Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3194
Peer-review started: December 21, 2020
First decision: December 30, 2020
Revised: January 22, 2021
Accepted: March 10, 2021
Article in press: March 10, 2021
Published online: May 6, 2021
Processing time: 122 Days and 10.9 Hours
It is difficult to restore the cognitive functions of patients with impaired cognition caused by brain injury. Diffusion tensor imaging can visualize the integrity of neural tracts in the white matter (WM) three-dimensionally. It is unclear whether encephalitis following scrub typhus damages the WM. For the first time, we aimed to report diffusion tensor tractography (DTT) findings in a chronic patient with cognitive impairment following scrub typhus encephalitis, which revealed injury to the Papez circuit of the WM.
A 70-year-old male patient was affected by encephalitis caused by scrub typhus that occurred 23 years ago. He had poor cognition and his clinical examination findings were as follows: Mini-Mental Status Examination score, 14; and handgrip strength (right/left, kg), 32.3/31.3. DTT revealed serious injuries of the left thalamocingulate tract and right mammillothalamic tract in the Papez circuit, and a partial injury of the anterior part of the fornix.
Using DTT, we found a relationship between cognitive impairment and the integrity of the Papez circuit following scrub typhus.
Core Tip: Scrub typhus is one of the most common causes of acute encephalitis in endemic countries. It is still debated whether white matter (WM) is injured in the encephalitis of the scrub typhus. Diffusion tensor tractography allows us to investigate the integrity of WM three-dimensionally. The Papez circuit consists mainly of the WM and is known to play a critical role in cognition. Using a diffusion tensor tractography, we detected the injury of the Papez circuit in a patient with cognitive impairment following encephalitis of scrub typhus 23 years ago.
- Citation: Kwon HG, Yang JH, Kwon JH, Yang D. Association between scrub typhus encephalitis and diffusion tensor tractography detection of Papez circuit injury: A case report. World J Clin Cases 2021; 9(13): 3194-3199
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3194.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3194
Scrub typhus is one of the most common etiologies of acute encephalitis syndrome in endemic countries[1,2]. Encephalitis caused by scrub typhus may lead to brain parenchymal damage, which has various neurological manifestations[3]. As a result of these neurological manifestations, cognitive functions can be seriously impaired. It is therefore important to reverse the cognitive impairment such that cognition returns to previous functional levels. The Papez circuit of the white matter (WM) plays a major role in cognition[4].
Using magnetic resonance imaging (MRI), few studies have previously demonstrated cognitive impairments following injuries to the Papez circuit in acute patients with brain injuries[5,6]. However, conventional MRI cannot reconstruct neural tracts of the deep brain structures, such as the Papez circuit, three- dimensionally. Recently, studies have reported that diffusion tensor tractography (DTT), derived from diffusion tensor imaging (DTI), can visualize neural tracts, including the Papez circuit, three-dimensionally[7-9]. Nonetheless, no study has investigated the injury of the Papez circuit in a patient following encephalitis of scrub typhus. In this study, using DTT, for the first time, we investigated the integrity of the Papez circuit related to cognitive dysfunctions in a chronic patient following encephalitis caused by scrub typhus.
A 70-year-old male patient visited our clinic because of poor cognitive function and gait disturbance.
He was diagnosed with encephalitis following scrub typhus 23 years ago. The patient complained of poor memory. The patient was initially admitted to a local hospital with a high fever and headache 23 years ago. He developed neurological symptoms as mental change, visual disturbance, and weakness, according to medical history. The first MRI was conducted 7-d after onset. He was then transferred to a tertiary hospital to treat his symptoms, and the diagnosis of scrub typhus encephalitis was confirmed.
No special previous medical history was reported.
No special personal and family histories were found.
Physical examination revealed narrow visual fields; however, he could read books and newspapers. His motor power on both extremities was intact without any pain. But fine movements of the left hand were poor, and he could only manage to walk indoors because of sensory impairment.
Motor and sensory findings tested using objective tools were as follows: handgrip strength (right/left, kg), 32.3/31.3; two-point discrimination test (right/left, mm), 5/absence; and monofilament test (right/left, mm), 3.22/absence. The patient had impaired cognition, with a mini-mental status examination score of 14 and a Montreal cognitive assessment score of 10[7-10]. For a more detailed evaluation of cognition, we performed a computerized neuropsychological test (Table 1).
| Visual span | Digit span | Memory | |||||
| Verbal learning | Visual learning | ||||||
| Forward (%) | Back (%) | Forward (%) | Back (%) | Frist trial (%) | Fifth trial (%) | First trial (%) | Fifth trial (%) |
| 3 (0.5) | 2 (0.5) | 3 (0.5) | 2 (0.5) | 0 (0.5) | 5 (0.5) | 6 (18) | 7 (50) |
DTI data were acquired with a 3.0 T scanner Intera (Philips, Ltd., Best, The Netherlands) with a six-channel head coil and single-shot echo-planar imaging. For each of the 32 non-collinear diffusion-sensitizing gradients, we acquired 80 contiguous slices parallel to the anterior commissure-posterior commissure line. The imaging parameters were as follows: acquisition matrix = 112 × 112, field of view = 224 mm × 224 mm, TR/TE = 8973/80 ms, parallel imaging reduction factor (SENSE factor) = 2, EPI factor = 49, b = 1000 s/mm2, NEX = 2, and slice thickness = 2.0 mm (acquired voxel size = 2 mm × 2 mm × 2 mm). Analysis of the DTI data was performed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL: www.fmrib.ox.ac.uk/fsl) based on the probabilistic tractography method. Head motion artifacts and image distortion due to eddy current were corrected by affine multiscale two-dimensional registration. To reconstruct the Papez circuit, the following locations were considered as the regions of interest (ROIs)[6]: The thalamocortical tract-cingulate gyrus (seed ROI), the anterior limb of the internal capsule (target ROI 1) and the anterior thalamic nuclei (target ROI 2) on axial images; the fornix - mammillary body (seed ROI) on axial images and the crus of the fornix (target ROI) on coronal images; the mammillothalamic tract-anterior thalamic nucleus (seed ROI) and the portion of the isolated mammillothalamic tract (target ROI 1), and the mammillary body (target ROI 2) on axial images; the cingulum - the middle portion of the cingulum (seed ROI) and the posterior portion of the cingulum (target ROI) on coronal images. Additionally, we reconstructed the corticospinal tract (CST) as described in a previous study as we believed it would help to confirm whether specific neural tracts can reflect their related functions[11].
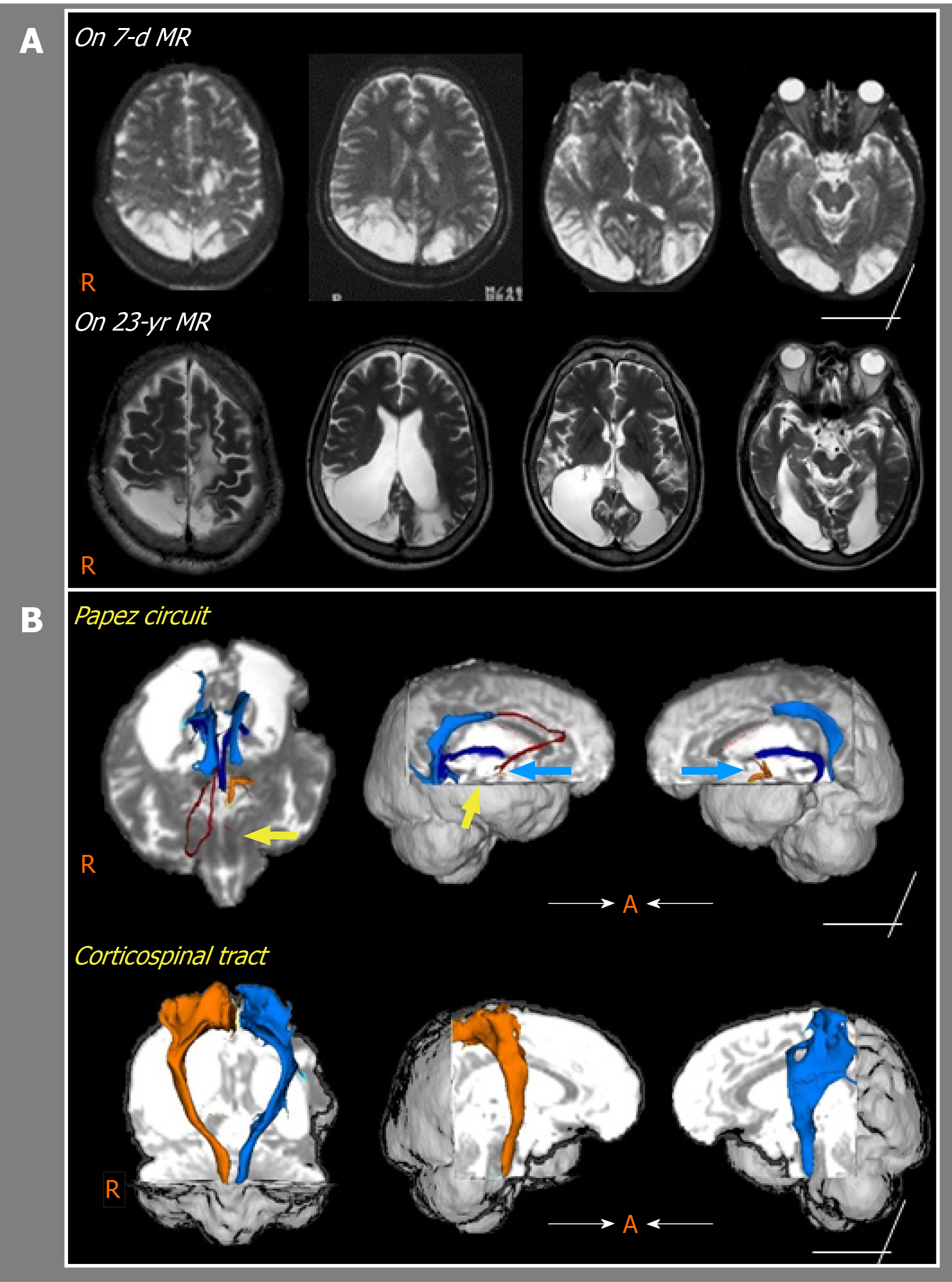
The 7-d MRI onset showed small lesions in the left frontal and parietal lobes, and large lesions in the right superior parietal lobule and both occipital lobes. Compared with the 7-d MRI, the 23-year MRI findings indicated expanded lesions of encephalomalacia with marked dilation of both ventricles (Figure 1A). Probabilistic DTT of the Papez circuit revealed that the left thalamocortical tract and right mammillothalamic tract could not be reconstructed; however, the anterior part of the fornix was found to be injured. Both CSTs were well preserved (Figure 1B).
Based on these findings, WM injury of the Papez circuit was identified.
We did not supply special treatment because his cognitive impairment was a chronic condition.
One-year follow-up showed that his cognitive function has not gotten worse since his first visit to our clinic.
In this study, using DTT, we found that cognitive impairments in a chronic patient with encephalitis of scrub typhus were strongly associated with Papez circuit injury. Pathologic changes of scrub typhus are known to cause vasculitis, which affects multiple organs, including the central nervous system. It has been debated for a long time whether the WM is injured in the encephalitis following scrub typhus because encephalitis occurring after scrub typhus and epidemic typhus is usually limited to the gray matter[12]. Only two case studies of scrub typhus encephalitis had abnormal MRI findings indicative of deep WM involvement[12,13]. Despite a follow-up MRI, we were unable to identify deep WM lesions in areas, such as the Papez circuit. Using DTT, our study findings exclusively detect the injury in two major WM areas — the left thalamocingulate tract and right mammillothalamic tract in the Papez circuit — whereas the CST was found to be intact. In our case, relative to the initial MRI findings, we found an expanded medial temporal lesion in the follow-up MRI. This finding suggests the possibility of a secondary Wallerian degeneration of the Papez circuit, following the direct WM lesions. However, we could not perform follow-up DTT to confirm this possibility. Secondary WM Wallerian degeneration, which is the degeneration of WM distal to a primary lesion, can be caused by various axonal injuries such as trauma, ischemia, and inflammation[14]. Previous studies on Wallerian degeneration of the Papez circuit have been reported in epileptic patients with temporal lobectomy[14-16]. Using DTT, these studies showed complete Wallerian degeneration of the fornix and cingulum within 2-4 mo postoperatively. Moreover, temporal lobe resection indirectly affects the fornix and cingulum contralaterally, demonstrating downstream Wallerian degeneration via the interhemispheric components[15,16]. In the Papez circuit, following encephalitis, concomitant injuries of the thalamocortical tract, mammillothalamic tract, and fornix were demonstrated, along with severe cognitive impairment. In line with our observations, previous studies have reported that thalamocortical tract injury in the Papez circuit is the most common cause of cognitive impairment following brain injury, including encephalitis[6]. Other studies have supported the view that mammillothalamic tract damage following a thalamic infarct is a strong predictor of memory impairment[17]. Regarding cognition following encephalitis, a 3.7-year follow-up study reported only a 12.8% incidence of dementia[18]. However, these findings have limited application in our patient, as viral encephalitis was the etiology in 86.4% (38/44) of the patients in this study[18].
Our patient showed intact bilateral motor power on hand grip strength assessment. By reconstructing the CST using DTT data, we found relative preservation of both CSTs. Overall, we simultaneously analyzed the relationship between the integrity of the Papez circuit and CST, and the neurological manifestations three-dimensionally. Thus, it seems possible to predict the level of cognition and motor function in a chronic patient with encephalitis, which might help develop rehabilitative strategies for restoring function and normal activities of daily life after brain injury. However, our study has a few limitations. The probabilistic DTT usually reconstructs the WM but not the gray matter; thus, we were not able to show the nucleus in the Papez circuit; without quantitative analysis, it may lead to false-negative results.
In the current study, using DTT, we demonstrated injuries to specific neural tracts of the Papez circuit in a chronic patient with encephalitis following scrub typhus. Our findings may be helpful for the elucidation of the pathophysiological mechanisms of encephalitis following scrub typhus.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozair A S-Editor: Gao CC L-Editor: A P-Editor: Xing YX
| 1. | Dittrich S, Rattanavong S, Lee SJ, Panyanivong P, Craig SB, Tulsiani SM, Blacksell SD, Dance DA, Dubot-Pérès A, Sengduangphachanh A, Phoumin P, Paris DH, Newton PN. Orientia, rickettsia, and leptospira pathogens as causes of CNS infections in Laos: a prospective study. Lancet Glob Health. 2015;3:e104-e112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Jain P, Prakash S, Khan DN, Garg RK, Kumar R, Bhagat A, Ramakrishna V, Jain A. Aetiology of acute encephalitis syndrome in Uttar Pradesh, India from 2014 to 2016. J Vector Borne Dis. 2017;54:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Mahajan SK, Mahajan SK. Neuropsychiatric Manifestations of Scrub Typhus. J Neurosci Rural Pract. 2017;8:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Weininger J, Roman E, Tierney P, Barry D, Gallagher H, Murphy P, Levins KJ, O'Keane V, O'Hanlon E, Roddy DW. Papez's Forgotten Tract: 80 Years of Unreconciled Findings Concerning the Thalamocingulate Tract. Front Neuroanat. 2019;13:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Chang MC, Yeo SS, Do Lee H, Jang SH. Diffusion tensor tractography in a patient with memory impairment following encephalitis. Acta Neurol Belg. 2016;116:629-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Park DK, Byun KH, Yang D. Which Neural Tract Plays a Major Role in Memory Impairment After Multiple Cerebral Infarcts? Ann Rehabil Med. 2018;42:617-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Brueggen K, Dyrba M, Kilimann I, Henf J, Hoffmann W, Thyrian JR, Teipel S. Hippocampal Mean Diffusivity for the Diagnosis of Dementia and Mild Cognitive Impairment in Primary Care. Curr Alzheimer Res. 2018;15:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Jiang S, Liu WH, Zhang MY. Update clinical application of diffusion tensor imaging. Curr Med Imaging. 2012;8:331-339. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Kljajevic V, Erramuzpe A. Dorsal White Matter Integrity and Name Retrieval in Midlife. Curr Aging Sci. 2019;12:55-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Saito H, Kashiwakura I, Tsushima M, Mariya Y. Association between Regional Cerebral Blood Flow and Mini-Mental State Examination Score in Patients with Alzheimer's Disease. Curr Med Imaging. 2020;16:1290-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Bucci M, Mandelli ML, Berman JI, Amirbekian B, Nguyen C, Berger MS, Henry RG. Quantifying diffusion MRI tractography of the corticospinal tract in brain tumors with deterministic and probabilistic methods. Neuroimage Clin. 2013;3:361-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Yum KS, Na SJ, Lee KO, Ko JH. Scrub typhus meningo-encephalitis with focal neurologic signs and associated brain MRI abnormal findings: literature review. Clin Neurol Neurosurg. 2011;113:250-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Shen Y, Bao B, Cheng Z. Mild encephalitis/encephalopathy with reversible splenial lesion complicated with scrub typhus. Neurol Sci. 2018;39:1997-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Liu M, Gross DW, Wheatley BM, Concha L, Beaulieu C. The acute phase of Wallerian degeneration: longitudinal diffusion tensor imaging of the fornix following temporal lobe surgery. Neuroimage. 2013;74:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Concha L, Beaulieu C, Wheatley BM, Gross DW. Bilateral white matter diffusion changes persist after epilepsy surgery. Epilepsia. 2007;48:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | McDonald CR, Hagler DJ Jr, Girard HM, Pung C, Ahmadi ME, Holland D, Patel RH, Barba D, Tecoma ES, Iragui VJ, Halgren E, Dale AM. Changes in fiber tract integrity and visual fields after anterior temporal lobectomy. Neurology. 2010;75:1631-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Kamali A, Zhang CC, Riascos RF, Tandon N, Bonafante-Mejia EE, Patel R, Lincoln JA, Rabiei P, Ocasio L, Younes K, Hasan KM. Diffusion tensor tractography of the mammillothalamic tract in the human brain using a high spatial resolution DTI technique. Sci Rep. 2018;8:5229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Hokkanen L, Launes J. Cognitive recovery instead of decline after acute encephalitis: a prospective follow up study. J Neurol Neurosurg Psychiatry. 1997;63:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |