Published online Apr 26, 2021. doi: 10.12998/wjcc.v9.i12.2923
Peer-review started: December 18, 2020
First decision: January 17, 2021
Revised: January 26, 2021
Accepted: February 22, 2021
Article in press: February 22, 2021
Published online: April 26, 2021
Processing time: 117 Days and 13.6 Hours
The incidence of breast cancer among women of reproductive age is increasing, as well as the desire for children at late childbearing age. Identifying factors that may be associated with fetal malformation and maternal and fetal prognosis has gained importance. We describe a 32-year-old woman with breast cancer who gave birth to a son with congenital bilateral cryptorchidism after treatment, with a literature review performed.
A 32-year-old woman with breast cancer who had been treated by surgery and radiotherapy experienced recurrence and underwent a second surgery, adjuvant chemotherapy, and targeted therapy. Her tumor cells were negative for estrogen receptor (ER) α, progesterone receptor (PR), and p53; positive for ERβ, human epidermal growth factor receptor-2 (HER2), epidermal growth factor receptor (EGFR), and Ki67. She had pathogenic BRCA gene mutations. She became pregnant within 2 years and delivered a boy with congenital bilateral cryptorchidism. The boy underwent bilateral orchidopexy. As of this writing, the woman and her son are both healthy.
HER2 overexpression, positivity for EGFR, Ki67, and ER, and PR negativity are associated with a poor prognosis in breast cancer. While no link has been established statistically between treatment for breast cancer and cryptorchidism in a subsequent pregnancy, this case suggests the possibility that ERβ and gene mutations may be contributing factors.
Core Tip: The number of young premenopausal women with breast cancer is increasing and they usually have a worse prognosis. Most studies focus on therapy for breast cancer. Only a few reports are published regarding fetal malformations that occur after cancer therapy. In this article, a patient got pregnant after breast cancer treatment and her son was diagnosed with congenital bilateral cryptorchidism. We conclude that there has no established link between treatments for breast cancer and cryptorchidism. Estrogen receptor β and gene mutations may be related to bilateral cryptorchidism.
- Citation: Hu WK, Liu J, Liu RX, Liu XW, Yin CH. Congenital bilateral cryptorchidism in an infant conceived after maternal breast cancer treatment: A case report. World J Clin Cases 2021; 9(12): 2923-2929
- URL: https://www.wjgnet.com/2307-8960/full/v9/i12/2923.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i12.2923
Breast cancer is a leading cause of death among women worldwide[1]. Although the median age at diagnosis is around 60 years, approximately 20% of patients are younger than 49 years, and 6% are younger than 40 years[2,3]. Annually, about 3000 women of childbearing age receive a new diagnosis of breast cancer[4], and the number is increasing in young premenopausal women in Asian countries[5].
Younger women with breast cancer tend to have a worse prognosis and therefore require more intensive therapy. Some studies have reported that fetal malformations occur after cancer therapy[6-8], but most experts believe that pregnancies are not at increased risk of fetal malformation or hypophrenia. However, information is limited. Of note, one study reported that pregnancy after trastuzumab treatment appeared safe for fetal and maternal prognosis, in patients with early breast cancer and positive for human epidermal growth factor receptor-2 (HER2)[9]. Yet, there remains concern that therapy given for breast cancer may lead to fetal congenital abnormalities in latter pregnancies.
A 24-year-old woman presented to the Surgery Department of Peking Union Medical College Hospital complaining of an increasing left breast mass.
The patient’s symptoms started a month ago with slight lancinating pain in the menstrual cycle of left breast mass.
The patient had appendectomy about ten years ago. The patient had accidental abortion two years ago and details were unclear.
The patient was an ex-smoker and had a free family history.
At surgery department of Peking Union Medical College Hospital, the patient’s temperature was 36.8 ℃, heart rate was 78 bpm, respiratory rate was 19 breaths per minute, blood pressure was 115/70 mmHg, and oxygen saturation in room air was 98%. The clinical physical examination revealed a mass in the upper outer quadrant of the left breast, about 3 cm in diameter, tough, immobile, and its boundary was obscure. Our clinical consideration was breast cancer.
Blood analysis and prothrombin and partial thromboplastin times were normal. D-dimers were slightly increased at 5.41 mg/L. Blood biochemistries, as well as urine analysis were normal. Electrocardiogram was also normal.
Imaging evaluation by ultrasound revealed a 27 mm × 14 mm suspected malignant mass in the lower outer quadrant around 4 o’clock direction without axillary lymphadenopathy (BI-RADS 4). A 21 mm × 8 mm nodule in the upper outer quadrant of the left breast around 2 o’clock direction and a 6 mm × 4 mm nodule in the upper outer quadrant of the right breast around 2 o’clock direction were observed (BI-RADS 3).
The final diagnosis of the presented case was intraductal carcinoma of the left breast.
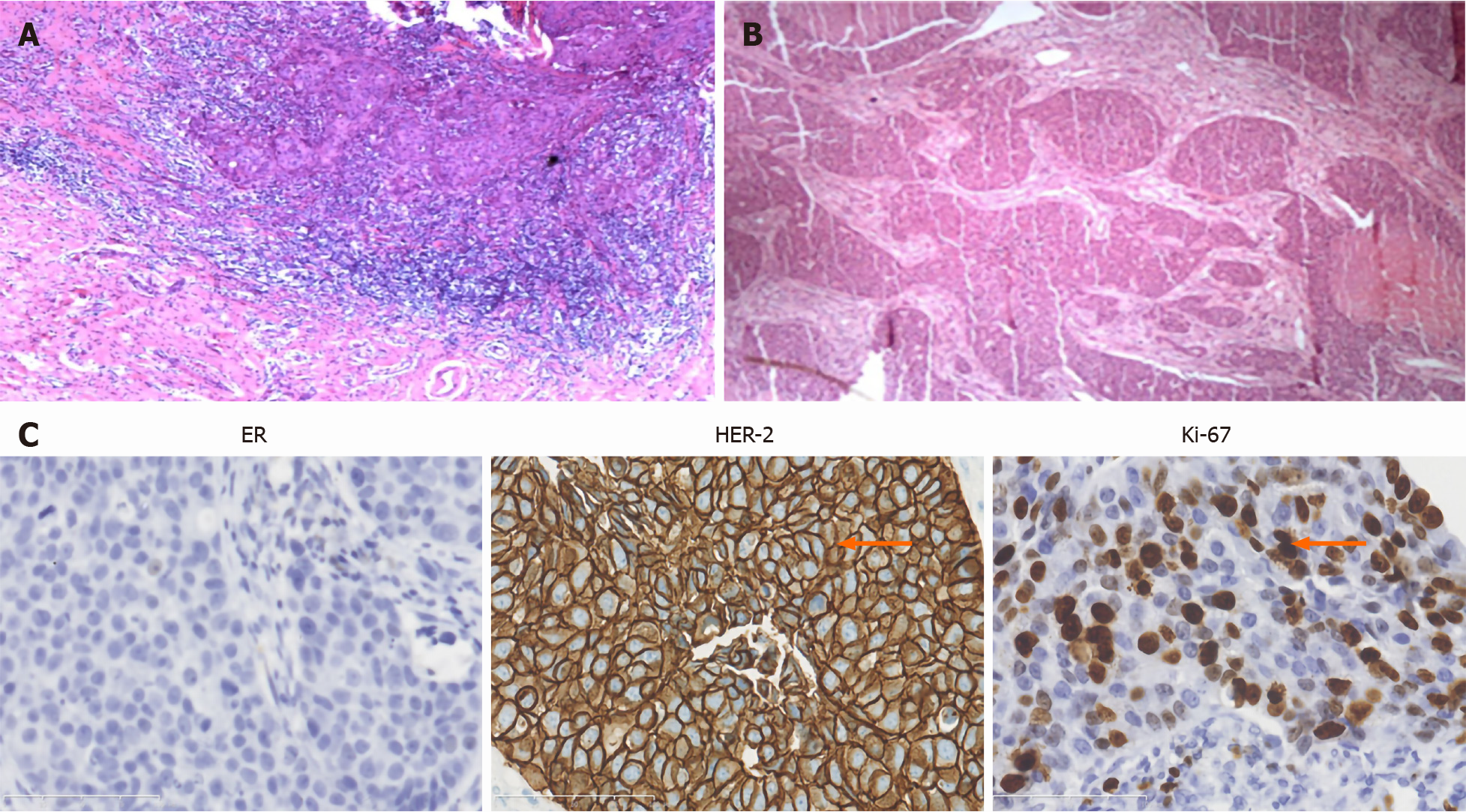
On April 23, 2013, a 32-year-old woman was confirmed to have a 21 mm × 8 mm high-grade intraductal carcinoma (Figure 1A) in the upper outer quadrant of the left breast, and a 6 mm × 4 mm nodule in the upper outer quadrant of the right breast without axillary lymphadenopathy through lumpectomy. The cells of the mass were negative for estrogen receptor (ER) α, progesterone receptor (PR), CD10, CK5/6, p63, and p53, but positive for ERβ (70%) and HER2 (3+). The Ki67 index was 25%.
The patient strongly preferred to retain her breasts. A local resection of the left breast and left sentinel lymph node biopsy (SLNB) were performed under local anesthesia on May 3, 2013 at Peking Union Medical College Hospital. The SLNB was negative. After surgery, the patient received 30 Gy radiation to the left breast.
On October 21, 2014, a 30 mm × 30 mm suspected malignant mass was discovered. A BRCA gene test showed BRCA1 (17q21) and BRCA2 (13q12-13) pathogenic mutations. She was given modified radical surgery of the left breast, mastectomy of the right breast, SLNB, and expander implantation in both breasts.
The pathology results revealed low-grade invasive ductal carcinoma (Figure 1B). The cells were negative for ERα, PR, p53, p63, CD10, CK14, and CK5/6, but positive for ERβ (90%), epidermal growth factor receptor (EGFR) (1+), and HER2 (3+). The Ki67 index was 50% (Figure 1C). SLNB showed two negative sentinel nodes.
The patient underwent postoperative adjuvant chemotherapy with pirarubicin + cyclophosphamide for four cycles (every 21 d), and then paclitaxel + trastuzumab for four cycles (every 21 d). Therapy was well tolerated, despite one instance of myelosuppression.
On December 14, 2016, the patient became pregnant and was closely monitored at Beijing Obstetrics and Gynecology Hospital. She did not receive any kind of therapy related to breast cancer during that time. All examinations were normal. A cardiology study of the fetus showed normal results during the entire gestation. At the 38th week of gestation, on September 10, 2017, the patient gave birth to a boy via spontaneous vaginal delivery (3250 g). Apgar scores at 1, 5, and 10 min after birth were 10/10. The child had congenital bilateral cryptorchidism.
During the first year, the child twice had fever and urinary tract infection. Computed tomography detected moderate hydronephrosis of the left side. Cystography showed vesicoureteral reflux on the left and membranous urethral stenosis. The child recovered after treatment for inflammation. The testicles did not spontaneously descend into the scrotal sac within one year. Therefore, the boy underwent bilateral orchidopexy on October 9, 2018 at Capital Institute of Pediatrics.
The last follow-up occurred on October 4, 2020. As of this writing, the boy is healthy. The mother is examined regularly and there has been no sign of recurrence.
In this case, the woman patient underwent surgery, radiotherapy, and chemotherapy in chronological order. These therapies can cause toxic reactions or fetal malformations. It is recommended that pregnancy should be delayed more than six months after the last breast cancer treatment[8]. In this case, the baby’s testicles did not descend, and the cryptorchidism (i.e., failure of the testis to descend to the scrotum within 4 mo of age) was treated by bilateral orchidopexy at the age of 13 mo.
We cannot draw a conclusion regarding the relationship between maternal cancer treatment and fetal malformation from this case alone.
BRCA mutation can elevate the risks of breast cancer and ovarian cancer. For women with BRCA1 and BRCA2 mutations, the risks of breast cancer development by age 80 are estimated at 72% and 69%, respectively[10]. Unmutated BRCA1 and BRCA2 can repair homologous DNA[11]. Clinical studies of the potential correlations between BRCA1 and BRCA2 mutations and outcomes in patients with breast cancer provide conflicting results[12].
Estrogen binds to ER, which promotes breast cancer progression and regulates the transcription of PR. ER is an important predictive biomarker for endocrine therapy[13], and ER-positive patients should receive endocrine therapy. ER-positive and PR-negative tumors are a distinct subset of breast cancers that have shown aggressive behavior, greater genomic instability, a higher proliferation rate, poor outcome, and tamoxifen resistance[14]. ER-positive and PR-negative patients have higher levels of epidermal growth factor receptor and HER2 compared with patients who are both ER- and PR-positive[14]. The percentage of patients with breast cancer with amplified ERBB2 (erb-b2 receptor tyrosine kinase 2) is 13% to 15%. ERBB2 activates the HER2 pathway.
HER2 overexpression promotes proliferation, metastasis, and adhesion of cancer cells. Endocrine therapy combined with anti-HER2 agents is the initial treatment, and the maintenance treatment, for ER- and HER2-positive patients[15]. In the present case, the patient was ER-positive and PR-negative. The patient would have received aromatase inhibitors as endocrine therapy, but she willingly refused.
Ki67 is related to proliferation, metastasis, and chemosensitivity. High Ki67 positivity correlates with tumor-node-metastasis stage, infiltration, aggressive behavior, and lymph node metastasis. P53 mutations contribute crucially to tumorigenesis and are associated with more aggressive behavior, infiltration, and worse overall survival. Elevated EGFR is linked to breast cancer pathogenesis and poor prognosis and is an important target of breast cancer treatment[16]. In the present case, the Ki67 index was 50%, p53 was negative, and EGFR had 1+ staining intensity.
In the present case, the baby had cryptorchidism and underwent bilateral orchidopexy at the age of 13 mo to avoid further consequences. Drug therapy for the mother consisted of anthracycline, cyclophosphamide, paclitaxel, and trastuzumab. Anthracyclines can cause irreversible cardiomyopathy and congestive heart failure[7]. Although the incidence of abnormalities among children born after their mothers are treated for cancer is similar to the average rate of 3%, we should still consider potential drug toxicity to a fetus.
Cyclophosphamide can cause immunotoxicity and urotoxicity[17]. The side effects of paclitaxel are peripheral neuropathy, born marrow suppression, and muscle toxicity. In addition, a study found that using paclitaxel in pregnant mice can lead to delayed testis descension[18]. A case was reported on a child with congenital hearing loss who was exposed to paclitaxel and cisplatin in utero[7].
The major side effect of trastuzumab (trade name Herceptin) is cardiotoxicity, which can persist 2 years after the end of therapy. The Herceptin Adjuvant trial is a large phase III randomized clinical trial in which 33 patients with breast cancer became pregnant after trastuzumab use, and one patient had a child with Down’s syndrome[6].
In conclusion, no link has been established between treatments and cryptorchidism. Further multicenter, randomized controlled studies are needed to draw definitive conclusions.
Normally, the testes begin to spontaneously descend into the scrotum at 28 wk of gestation and complete descension at birth. Testes in an abnormal location after birth can influence their normal function. The incidence of cryptorchidism among full-term newborn boys is 2% to 4%[19]. The etiology of cryptorchidism is complex. The following discusses mainly genes and different hormonal environments[20].
Cryptorchidism may be related to gene mutations. For example, insulin-like factor 3 (INSL3) participates in testicular descent, and gene mutation may be related to bilateral cryptorchidism[21]. HOXA10 gene mutation may influence the pathogenesis of cryptorchidism[22,23]. Haploinsufficiency of the MKX gene may affect the process of testis descension or lead to cryptorchidism[24]. Nuclear receptor subfamily 5 group A member 1 regulates sex determination, testis descension, differentiation, and steroidogenesis. Associations have been determined among WT1 abnormalities, renal abnormalities, and cryptorchidism[25].
In addition, testis-derived testosterone, estrogen, and INSL3 may affect testis descension[21]. Estrogen can decrease the secretion of testosterone and INSL3, resulting in cryptorchidism. ERα and ERβ mediate estrogenic effects. ERα participates in the development of the male reproductive tract and may increase the risk of cryptorchidism[26]. A study concluded that oxytocin receptor expression, ERβ, and their correlation may be involved in the pathogenesis of cryptorchidism[27], but was based on animal models, and no additional studies on ERβ and cryptorchidism are available. The patient in the present case had tumor cells that were ERα-negative and ERβ-positive. ERβ may eventually be proven to influence cryptorchidism.
In this case, the baby was a term infant without a family history of cryptorchidism. There was no exposure during the pregnancy to chemicals associated with infant reproductive hormone levels. Furthermore, no link has been established between treatments for breast cancer and cryptorchidism. We conclude that the cryptorchidism in this infant was likely related to ERβ and gene mutations. Elevated levels of HER2 and Ki67 promote proliferation and metastasis, and EGFR overexpression, ER positivity, and PR negativity have been linked to a poor prognosis. Our patient remains living without progression, and she and her baby are healthy.
Our deepest gratitude goes to the anonymous reviewers and editors for their careful work and thoughtful suggestions that have helped improve this paper substantially.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kapritsou M S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1625] [Cited by in RCA: 1651] [Article Influence: 235.9] [Reference Citation Analysis (0)] |
| 2. | Erić I, Petek Erić A, Kristek J, Koprivčić I, Babić M. Breast cancer in young women: Pathologic and immunohistochemical features. Acta Clin Croat. 2018;57:497-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Winters S, Martin C, Murphy D, Shokar NK. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci. 2017;151:1-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (1)] |
| 4. | Elies A, Salakos E, Rouzier R. [Desire for pregnancy and breast cancer]. Bull Cancer. 2019;106:S53-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Iyengar NM, Chen IC, Zhou XK, Giri DD, Falcone DJ, Winston LA, Wang H, Williams S, Lu YS, Hsueh TH, Cheng AL, Hudis CA, Lin CH, Dannenberg AJ. Adiposity, inflammation, and breast cancer pathogenesis in Asian women. Cancer Prev Res (Phila). 2018;11:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Azim HA Jr, Metzger-Filho O, de Azambuja E, Loibl S, Focant F, Gresko E, Arfi M, Piccart-Gebhart M. Pregnancy occurring during or following adjuvant trastuzumab in patients enrolled in the HERA trial (BIG 01-01). Breast Cancer Res Treat. 2012;133:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Geijteman ECT, Wensveen CWM, Duvekot JJ, van Zuylen L. A child with severe hearing loss associated with maternal cisplatin treatment during pregnancy. Obstet Gynecol. 2014;124:454-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | China Guideline Committee of Clinical Practice and Fertility Preservation for Breast Cancer in Young Women. [Chinese consensus guidelines for breast cancer in young women: clinical practice and fertility preservation]. Zhonghua Zhong Liu Za Zhi. 2019;41:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Lambertini M, Martel S, Campbell C, Guillaume S, Hilbers FS, Schuehly U, Korde L, Azim HA Jr, Di Cosimo S, Tenglin RC, Huober J, Baselga J, Moreno-Aspitia A, Piccart-Gebhart M, Gelber RD, de Azambuja E, Ignatiadis M. Pregnancies during and after trastuzumab and/or lapatinib in patients with human epidermal growth factor receptor 2-positive early breast cancer: Analysis from the NeoALTTO (BIG 1-06) and ALTTO (BIG 2-06) trials. Cancer. 2019;125:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Kotsopoulos J. BRCA Mutations and breast cancer prevention. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Mahdavi M, Nassiri M, Kooshyar MM, Vakili-Azghandi M, Avan A, Sandry R, Pillai S, Lam AK, Gopalan V. Hereditary breast cancer; Genetic penetrance and current status with BRCA. J Cell Physiol. 2019;234:5741-5750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Gorodetska I, Kozeretska I, Dubrovska A. BRCA Genes: The role in genome stability, cancer stemness and therapy resistance. J Cancer. 2019;10:2109-2127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Nicolini A, Ferrari P, Duffy MJ. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin Cancer Biol. 2018;52:56-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 14. | Thakkar JP, Mehta DG. A review of an unfavorable subset of breast cancer: estrogen receptor positive progesterone receptor negative. Oncologist. 2011;16:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Primers. 2019;5:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 1772] [Article Influence: 295.3] [Reference Citation Analysis (0)] |
| 16. | Kjaer IM, Bechmann T, Brandslund I, Madsen JS. Prognostic and predictive value of EGFR and EGFR-ligands in blood of breast cancer patients: a systematic review. Clin Chem Lab Med. 2018;56:688-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Sakthivel KM, Guruvayoorappan C. Acacia ferruginea inhibits cyclophosphamide-induced immunosuppression and urotoxicity by modulating cytokines in mice. J Immunotoxicol. 2015;12:154-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Borovskaya TG, Shchemerova YA, Poluektova ME, Vychuzhanina AV, Goldberg VE, Kinsht DN, Yershov KI, Madonov PG. Mechanisms of reparative regeneration of rat testis after injection of paclitaxel. Bull Exp Biol Med. 2014;156:483-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Chul Kim S, Kyoung Kwon S, Pyo Hong Y. Trends in the incidence of cryptorchidism and hypospadias of registry-based data in Korea: a comparison between industrialized areas of petrochemical estates and a non-industrialized area. Asian J Androl. 2011;13:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Gurney JK, McGlynn KA, Stanley J, Merriman T, Signal V, Shaw C, Edwards R, Richiardi L, Hutson J, Sarfati D. Risk factors for cryptorchidism. Nat Rev Urol. 2017;14:534-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Huang X, Jia J, Sun M, Li M, Liu N. Mutational screening of the INSL3 gene in azoospermic males with a history of cryptorchidism. Andrologia. 2016;48:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Cheng Z, Wang M, Xu C, Pei Y, Liu JC, Huang H, He D, Lu P. Mutational analysis of HOXA10 gene in Chinese patients with cryptorchidism. Andrologia. 2017;49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Lu P, Wang Y, Wang F, Huang J, Zeng Y, He D, Huang H, Cheng Z. Genetic analysis of HOXA11 gene in Chinese patients with cryptorchidism. Andrologia. 2018;50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Mroczkowski HJ, Arnold G, Schneck FX, Rajkovic A, Yatsenko SA. Interstitial 10p11.23-p12.1 microdeletions associated with developmental delay, craniofacial abnormalities, and cryptorchidism. Am J Med Genet A. 2014;164A:2623-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Seabra CM, Quental S, Neto AP, Carvalho F, Gonçalves J, Oliveira JP, Fernandes S, Sousa M, Barros A, Amorim A, Lopes AM. A novel Alu-mediated microdeletion at 11p13 removes WT1 in a patient with cryptorchidism and azoospermia. Reprod Biomed Online. 2014;29:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Deng C, Dai R, Li X, Liu F. A allele of SNP12 in estrogen receptor 1 was a risk factor for cryptorchidism in Asians: a systematic review with meta-analysis and trial sequential analysis. Pediatr Surg Int. 2016;32:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Prapaiwan N, Manee-In S, Moonarmart W, Srisuwatanasagul S. The expressions in oxytocin and sex steroid receptors in the reproductive tissues of normal and unilateral cryptorchid dogs. Theriogenology. 2017;100:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |