | 2.1% | 4% |
| Length of stay, d, mean ± SD | 5.5 ± 6.9 | 5.5 ± 7.4 | 5.1 ± 6.3 | 6.9 ± 8.7 | 6.2 ± 7.6 | 5 ± 5.4 | 5.4 ± 5.9 | 5.8 ± 6.3 | 4.9 ± 5.6 | 6 ± 6.7 | 4.9 ± 4.6 | 5.8 ± 8.6 | 7.1 ± 8.8 |
| Total hospitalization cost, $, mean ± SD | 14389 ± 21148 | 17661 ± 22624 | 14213 ± 19367 | 19190 ± 39030 | 17052 ± 29497 | 13744 ± 16245 | 14566 ± 18344 | 14343 ± 17693 | 14726 ± 18097 | 14742 ± 18924 | 13368 ± 14943 | 16008 ± 25989 | 19874 ± 29132 |
| Variables | Odds ratio | 95%CI | P value |
| Lupus | 0.66 | 0.65-0.67 | < 0.0001 |
| Ankylosing spondylitis | 1.03 | 1.01-1.06 | 0.03 |
| Sjogren’s syndrome | 1.03 | 1.02-1.05 | 0.0002 |
| Dermatopolymyositis | 1.19 | 1.15-1.22 | < 0.0001 |
| Scleroderma | 1.26 | 1.24-1.29 | < 0.0001 |
| Polymyalgia rheumatica | 3.00 | 2.97-3.03 | < 0.0001 |
| Vasculitis | 1.34 | 1.31-1.37 | < 0.0001 |
| Rheumatoid arthritis | 1.41 | 1.40-1.42 | < 0.0001 |
| Gout | 2.70 | 2.69-2.71 | < 0.0001 |
| Pseudogout | 2.24 | 2.19-2.29 | < 0.0001 |
| Reactive arthritis | 0.83 | 0.77-0.90 | < 0.0001 |
| Psoriatic arthropathy | 1.04 | 1.02-1.05 | 0.0002 |
| Enteropathic arthropathy | 0.20 | 0.14-0.29 | < 0.0001 |
| Variables | Odds ratio | 95%CI | P value |
| Lupus | 1.05 | 1.04-1.06 | < 0.0001 |
| Ankylosing spondylitis | 0.96 | 0.93-0.99 | 0.02 |
| Sjogren’s syndrome | 0.94 | 0.92- 0.95 | < 0.0001 |
| Dermatopolymyositis | 1.14 | 1.10-1.18 | < 0.0001 |
| Scleroderma | 0.96 | 0.93-0.98 | < 0.0001 |
| Polymyalgia rheumatica | 1.15 | 1.14-1.16 | < 0.0001 |
| Vasculitis | 1.19 | 1.16-1.22 | < 0.0001 |
| Rheumatoid arthritis | 1.05 | 1.04-1.05 | < 0.0001 |
| Gout | 1.25 | 1.25-1.26 | < 0.0001 |
| Pseudogout | 1.05 | 1.03-1.08 | < 0.0001 |
| Reactive arthritis | 1.02 | 0.93-1.11 | 0.67 |
| Psoriatic arthropathy | 1.12 | 1.10-1.14 | < 0.0001 |
| Enteropathic arthropathy | 0.44 | 0.30-0.65 | < 0.0001 |
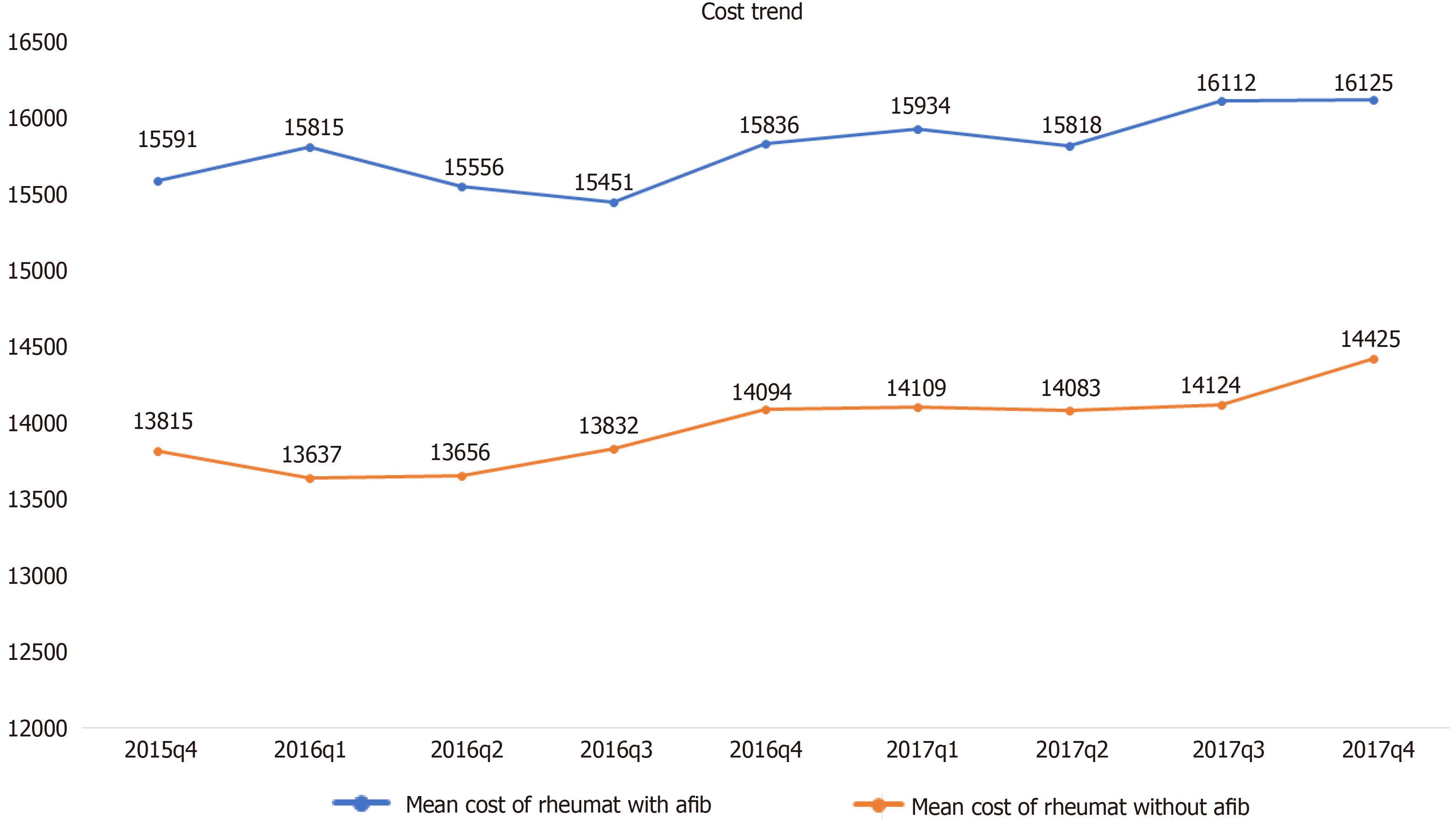
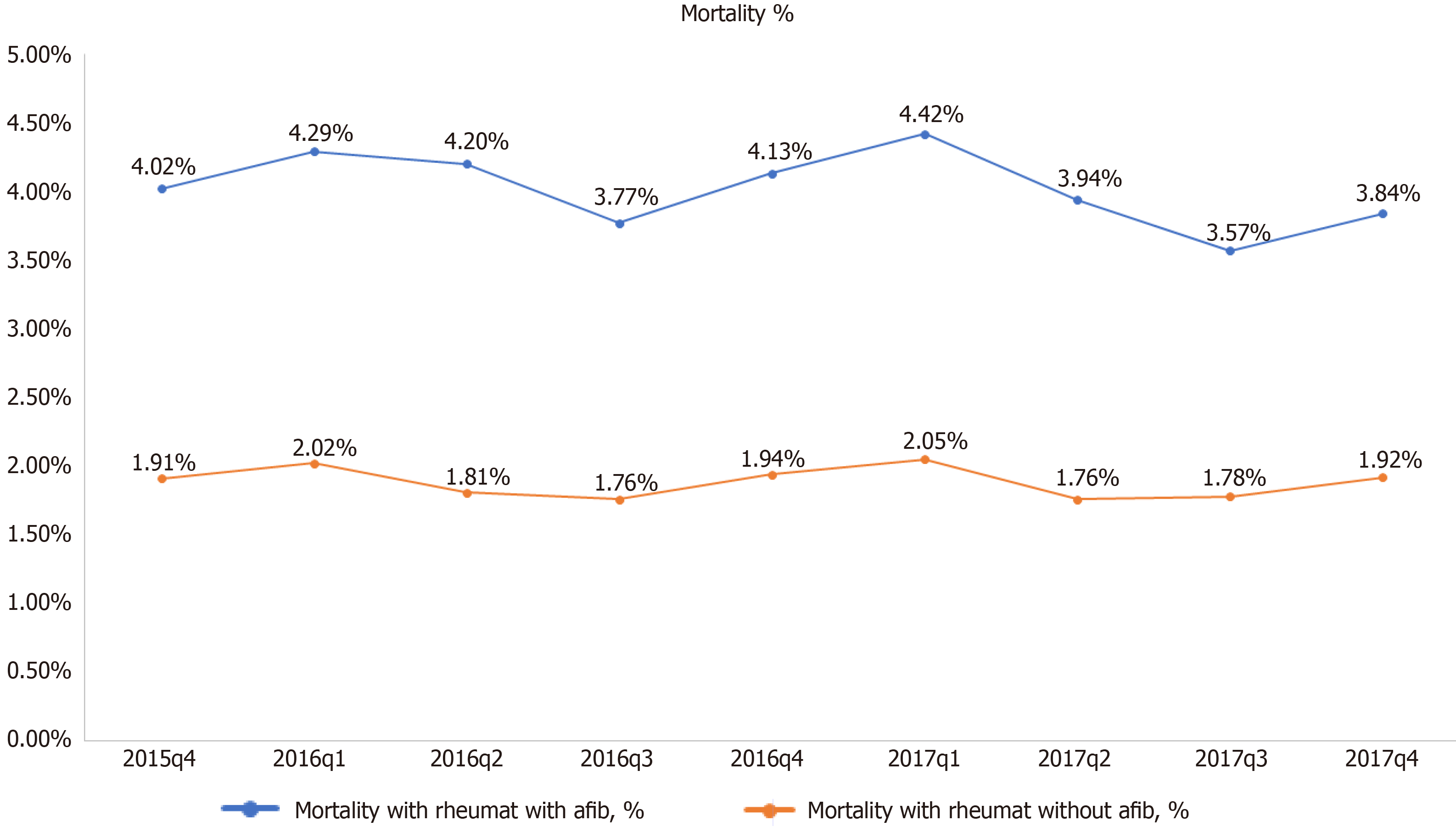
Mortality was twofold higher among RD hospitalizations with AF compared to RD hospitalizations without AF (4% vs 1.9%). Other secondary outcomes, including the length of the stay and hospitalization cost, were also higher in hospitalizations with RD with AF. The mortality, length of stay, and cost were found to be highest in scleroderma, followed by vasculitis and dermatopolymyositis (Table 2). Mean cost of hospital stay was highest in the last quartile of 2016 for RD with AF patients (Figure 3). RD with AF had highest percentages of inpatient mortality in the first quartile of 2016 and 2017 (Figure 4).
The present study utilized the NIS data to identify the prevalence and association of AF with different RD. The prevalence of AF was significantly higher in patients hospitalized with RD compared to without. The prevalence of AF was found to be higher in males compared to females. After controlling for risk factors known to be associated with AF (hypertension, coronary artery disease, obstructive sleep apnea, pulmonary hypertension, congestive heart failure, valvular heart disease, age > 65), this study showed the strongest association was with gout, vasculitis and polymyalgia rheumatica. These were followed by dermatopolymyositis, psoriatic arthropathy, lupus, RA and pseudogout. Conversely, enteropathic arthropathy, scleroderma, ankylosing spondylitis, and Sjorgen’s syndrome had decreased association of AF.
In this study, the prevalence of AF in RD hospitalizations was 23.9%, while the prevalence of AF in the overall included study population was 14.3%. One hypothesis for developing AF in RD was thought to be atrial fibrosis, which can develop in patients with chronic inflammatory diseases such as RD. Inflammatory markers are known to play a role in the development of AF, such as tumor necrosis factor-alpha, C-reactive protein, platelet-derived growth factor, and IL-6[14-17]. These inflammatory markers can cause inflammation in the heart, which can lead to structural and electrical remodeling of the heart, especially in the atrial myocardium[18,19]. Studies wherein cardiac biopsies were conducted, found inflammatory infiltrates in the myocardium of patients with AF[14,20]. However, these studies were not conducted explicitly on RD patients. Further studies are needed to determine which inflamma
The prevalence of AF varied among each RD studied, which might be due to differences in age, the severity of the disease, and other risk factors known to be associated with AF. Lindhardsen et al[6] reported the incidence of AF was 40% higher in RA compared to the general population. In contrast, Kim et al[7] reported that there is no association between RA and AF that existed when compared to the general population after adjusting for cardiovascular disease, diabetes, healthcare utilization, and medications. Singh et al[8] found that gout increased the risk of AF by 71%-90%. The study also showed that the incidence of AF was higher in gout patients (43.4 vs 16.3 per 1000 patient-years) compared to those without gout. Studies by Kuo et al[21] and Kim et al[7] found that the incidences of AF in gout patients were 9% and 13%, respectively, which was much lower than that reported by Singh et al[8]. A study by Myung et al[22] found the prevalence of AF to be 9% in 235 Lupus patients. Similarly, Lim et al[9] reported incidence of AF of 2.27% in lupus patients vs 0.59% of patients in the control group.
Of the RD, the highest prevalence of AF was found in patients with polymyalgia rheumatica (33.2%), followed by 30.2% with gout, and 27.1% with pseudogout. While the exact reason is not known for having the highest rates of AF with polymyalgia rheumatica, we found that the average age for this population was highest among RD. Additionally, the highest number of people with age above 80 were found in this population (> 55%). Higher age is associated with higher incidence and prevalence of AF in previously published articles[23]. The burden of coronary artery disease, chronic pulmonary disease, and OSA was significantly elevated in patients with gout and polymyalgia rheumatica, which may attribute to higher AF. The prevalence of AF in enteropathic arthropathy were comparatively lower than other RD in our study. Adjusted odds ratios of enteropathic arthropathy showed decrease association of AF. The exact pathophysiology of decrease prevalence and association of AF in enteropathic arthropathy patients are unknown and further studies are needed.
Studies suggest that uric acid plays a role in the atrial remodeling processes, which increase the risk of AF[21]. The high association of AF in gout could be due to hyperuricemia and systemic inflammation. Studies have shown that a 1 standard deviations increase in serum uric acid level increases the risk of AF 1.56-fold in black Americans[21]. Obesity can increase the risk of AF significantly[23]. Obesity was observed highest among patients with gout. The highest association of AF in gout patients could also be due to the involvement of the coronary arteries or MI[24]. Second highest association of AF was found in vasculitis patients, which can be due to inflammatory changes in the myocardium, which can further alter the conduction pathway triggering arrhythmias[25].
Studies have shown that AF patient have high mortality and morbidity as compare to the no AF group[24]. RD can also lead to atherosclerosis, thrombus formation, vasculitis, myocardial inflammation, and/or fibrosis which increases morbidity and mortality[26]. Mortality among patients with RD with AF is higher than those without AF.
This observational study has some limitations. We were not able to determine whether patients developed AF before or after the development of the RD, given the nature of the database. However, our study sample size is large and is representative of all United States hospitals. After adjusting for other comorbidities, we still demonstrated that RD could be associated with AF. We relied primarily on diagnosis codes for RA and AF, which could potentially lead to exposure and outcome misclassification; however, both the ICD codes for RA and AF have been validated and used in several studies[6]. We did not investigate disease severity, which could affect the development of AF. We included only common RD prevalent in the community for this study. Our population is inpatient, we did not take outpatient. The data sample is large and there is a chance that the patient who is hospitalized one time, can be admitted again with the same diagnosis and their information may be repeated in data. Finally, we did not have a medication list given the nature of the database.
The present study showed that the prevalence of AF is significantly higher in hospitalizations with RD compared to those without. The highest association of AF was found with gout followed by vasculitis and polymyalgia rheumatica. Further, mortality was significantly higher in hospitalizations with RD and AF. Length of stay and hospitalization cost was also significantly higher among RD hospitalizations with AF.
Atrial fibrillation (AF) can be induced by systemic inflammation in certain diseases such as rheumatic diseases (RD). A relationship between rheumatoid arthritis and AF was evaluated in the Danish cohort study, while other studies have shown a lack of relationship between RA and AF.
Arrhythmia should be excluded in RD patients with cardiac risk factors. If the patient develops any concerning cardiac symptoms, RD patients should be monitored for arrhythmia using cardiac monitors.
The purpose of this study was to determine the association of AF among hospitalized RD patients and to determine morbidity and mortality associated with AF in hospitalized patients with RD.
National Inpatient Sample database from October 2015 to December 2017 was used for the current study. International classification of disease, 10th revision, clinical modification codes were used to identify diagnosis RD and AF patients. The analysis was conducted using statistical analysis system statistical software version 9.4. Both univariate and multivariable regression analysis was used to identify the association between AF and RD.
Higher AF prevalence was found in RD patients. After adjusting the risk factors using multivariate regression analysis, the strongest association of AF was found with gout, vasculitis and polymyalgia. Conversely, enteropathic arthropathy, scleroderma, ankylosing spondylitis, and Sjorgen’s syndrome had decreased association with AF.
The study showed a higher association of AF with gout and vasculitis. Overall mortality, length of stay and hospitalization cost was higher among RD patients with AF.
Further randomized controlled trial should be directed to evaluate long-term outcomes of arrhythmias in RD patients.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cismaru G S-Editor: Zhang L L-Editor: A P-Editor: Xing YX
| 1. | Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14:195-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 112] [Reference Citation Analysis (0)] |
| 2. | Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, McManus DD, Ko D, Weng LC, Lunetta KL, Frost L, Benjamin EJ, Trinquart L. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ. 2018;361:k1453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 250] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 3. | Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26:2083-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 302] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Liu T, Li G, Li L, Korantzopoulos P. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol. 2007;49:1642-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 641] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 6. | Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Svendsen JH, Torp-Pedersen C, Hansen PR. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. BMJ. 2012;344:e1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Kim SC, Liu J, Solomon DH. The risk of atrial fibrillation in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73:1091-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Singh JA, Cleveland JD. Gout and the risk of incident atrial fibrillation in older adults: a study of US Medicare data. RMD Open. 2018;4:e000712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Lim SY, Bae EH, Han KD, Jung JH, Choi HS, Kim CS, Ma SK, Kim SW. Systemic lupus erythematosus is a risk factor for atrial fibrillation: a nationwide, population-based study. Clin Exp Rheumatol. 2019;37:1019-1025. [PubMed] |
| 10. | Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. NIS database documentation archive. Rockville, MD, June 2016 [cited 14 March 2021]. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp. |
| 11. | Kalra R, Patel N, Doshi R, Arora G, Arora P. Evaluation of the Incidence of New-Onset Atrial Fibrillation After Aortic Valve Replacement. JAMA Intern Med. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Anantha-Narayanan M, Doshi RP, Patel K, Sheikh AB, Llanos-Chea F, Abbott JD, Shishehbor MH, Guzman RJ, Hiatt WR, Duval S, Mena-Hurtado C, Smolderen KG. Contemporary Trends in Hospital Admissions and Outcomes in Patients With Critical Limb Ischemia: An Analysis From the National Inpatient Sample Database. Circ Cardiovasc Qual Outcomes. 2021;14:e007539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Dave M, Kumar A, Majmundar M, Adalja D, Shariff M, Shah P, Desai R, Patel K, Jagirdhar GSK, Vallabhajosyula S, Gullapalli N, Doshi R. Frequency, Trend, Predictors, and Impact of Gastrointestinal Bleeding in Atrial Fibrillation Hospitalizations. Am J Cardiol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 742] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 15. | Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 411] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 16. | Schirone L, Forte M, Palmerio S, Yee D, Nocella C, Angelini F, Pagano F, Schiavon S, Bordin A, Carrizzo A, Vecchione C, Valenti V, Chimenti I, De Falco E, Sciarretta S, Frati G. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid Med Cell Longev. 2017;2017:3920195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 17. | Ungprasert P, Srivali N, Kittanamongkolchai W. Risk of incident atrial fibrillation in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int J Rheum Dis. 2017;20:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Lee SH, Chen YC, Chen YJ, Chang SL, Tai CT, Wongcharoen W, Yeh HI, Lin CI, Chen SA. Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007;80:1806-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Musa H, Kaur K, O'Connell R, Klos M, Guerrero-Serna G, Avula UM, Herron TJ, Kalifa J, Anumonwo JM, Jalife J. Inhibition of platelet-derived growth factor-AB signaling prevents electromechanical remodeling of adult atrial myocytes that contact myofibroblasts. Heart Rhythm. 2013;10:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 995] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 21. | Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Impact of gout on the risk of atrial fibrillation. Rheumatology (Oxford). 2016;55:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Myung G, Forbess LJ, Ishimori ML, Chugh S, Wallace D, Weisman MH. Prevalence of resting-ECG abnormalities in systemic lupus erythematosus: a single-center experience. Clin Rheumatol. 2017;36:1311-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56-e528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4294] [Cited by in RCA: 5861] [Article Influence: 976.8] [Reference Citation Analysis (5)] |
| 24. | Lee E, Choi EK, Jung JH, Han KD, Lee SR, Cha MJ, Lim WH, Oh S. Increased risk of atrial fibrillation in patients with Behçet's disease: A nationwide population-based study. Int J Cardiol. 2019;292:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Hancock AT, Mallen CD, Muller S, Belcher J, Roddy E, Helliwell T, Hider SL. Risk of vascular events in patients with polymyalgia rheumatica. CMAJ. 2014;186:E495-E501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Seferović PM, Ristić AD, Maksimović R, Simeunović DS, Ristić GG, Radovanović G, Seferović D, Maisch B, Matucci-Cerinic M. Cardiac arrhythmias and conduction disturbances in autoimmune rheumatic diseases. Rheumatology (Oxford). 2006;45 Suppl 4:iv39-iv42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |