Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3185
Peer-review started: December 10, 2020
First decision: January 27, 2021
Revised: January 30, 2021
Accepted: February 25, 2021
Article in press: February 25, 2021
Published online: May 6, 2021
Processing time: 132 Days and 23.1 Hours
Intrahepatic bile duct papilloma (IPNB) is a rare benign tumour from the bile duct epithelium and has a high malignant transformation rate. Early radical resection can obviously improve the prognosis of patients, but it is difficult to be sure of the diagnosis of IPNB before operating.
This study included 28 patients with intraductal papilloma admitted to the First Hospital of Jilin University from January 2010 to November 2020 and recorded their clinical manifestations, imaging features, complications and prognosis. There were 12 males and 16 females with an average age of 61.36 ± 8.03 years. Most patients had symptoms of biliary obstruction. Biliary dilatation and cystic mass could be seen on imaging. After surgery, IPNB was diagnosed by pathology.
IPNB is a rare benign tumour in the bile duct. Early diagnosis and timely R0 resection can improve the prognosis of IPNB.
Core Tip: Intrahepatic bile duct papilloma (IPNB) is a rare benign tumour in the bile duct. An early radical operation can improve the prognosis. This study analysed the clinical manifestations, imaging features, complications and prognosis of IPNB. IPNB should be considered in patients with bile duct recurrent infection, cholestasis and biliary obstruction, if imaging examination shows cystic dilatation of the bile duct, if there are mural nodules or papillary tumours with delayed enhancement, if the cystic-solid tumours communicate with the bile duct and if the upstream and downstream bile ducts dilate.
- Citation: Yi D, Zhao LJ, Ding XB, Wang TW, Liu SY. Clinical characteristics of intrahepatic biliary papilloma: A case report. World J Clin Cases 2021; 9(13): 3185-3193
- URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3185.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3185
Intrabiliary papilloma is a rare benign tumour from the bile duct epithelium that has been gradually recognized by surgeons in recent years. In 2019, the World Health Organization defined it as “a grossly visible premalignant neoplasm with intraductal papillary or villous growth of biliary-type epithelium”[1]. Therefore, intrahepatic bile duct papilloma (IPNB) is a precancerous lesion or early tumour lesion that is characterized by an intraluminal papillary tumour with a fibrovascular core in the dilated bile duct[2]. However, it has been reported that the malignant transformation rate of IPNB can be as high as 41%-83%[3]. Because it takes 1-2 years for precancerous lesions to transform into invasive carcinoma, there is sufficient time for surgical treatment.
Kim et al[4] showed that the 3-year overall survival rate of patients with IPNB who underwent R0 surgery was 91.3%, and the 5-year overall survival rate was as high as 68.8%. Therefore, early diagnosis, active R0 resection and avoidance of positive biliary tract margins are the primary ways to ensure long-term survival in patients with IPNB[1]. However, it is difficult to diagnose IPNB before surgery, and it is often misdiagnosed as cholangiocarcinoma before surgery, which leads to increased fear of patients and their families, poor willingness to actively treat and even the opportunity for surgical treatment. Therefore, patients with IPNB lose the best treatment time, and at the same time, their quality of life and survival time are reduced.
This study summarized and analysed the clinical data of 28 patients diagnosed with IPNB for the first time at the First Hospital of Jilin University from January 2010 to November 2020 to improve doctors’ understanding of IPNB and improve the preoperative diagnosis rate.
Eighteen patients (64.29%) showed epigastric pain, and three of them had yellow staining of the skin and sclera. Another 4 patients (14.29%) only showed yellow staining of the skin and sclera. Five patients (17.86%) had no obvious clinical manifestations.
Five patients (17.86%) came to see a doctor because of abdominal diseases found in physical examination.
Seven patients (25%) were referred to our hospital for jaundice reduction due to obstructive jaundice. Among them, 5 patients (17.86%) underwent ultrasound-guided intrahepatic bile duct puncture and drainage in local hospitals, and 2 patients (7.14%) underwent endoscopic retrograde cholangiopancreatography nasobiliary drainage in local hospitals. The other 16 patients (57.14%) did not receive other treatment, and they visited our hospital for the first time.
Four patients (14.29%) had undergone cholecystectomy for gallstones. One patient had undergone laparoscopic cholecystectomy for choledocholithiasis. One patient (3.57%) had undergone a radical operation for colon cancer.
All patients had no personal family history.
Eighteen patients (28.57%) had upper abdominal tenderness. Seven patients (25%) had yellow staining of the skin and sclera.
The CA19-9 of 13 patients was higher than normal, among which 12 patients (92.3%) were diagnosed as IPNB invasive carcinoma and 1 patient (7.7%) was IPNB high-grade intraepithelial neoplasia. The total bilirubin level of 11 patients (39.29%) increased.
Twenty-six patients underwent abdominal enhanced computed tomography (CT) examination. Ten patients (35.71%) were examined by cholangiography. According to the results of the imaging examination, 12 cases (42.86%) showed cystic and solid tumours communicating with the bile duct, 8 cases (28.57%) showed soft tissue nodules, and 23 cases (82.14%) showed obvious dilatation of the bile duct. However, only 4 patients (14.29%) were diagnosed as intrahepatic bile duct papilloma by imaging doctor before operation. Seventeen cases (60.71%) were considered cholangiocarcinoma or liver cancer, 4 cases (14.29%) as bile duct dilatation, 1 case (3.57%) as bile duct cystadenocarcinoma, and 1 case (3.57%) as intrahepatic bile duct stones.
Thirteen patients (46.43%) had bile duct stones, 7 patients (25%) had hepatitis, 3 patients (10.71%) had gallstones, 2 patients (7.14%) had sclerosing cholangitis, and 1 patient (3.57%) had bile duct cysts.
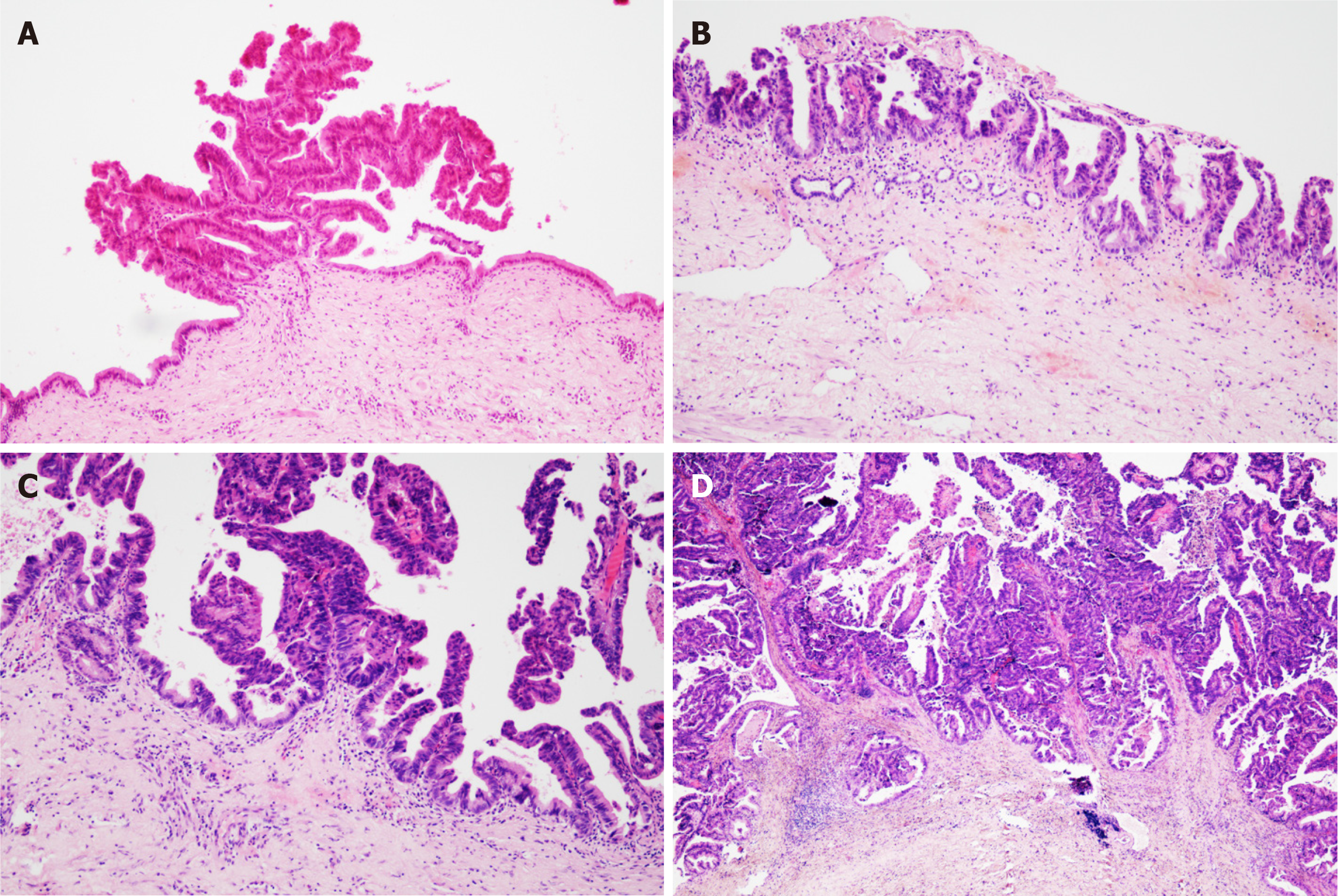
All cases were confirmed as papillary tumours of the intrahepatic bile duct by postoperative pathology. Among them, 11 cases (39.29%) were high-grade intraepithelial neoplasia, and 17 cases (60.71%) were IPNB invasive carcinoma (Figure 1).
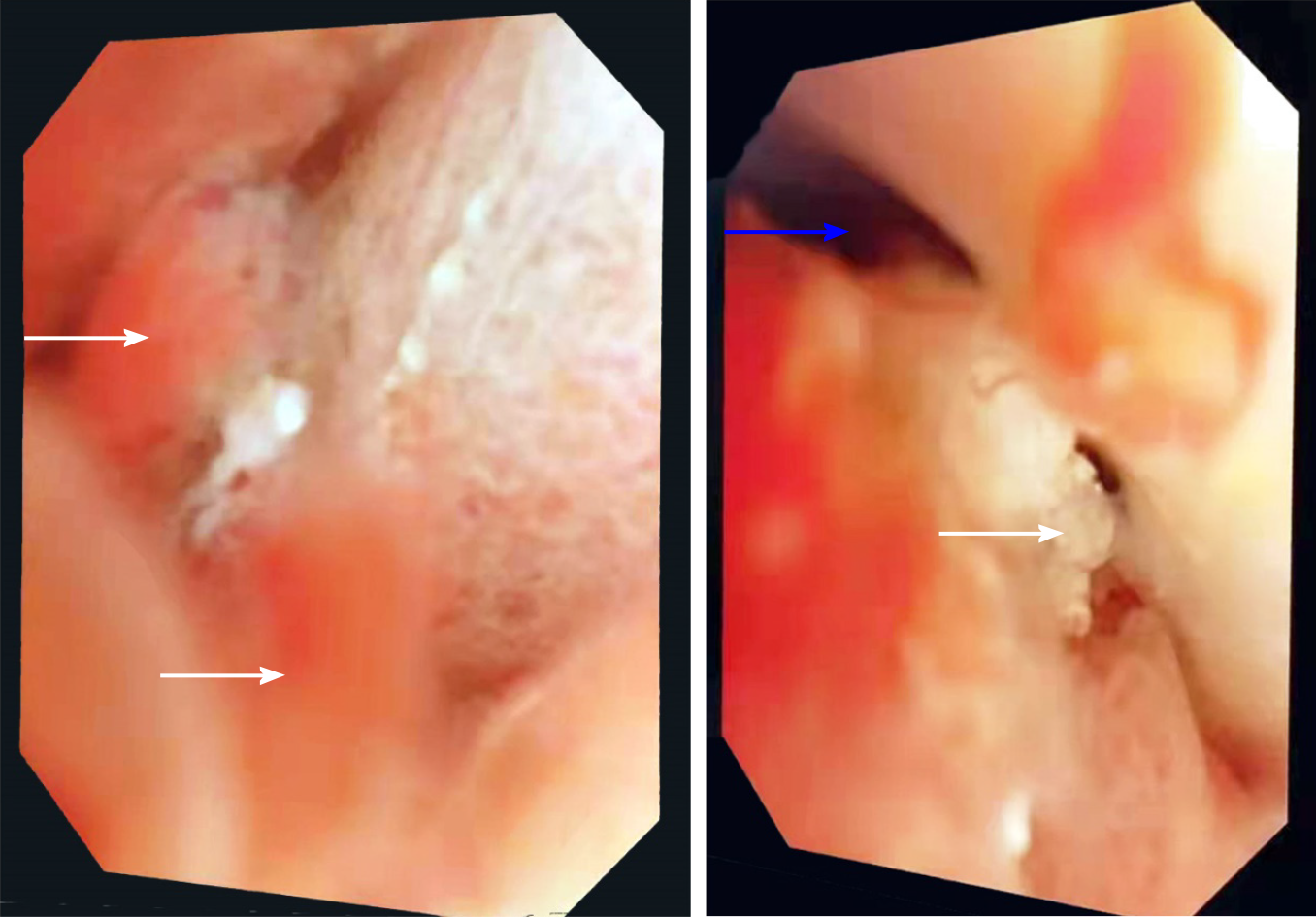
All patients underwent radical surgical resection. Rapid pathological examination was performed during operation to realize R0 resection. Choledochoscopy was performed in some patients during operation (Table 1 and Figure 2).
| Number | Gender | Age | CA19-9 | CEA | Chief complaint | Accompanying diseases | Pathological classification | Operation mode | ||
| Abdominal pain | Jaundice | No symptoms | ||||||||
| 1 | Female | 71 | Normal | Normal | + | + | I | 1234 | ||
| 2 | Female | 65 | Normal | Normal | + | a | II | 1 | ||
| 3 | Female | 74 | ↑ | ↑ | + | ab | II | 1234 | ||
| 4 | Male | 45 | ↑ | Normal | + | b | II | 1234 | ||
| 5 | Male | 71 | ↑ | Normal | + | II | 1235 | |||
| 6 | Female | 66 | Normal | Normal | + | a | II | 1235 | ||
| 7 | Female | 62 | Normal | Normal | + | b | I | 1246 | ||
| 8 | Male | 63 | ↑ | Normal | + | II | 1246 | |||
| 9 | Male | 57 | Normal | Normal | + | e | I | 135 | ||
| 10 | Female | 58 | Normal | Normal | + | I | 1246 | |||
| 11 | Female | 82 | ↑ | Normal | + | b | II | 1235 | ||
| 12 | Female | 67 | Normal | Normal | + | ab | I | 1246 | ||
| 13 | Female | 65 | Normal | Normal | + | I | 1 | |||
| 14 | Male | 52 | ↑ | Normal | + | + | abe | II | 1246 | |
| 15 | Female | 60 | Normal | Normal | + | b | II | 1235 | ||
| 16 | Male | 59 | Normal | Normal | + | + | b | II | 1235 | |
| 17 | Female | 70 | Normal | Normal | + | I | 12 | |||
| 18 | Female | 60 | ↑ | Normal | + | b | II | 124 | ||
| 19 | Male | 60 | Normal | Normal | + | c | II | 12 | ||
| 20 | Female | 58 | ↑ | Normal | + | ab | II | 14 | ||
| 21 | Male | 52 | Normal | Normal | + | I | 1246 | |||
| 22 | Male | 57 | ↑ | Normal | + | b | II | 123 | ||
| 23 | Female | 61 | ↑ | Normal | + | b | II | 135 | ||
| 24 | Male | 57 | ↑ | Normal | + | II | 1 | |||
| 25 | Female | 50 | ↑ | Normal | + | c | II | 1246 | ||
| 26 | Male | 51 | Normal | Normal | + | ab | I | 135 | ||
| 27 | Female | 58 | Normal | Normal | d | I | 1246 | |||
| 28 | Male | 67 | ↑ | ↑ | + | c | I | 1246 | ||
Patients were followed up for 3 years after discharge. Three patients died of multifunctional organ failure. The other patients had no recurrence and a good quality of life. The 3-year survival rate was 89.3%.
IPNB is a rare benign tumour in the bile duct that can secrete mucin in different proportions. It is considered the counterpart in the biliary system of the pancreas intraductal papilloma[5], which can affect any part of the biliary system, but it has been reported that it occurs in the hilar region[6]. In this study, 11 patients’ lesions occurred in the intrahepatic bile duct combined with the hilar region, and 16 patients’ lesions were only located in the left intrahepatic bile duct but did not invade the hilar region, which may be due to the fluidity of mucus secreted by IPNB and the dynamic evolution of the disease. According to the covered epithelial cells, IPNB can be divided into gastric type, intestinal type, pancreaticobiliary type and eosinophilic type[1]. According to pathological results, it can be divided into low-grade intraepithelial neoplasia, high-grade intraepithelial neoplasia and invasive tumour, and the former two are precancerous lesions. Wan-Joon Kim et al[4] others believe that this is a dynamic transition process from benign to malignant. According to the location, size and mucin secretion degree of IPNB, some patients have biliary obstruction symptoms to different degrees, such as abdominal pain, yellow skin and sclera, fever, etc.[7], and some patients have no obvious symptoms. The patients collected in this study are consistent with the above literature reports.
The pathogenesis of IPNB is not clear, but it may be related to repeated infection of the biliary tract and cholestasis[3]. It is worth mentioning that bile duct stones, Clonorchis sinensis infection, primary sclerosing cholangitis and biliary malformation are risk factors for IPNB[8]. Among the cases included in this study, 13 patients suffered from bile duct stones, 2 patients suffered from sclerosing cholangitis and 1 patient suffered from bile duct cysts. Long-term stimulation of the above factors can lead to hyperplasia of bile duct epithelial columnar cells and heterotopic tissues and further lead to papillary hyperplasia and the formation of papilloma.
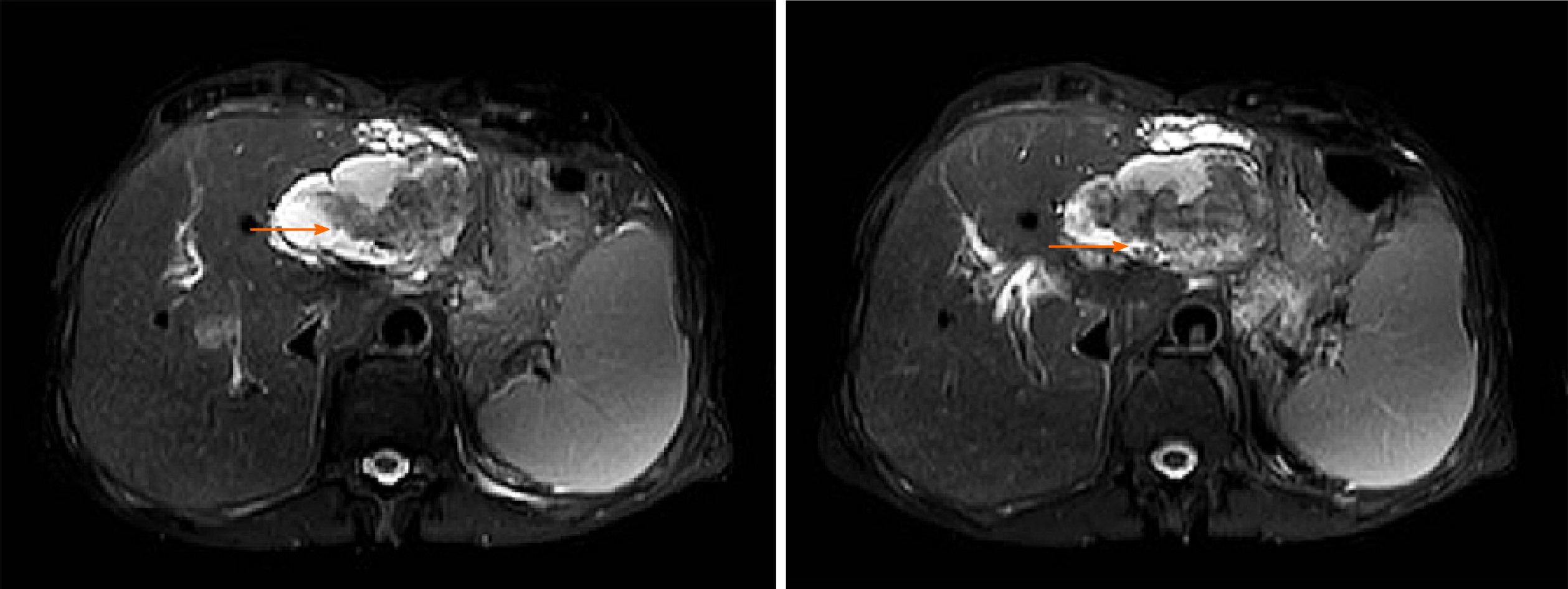
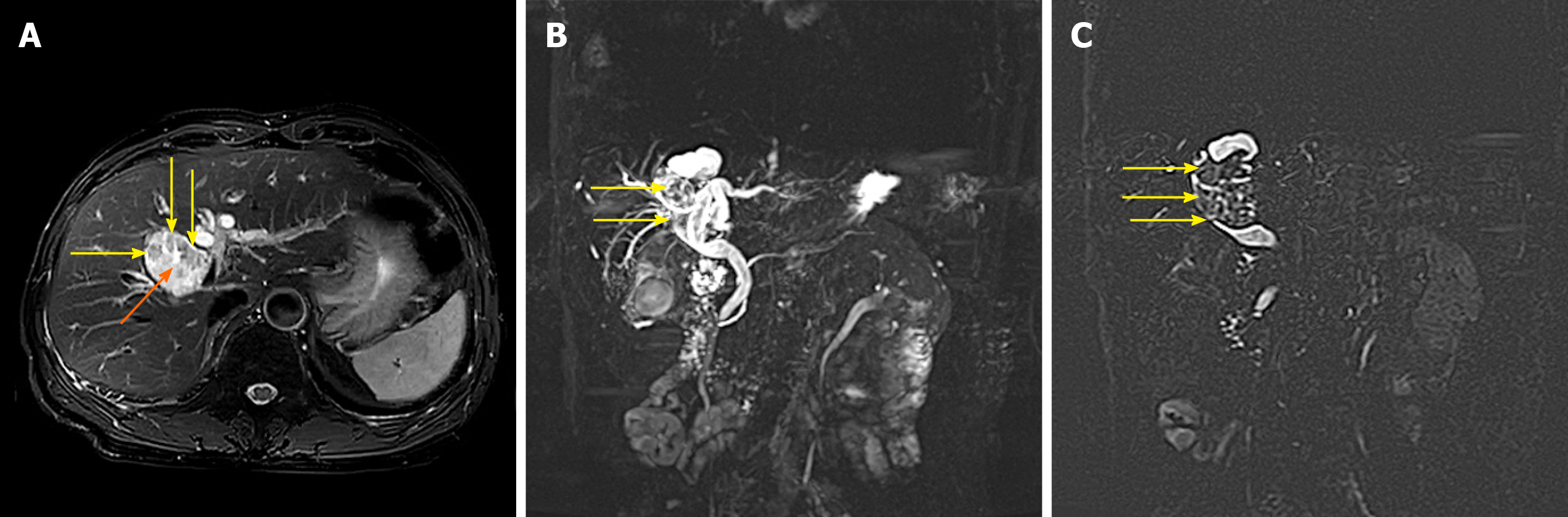
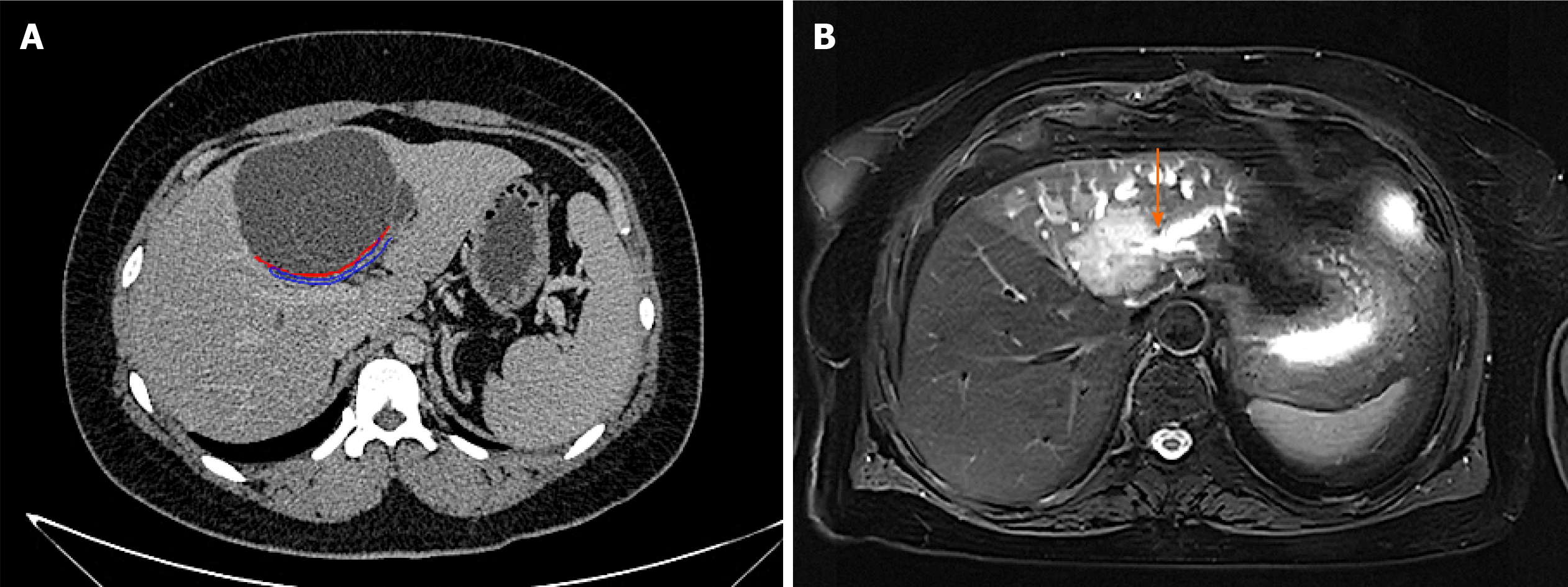
The preoperative diagnosis of IPNB is mainly based on magnetic resonance imaging, CT and magnetic resonance cholangiopancreatography. Because of the different locations, sizes, shapes and mucin secretions of intrahepatic bile duct tumours, they present different imaging manifestations[6], such as: (1) A tumour that is cystic in appearance, in which polypoid, lobulated or coral-like wall nodules can be seen, while in some patients, lump-like or papillary lesions can be seen; (2) The downstream bile duct is obviously dilated; and (3) Slight dilatation of the upstream bile duct is observed, which is also the characteristic manifestation of IPNB in CT examination[4,9]. However, in a few patients, nodule-like lesions are not obvious and are difficult to find by preoperative CT examination, which is also why preoperative diagnosis is difficult at present[6,10]. Siripongsakun et al[11] and other studies show that magnetic resonance imaging, as the most sensitive preoperative examination method of IPNB, has the following imaging features: (1) Cystic dilatation of the bile duct and small nodules in the catheter is observed; and (2) The lesion communicates with the bile duct, and the nodule grows into the bile duct cavity and is connected with the bile duct wall through the fibrovascular stalk[2,12], showing a floating sign[13] (Figure 3).
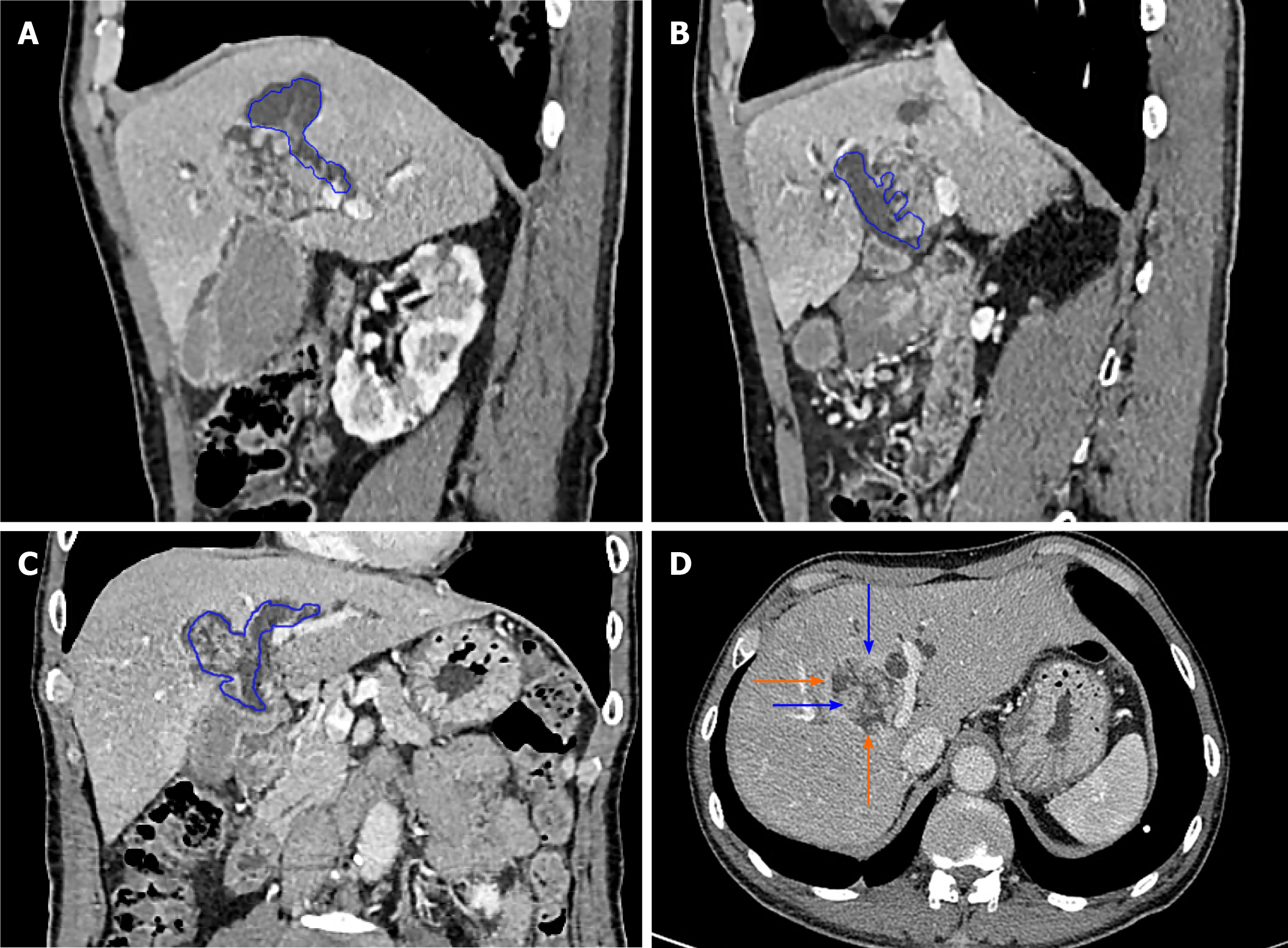
The imaging characteristics of the patients included in this study are consistent with the above report. CT showed that the cystic solid tumour had a smooth cystic wall in which the wall nodules distributed along the bile duct wall projected into the lumen but not out of the bile duct wall. The enhancement of parietal nodules was delayed, the enhancement of papilla was not obvious in the arterial phase, and the enhancement was delayed in the portal phase. Cystic components were not enhanced. Enlargement of the proximal and distal bile ducts was apparent. Magnetic resonance cholangiopancreatography and CT three-dimensional reconstruction characteristically showed that the tumour communicated with the bile duct (Figures 4 and 5). The mucus of the tumour can be distributed in any part of the bile duct, which leads to poor circulation of bile and dilatation of the bile duct, but the mucus cannot completely block all bile ducts, so there is communication with the bile duct. However, because cholangiocarcinoma can cause bile duct dilatation, the bile in the dilated bile duct is difficult to distinguish from IPNB mucus, so preoperative diagnosis is often misdiagnosed as space-occupying lesions of the bile duct.
To improve the accuracy of preoperative diagnosis, IPNB should be differentiated from cholangiocarcinoma and mucinous cystic tumours of the liver. Cholangiocarcinoma is characterized by an ill-defined mass invading into the liver, the distal bile duct showing dead tree-like changes and the tumour showing delayed enhancement[14] (Figure 5). Mucinous cystic tumours of the liver (MCN) tend to occur in women[15], and most of these MCN do not communicate with the bile duct and have ovarian stroma[3] (Figure 6). In contrast, IPNB communicates with the bile duct and lacks ovarian stroma.
The level of serum carbohydrate antigen 19-9 (CA19-9) is increased in malignant tumours of the bile duct[16,17]. In this study, 70.59% of patients with IPNB invasive carcinoma had higher CA19-9 than normal. The CA19-9 of 9.09% patients with IPNB high-grade intraepithelial neoplasia was higher than the normal level (Chi-square test, P < 0.05); therefore CA19-9 level in patients with IPNB invasive cancer is usually higher than normal level.
In summary, when patients have the following characteristics, doctors should be aware of the possibility of IPNB: (1) Symptoms of biliary obstruction; (2) Other diseases related to repeated infection of the biliary tract and cholestasis; and (3) Cystic dilatation of the bile duct in which wall nodules or papillary masses with delayed enhancement can be seen, cystic solid masses communicate with the bile duct and the upstream and downstream bile ducts are dilated. R0 surgery should be performed actively for patients with IPNB to obtain a good prognosis.
The authors thank all the patients who participated in the survey.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hokuto D, Jabi R, Minaga K S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li JH
| 1. | Uemura S, Higuchi R, Yazawa T, Izumo W, Matsunaga Y, Shiihara M, Ota T, Furukawa T, Yamamoto M. Prognostic Factors for Surgically Resected Intraductal Papillary Neoplasm of the Bile Duct: A Retrospective Cohort Study. Ann Surg Oncol. 2021;28:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Matono R, Ninomiya M, Morita K, Tomino T, Oshiro Y, Yokota T, Nishizaki T. Branch-type intraductal papillary neoplasm of the bile duct treated with laparoscopic anatomical resection: a case report. Surg Case Rep. 2020;6:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Pattarapuntakul T, Ovartlarnporn B, Sottisuporn J. Mucinous cystic neoplasm of the liver with extrahepatic growth presenting with ascending cholangitis diagnosed by endoscopic ultrasound features: a case report. J Med Case Rep. 2018;12:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Kim WJ, Hwang S, Lee YJ, Kim KH, Park KM, Ahn CS, Moon DB, Ha TY, Song GW, Jung DH, Park GC, Kim MH, Lee SK, Seo DW, Park DH, Lee SS, Lee SG. Clinicopathological Features and Long-Term Outcomes of Intraductal Papillary Neoplasms of the Intrahepatic Bile Duct. J Gastrointest Surg. 2016;20:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Matsumoto T, Kubota K, Hachiya H, Sakuraoka Y, Shiraki T, Shimizu T, Mori S, Iso Y, Kato M, Yamagishi H, Imai Y, Aoki T. Impact of Tumor Location on Postoperative Outcome of Intraductal Papillary Neoplasm of the Bile Duct. World J Surg. 2019;43:1313-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Park HJ, Kim SY, Kim HJ, Lee SS, Hong GS, Byun JH, Hong SM, Lee MG. Intraductal Papillary Neoplasm of the Bile Duct: Clinical, Imaging, and Pathologic Features. AJR Am J Roentgenol. 2018;211:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Jabbour E, Garcia-Manero G, Ravandi F, Faderl S, O'Brien S, Fullmer A, Cortes JE, Wierda W, Kantarjian H. Prognostic factors associated with disease progression and overall survival in patients with myelodysplastic syndromes treated with decitabine. Clin Lymphoma Myeloma Leuk. 2013;13:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Aslam A, Wasnik AP, Shi J, Sahai V, Mendiratta-Lala M. Intraductal papillary neoplasm of the bile duct (IPNB): CT and MRI appearance with radiology-pathology correlation. Clin Imaging. 2020;66:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Komori T, Inoue D, Zen Y, Yoneda N, Kitao A, Kozaka K, Yokka A, Toshima F, Matsubara T, Kobayashi S, Gabata T. CT imaging comparison between intraductal papillary neoplasms of the bile duct and papillary cholangiocarcinomas. Eur Radiol. 2019;29:3132-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Zaccari P, Cardinale V, Severi C, Pedica F, Carpino G, Gaudio E, Doglioni C, Petrone MC, Alvaro D, Arcidiacono PG, Capurso G. Common features between neoplastic and preneoplastic lesions of the biliary tract and the pancreas. World J Gastroenterol. 2019;25:4343-4359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Siripongsakun S, Sapthanakorn W, Mekraksakit P, Vichitpunt S, Chonyuen S, Seetasarn J, Bhumiwat S, Sricharunrat T, Srittanapong S. Premalignant lesions of cholangiocarcinoma: characteristics on ultrasonography and MRI. Abdom Radiol (NY). 2019;44:2133-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Kim JR, Jang KT, Jang JY, Lee K, Kim JH, Kim H, Kim SW, Kwon W, Choi DW, Heo J, Han IW, Hwang S, Kim WJ, Hong SM, Kim DS, Yu YD, Kim JY, Nah YW, Park HW, Choi HJ, Han HS, Yoon YS, Park SJ, Hong EK, Seo HI, Park DY, Kang KJ, Kang YN, Yu HC, Moon WS, Lim CS, Bae JM, Jo S, Lee W, Roh YH, Jeong JS, Jeong CY, Lee JS, Song IS, Kim KH, Kim HG, Cho CH, Joo SH, Won KY, Kim HJ, Choi JH, Chu CW, Lee JH, Park IY, Lee H, Lee SE, Kim HS, Lee HK, Cho MS, Han KM. Clinicopathologic analysis of intraductal papillary neoplasm of bile duct: Korean multicenter cohort study. HPB (Oxford). 2020;22:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Zhang H, Zhong Z, Kong G, Khan J, Zou L, Jiang Y, Liu X, Tang Y, Jiang B, Peng C, Song Y, Liu S. Clinicopathological findings and imaging features of intraductal papillary neoplasms in bile ducts. PeerJ. 2020;8:e10040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Joo I, Lee JM, Yoon JH. Imaging Diagnosis of Intrahepatic and Perihilar Cholangiocarcinoma: Recent Advances and Challenges. Radiology. 2018;288:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Frezin J, Komuta M, Zech F, Annet L, Horsmans Y, Gigot JF, Jouret-Mourin A, Hubert C. Mucin-producing hepatic cystic neoplasms: an uncommon but challenging disease often misdiagnosed and mismanaged. Acta Chir Belg. 2020;120:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Fang T, Wang H, Wang Y, Lin X, Cui Y, Wang Z. Clinical Significance of Preoperative Serum CEA, CA125, and CA19-9 Levels in Predicting the Resectability of Cholangiocarcinoma. Dis Markers. 2019;2019:6016931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Zheng BH, Yang LX, Sun QM, Fan HK, Duan M, Shi JY, Wang XY, Zhou J, Fan J, Ma ZY, Gao Q. A New Preoperative Prognostic System Combining CRP and CA199 For Patients with Intrahepatic Cholangiocarcinoma. Clin Transl Gastroenterol. 2017;8:e118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |