Published online Apr 26, 2021. doi: 10.12998/wjcc.v9.i12.2908
Peer-review started: December 10, 2020
First decision: January 29, 2021
Revised: February 9, 2021
Accepted: March 3, 2021
Article in press: March 3, 2021
Published online: April 26, 2021
Processing time: 125 Days and 17.6 Hours
Salivary duct carcinoma (SDC) is a rare, extremely aggressive malignancy that arises in the submandibular gland. It can metastasize locally early and therefore is an important differential diagnosis of metastatic disease in cervical lymph nodes or specific lymphadenitis such as tuberculous cervical lymphadenitis.
We report a case of SDC in the submandibular gland that presented diagnostic difficulty. The lesion was coincidentally discovered through examination of the radiolucent area of the maxilla. Imaging failed to confirm the possibility of specific inflammation, leading us to execute an open biopsy to verify the diagnosis. The surgical specimen showed that the submandibular gland was primarily replaced with a calcified body. Following histological analysis and confirmation, we performed surgical resection, radiotherapy, and various chemotherapies.
Radiographic imaging characteristics of lymph node metastases of salivary gland cancer, especially of SDC, may resemble other cervical lymphadenitis; calcification at the submandibular gland is the landmark of SDC occurring at the subman-dibular gland.
Core Tip: Salivary duct carcinoma (SDC) arising in the submandibular gland is particularly rare, and the related lymph node metastasis is difficult to distinguish from tuberculous cervical lymphadenitis, which complicates the establishment of a definitive diagnosis. We report our experience with a case of SDC in the submandibular gland, in which the similarities in the radiographic and imaging characteristics of lymph node metastatic SDC indeed mimicked those of tuberculous cervical lymphadenitis. However, calcification at the submandibular grand proved to be a critical finding that enabled a definitive diagnosis.
- Citation: Uchihashi T, Kodama S, Sugauchi A, Hiraoka S, Hirose K, Usami Y, Tanaka S, Kogo M. Salivary duct carcinoma of the submandibular gland presenting a diagnostic challenge: A case report. World J Clin Cases 2021; 9(12): 2908-2915
- URL: https://www.wjgnet.com/2307-8960/full/v9/i12/2908.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i12.2908
Salivary duct carcinoma (SDC) is highly aggressive, with high rates of recurrence, metastasis to cervical lymph nodes, and distant metastases. SDC mainly occurs in the parotid gland and rarely in the submandibular gland; SDC arising at the sub-mandibular gland accounts for approximately 10% of all SDC[1]. SDC is histologically similar to invasive mammary ductal carcinoma and is characterized by cribriform and central necrosis (also referred to as comedo necrosis)[1-3]. Representative subjective symptoms of SDC include pain, facial palsy, and a palpable mass[4].
Peripheral cervical lymphadenopathy is often involved not only with metastatic cervical lymph nodes but also with various forms of lymphadenitis, including tuberculous cervical lymphadenitis. Although ultrasonography is a reliable diagnostic method for cervical lymph node lesions, difficulty in ultrasonographic differentiation between tuberculous lymphadenitis and metastatic lymph nodes has been reported[5,6]. Ultrasonographic features of tuberculous lymphadenitis have been described as involvement of multiple nodes, a tendency toward fusion, a hypoechoic mass with posterior enhancement, and the presence of strong echo or calcification[5,6]. However, it is difficult to distinguish metastatic lymph nodes using only this modality; consequently, other radiographic imaging, and clinical findings are required for a proper diagnosis.
Here, we report a case of SDC that developed in the submandibular gland and for which the clinical findings, including radiographic features, were insufficient for a proper diagnosis. In addition, we highlight the challenges faced during the differential diagnosis of the lesion based on imaging characteristics.
A 46-year-old man was referred to our institution for evaluation of a cystic lesion in the maxilla, which was identified at another medical institution.
One year prior to visiting our hospital, the patient became aware of swelling in the alveolar part of the maxillary anterior tooth and received incision and drainage treatment. The patient later visited our institution to have a follow-up examination owing to persisted swelling.
The patient had a clear medical history.
Initial physical examination revealed swelling of the alveolar part of the maxillary anterior tooth and purulent discharge from the maxillary mesial palate.
The patient had normal laboratory examinations without any abnormal data.
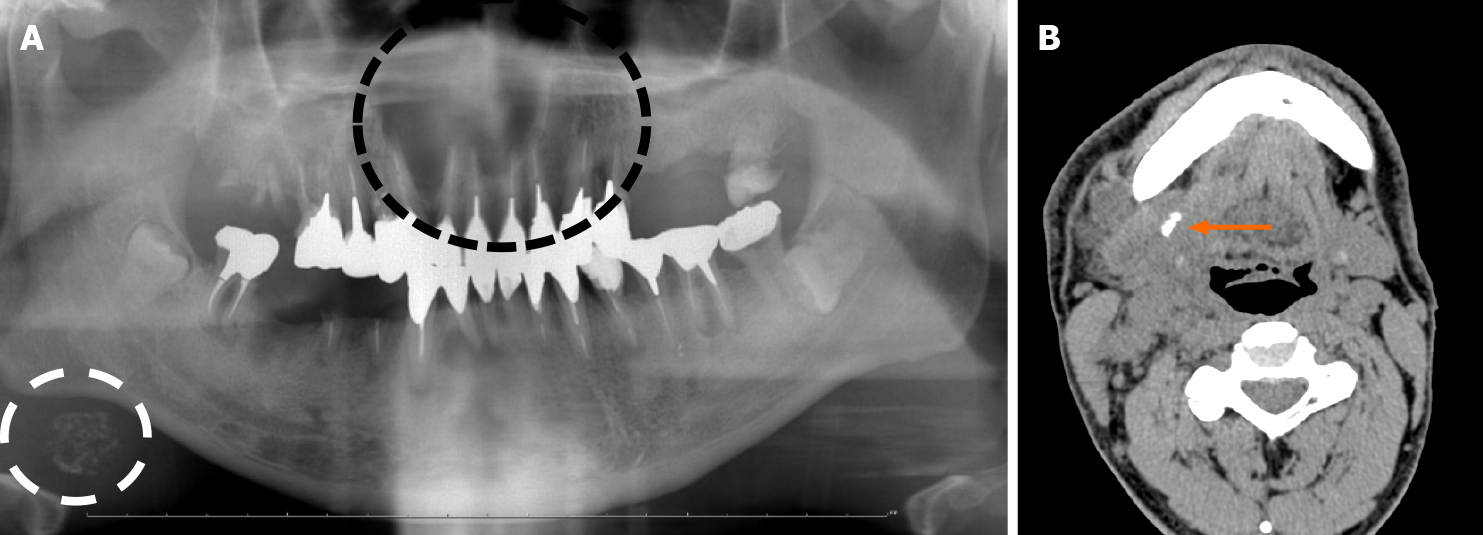
Panoramic radiographic images revealed well-defined and unilocular transmission images of the left maxillary lateral incisor to the right maxillary second premolar, as well as an oval radiopaque lesion mimicking sialolithiasis under the right side of the mandible (Figure 1A). Non-contrast computed tomography (CT) showed a similar lesion as mentioned above. The maxillary cyst was diagnosed as a radicular cyst on diagnostic imaging. A calcified body was observed near the opening of the subman-dibular gland, but not in the cervical lymph nodes (Figure 1B). It showed a mass composed of multiple small foci of calcification with a non-layer structure, which is a typical feature of sialolithiasis[7,8]. Swelling of the peripheral lymph nodes and the mass that appeared to be the submandibular gland were also observed without any symptoms at this time, although a slight palpable solid lesion existed.
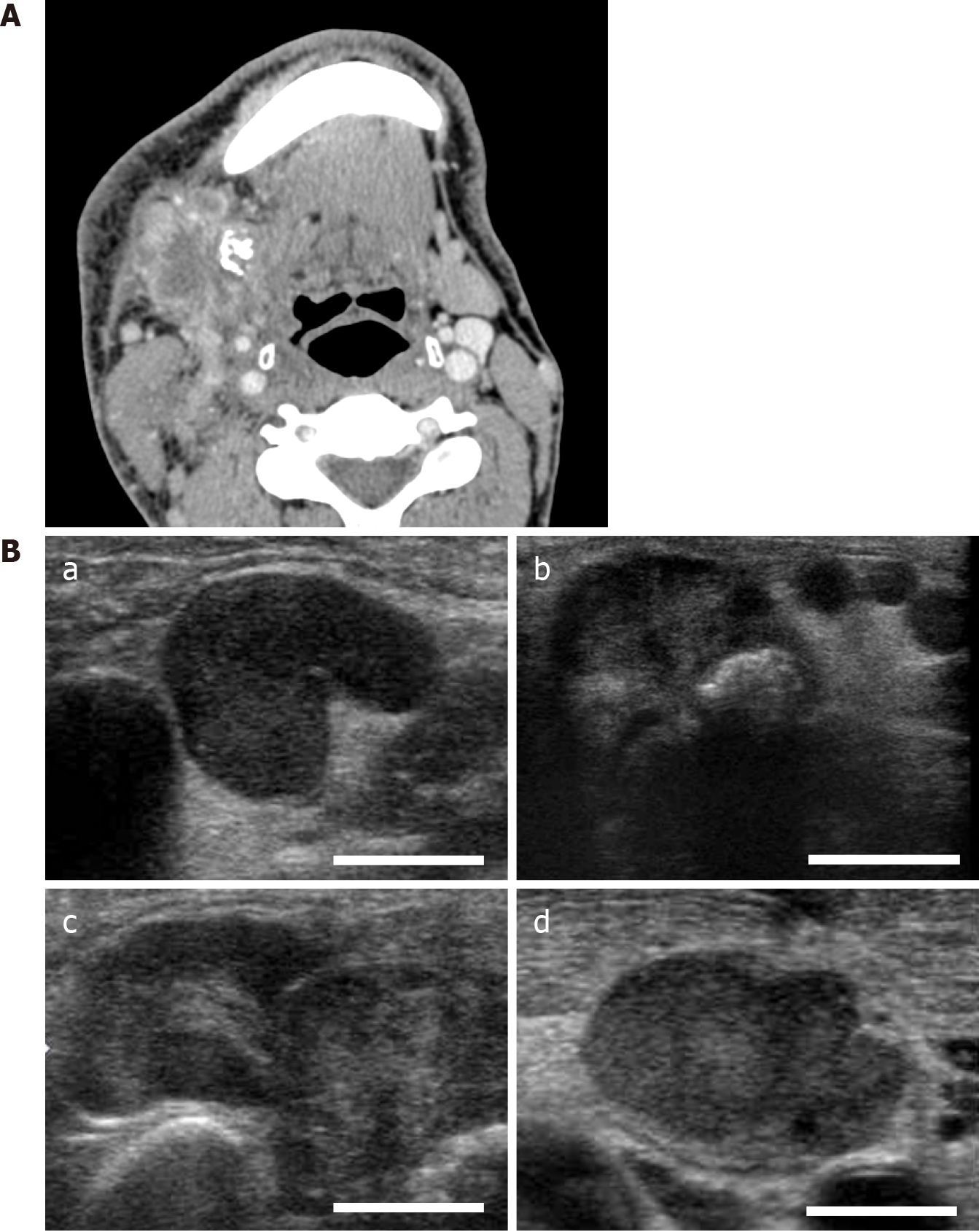
Considering the possibility of a tumor, we performed contrast-enhanced CT (CECT) and ultrasonography. CECT images revealed that the peripheral lymph nodes, existing in the submandibular lymph node, superior internal jugular node, and mid-internal jugular node, exhibited a central area of low attenuation state with rim enhancement. In addition, fusion of these lymph nodes was present at multiple sites (Figure 2A). All the affected nodes mimicked a similar pattern, suggestive of tuberculous cervical lymphadenitis, for which the typical observation mainly displays low attenuation at the center with rim enhancement; this represents the central area of necrosis, although this finding is also similar to that of metastatic lymph nodes[9,10]. Ultrasonography revealed the findings of the imaging, such as preserved oval shape, absence of peripheral halo, and internal echogenicity (Figure 2B), which is suspicious of tuber-culous cervical lymphadenitis or metastatic lymph nodes, whereas a QuantiFERON Gold blood test yielded a negative result, and there was no suspicious lesion on chest radiography. This ultrasonographic finding is rather atypical of metastatic disease (preservation of oval shape, existence of hilus in the enlarged lymph nodes, and relatively well-defined margins).
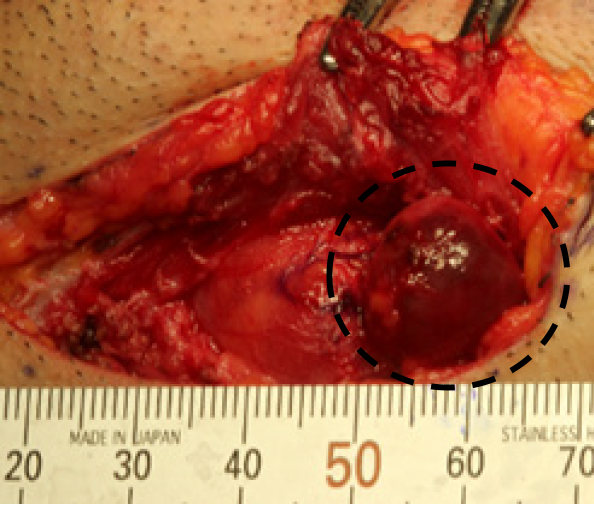
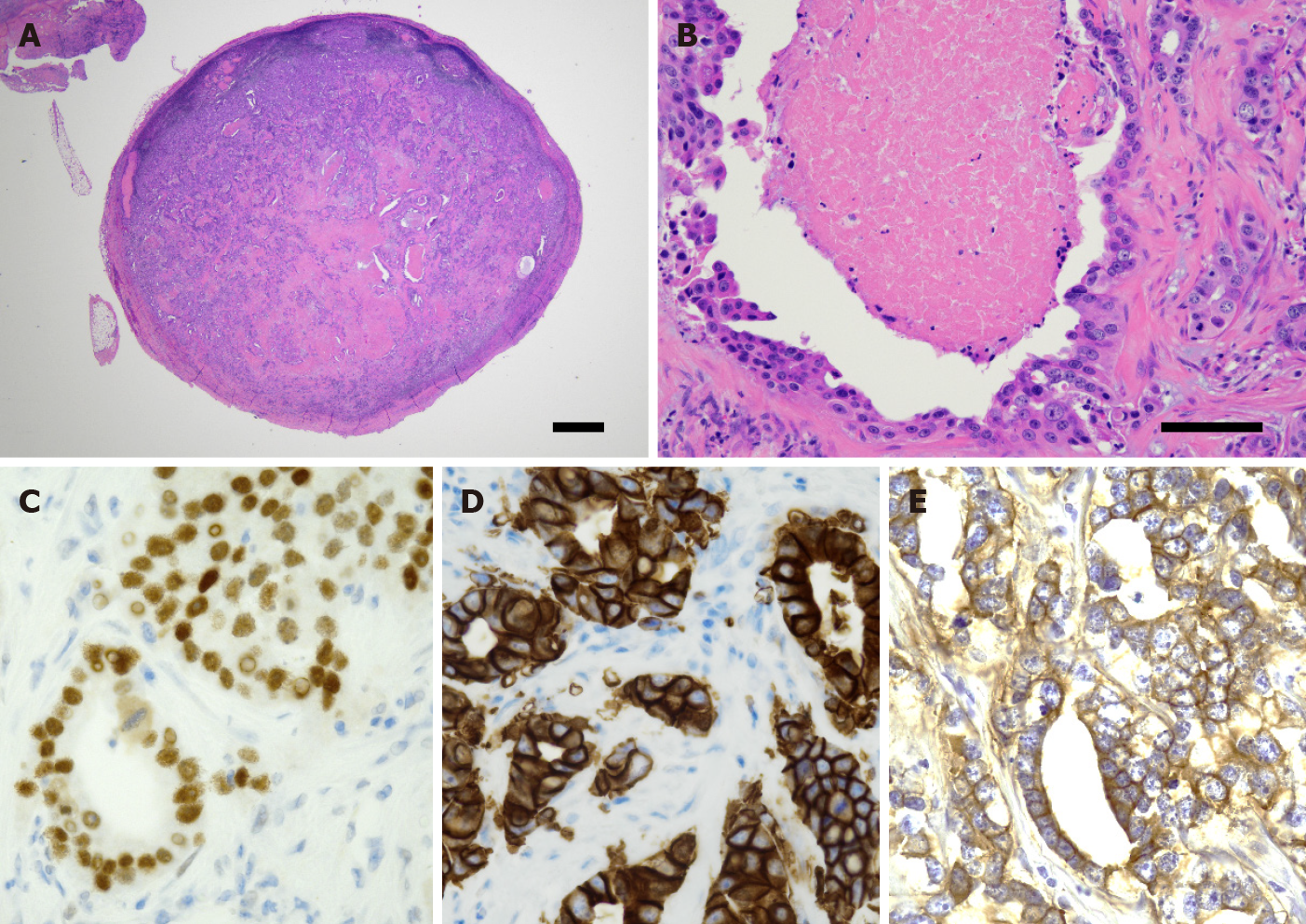
Excisional biopsy of the cervical lymph node was performed under general anesthesia to make a definitive diagnosis (Figure 3). The biopsy specimen revealed a gland-forming epithelial lesion with comedo-type necrosis inside the lymph node (Figure 4A). Higher magnification showed that the tumor cells had abundant pink cytoplasm and large pleomorphic nuclei with prominent nucleoli and coarse chromatin (Figure 4B). Tuberculous cervical lymphadenitis was not evident, and pathological diagnosis of metastatic SDC was suggested. To confirm the diagnosis, immunohistochemical staining was performed. The tumor was positive for pan-cytokeratin (AE1/AE3), cytokeratin 7, androgen receptor (AR), human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor (EGFR) (Figure 4C-E), but negative for cytokeratin 20, p63, estrogen receptor, thyroid transcription factor 1, and CDX2. 18F-fluorodeoxyglucose positron emission tomography-CT revealed no detectable tumor except for an ill-defined lesion in the submandibular salivary gland with heterogeneously increased 18F-fluorodeoxyglucose uptake.
These data suggest that the tumor of the cervical lymph node was a metastatic tumor from the submandibular gland, and the final diagnosis of metastatic SDC was achieved. This lesion was classified as cT4aN3bM0 based on the Tumor-Node-Metastasis Classification of Malignant Tumors, 8th edition.
According to the diagnosis mentioned above, the patient underwent surgery with ipsilateral radical neck dissection in addition to primary tumor resection, segmental mandibulectomy, and cystectomy, followed by radiotherapy (70 Gy). Histologically, the tumor had a similar appearance to the biopsy material. In addition, atrophic salivary gland tissue was evident adjacent to the tumor, suggesting that the tumor was arising in the salivary glands. Incidentally, the cystic lesion was diagnosed as a radicular cyst based on pathological findings; the cyst was not correlated with SDC. This primary combined treatment revealed tumor elimination based on CECT imaging.
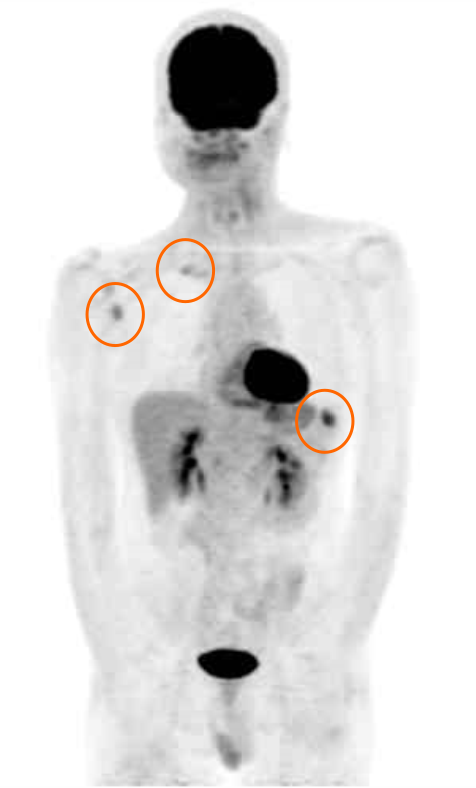
Due to the poor prognosis of this disease even after surgery and radiotherapy[1], chemotherapy with cetuximab based on EGFR-positive lesions has been reported previously[11]. Accordingly, as treatment, we provided weekly paclitaxel in addition to cetuximab for 4 wk. Nevertheless, distant bone metastases, such as the tenth rib bone, appeared (Figure 5). Weekly administration of tegafurgimeraciloteracil potassium (120 mg/m2) and cetuximab (250 mg/m2) for 4 wk, followed by drug withdrawal for 2 wk, and with injection of denosumab (120 mg) every 4 wk, was repeated. Five rounds of this cycle resulted in a partial response from the patient.
At approximately 2 years post-surgery, multiple distant metastases, including lung and bone, were observed on positron emission tomography, although primary and neck lesions had no evidence of recurrence. In the present study, after which 2 years and 7 mo have passed after primary treatment, the patient remains alive with chemotherapy using cisplatin and docetaxel.
SDC is a rare and highly aggressive type of cancer, accounting for 1%-3% of all salivary cancers, and SDC arising in the submandibular gland is even more rare[1,11]. Previous reports indicated that the major presenting symptom of SDC arising at the submandibular gland is a fixed painful or painless mass. In addition, the presence of calcification in an ill-defined salivary gland mass may be a good CT criterion that raises the possibility of SDC[12-14]. In the present case, the initial objective symptom at the submandibular grand was a painless slight palpable mass, and CT imaging showed multiple calcification lesions, which seemed to occupy the submandibular grand and mimicked sialolithiasis, with swelling of the peripheral lymph nodes.
The location, structure, and number of lesions are important elements during imaging evaluation of calcified lesions in the cervical lymph nodes or submandibular gland. Differential diagnoses include sialolithiasis, calcific substance produced by the tumor, peripheral calcification derived from tuberculosis[9], or malignant tumors, such as squamous cell carcinoma and papillary thyroid carcinoma[15]. In this case, the calcified body was coincidentally found on panoramic radiography due to the completely different chief complaint without any subjective symptoms. Although the findings of CT with or without contrast-enhancement and ultrasonography excluded the possibility of sialolithiasis or papillary thyroid carcinoma, the central area of low attenuation with rim enhancement observed in multiple cervical lymph nodes made the diagnosis difficult.
In this case, open biopsy was necessary for an early definitive diagnosis for extracting the suspected lymph node and obtaining an accurate histological analysis, especially for salivary gland cancer comprising various histological subtypes. The histology results were consistent with a diagnosis of SDC and included immuno-histochemical analyses such as EGFR, HER2, and AR, which are characteristically present in SDC[16,17].
Surgical resection with neck dissection followed by radiotherapy is often chosen for patients with SDC[11,16]. Therefore, we performed this procedure by adding various chemotherapy regimens using cetuximab, which is used to treat EGFR-positive tumors. The surgical specimen was immunohistochemically positive for HER2 and AR, indicating that trastuzumab or hormonotherapy might be effective for this lesion, although the patient strongly declined uninsured treatments. Despite the poor prognosis for patients with SDC, the patient is still alive 2 years and 7 mo after the definitive diagnosis although innumerable lymph node metastatic lesions were practically existed in the surgical specimen of the operation.
Approximately 3.5 mo elapsed from the first visit to extensive surgical resection. In this interim, the lesion seemed to exhibit rapid growth. Indeed, swelling in the submandibular region was increased at the time of the operation. In this case, the surgical specimen showed that the tumor invaded the adjacent tissue and that the “submandibular gland region” was mostly replaced with a calcified body. The findings of cervical lymph nodes on ultrasonography and CECT imaging initially confused the distinction between tuberculous cervical lymphadenitis and metastatic lesions. However, a calcified body existed in “the submandibular gland region”, rather than in the lymph nodes. Considering the high rate of calcification that occurs in SDC, especially in cases arising at the submandibular gland as indicated previously[12], we should have emphasized the malignancy.
In conclusion, radiographic and imaging characteristics of lymph node metastatic SDC may mimic those of tuberculous cervical lymphadenitis, and calcification at the submandibular gland is the key finding of SDC arising at the submandibular gland.
We thank Dr. Yasuhiro Fukuda for the histological analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozcan C S-Editor: Zhang L L-Editor: A P-Editor: Yuan YY
| 1. | Jaehne M, Roeser K, Jaekel T, Schepers JD, Albert N, Löning T. Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103:2526-2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Delgado R, Vuitch F, Albores-Saavedra J. Salivary duct carcinoma. Cancer. 1993;72:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Zainab H, Sultana A, Jahagirdar P. Denovo High Grade Salivary Duct Carcinoma: A Case Report and Review of Literature. J Clin Diagn Res. 2017;11:ZD10-ZD12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Beck ACC, Lohuis PJFM, Al-Mamgani A, Smit LA, Klop WMC. Salivary duct carcinoma: evaluation of treatment and outcome in a tertiary referral institute. Eur Arch Otorhinolaryngol. 2018;275:1885-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Ahuja A, Ying M, Evans R, King W, Metreweli C. The application of ultrasound criteria for malignancy in differentiating tuberculous cervical adenitis from metastatic nasopharyngeal carcinoma. Clin Radiol. 1995;50:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Ying M, Ahuja AT, Evans R, King W, Metreweli C. Cervical lymphadenopathy: sonographic differentiation between tuberculous nodes and nodal metastases from non-head and neck carcinomas. J Clin Ultrasound. 1998;26:383-389. [PubMed] [DOI] [Full Text] |
| 7. | Im YG, Kook MS, Kim BG, Kim JH, Park YJ, Song HJ. Characterization of a submandibular gland sialolith: micromorphology, crystalline structure, and chemical compositions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124:e13-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Nolasco P, Anjos AJ, Marques JM, Cabrita F, da Costa EC, Maurício A, Pereira MF, de Matos AP, Carvalho PA. Structure and growth of sialoliths: computed microtomography and electron microscopy investigation of 30 specimens. Microsc Microanal. 2013;19:1190-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Moon WK, Han MH, Chang KH, Im JG, Kim HJ, Sung KJ, Lee HK. CT and MR imaging of head and neck tuberculosis. Radiographics. 1997;17:391-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | King AD, Tse GM, Ahuja AT, Yuen EH, Vlantis AC, To EW, van Hasselt AC. Necrosis in metastatic neck nodes: diagnostic accuracy of CT, MR imaging, and US. Radiology. 2004;230:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Kawahara K, Hiraki A, Yoshida R, Arita H, Matsuoka Y, Yamashita T, Koga KI, Nagata M, Hirosue A, Fukuma D, Nakayama H. Salivary duct carcinoma treated with cetuximab-based targeted therapy: A case report. Mol Clin Oncol. 2017;6:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Weon YC, Park SW, Kim HJ, Jeong HS, Ko YH, Park IS, Kim ST, Baek CH, Son YI. Salivary duct carcinomas: clinical and CT and MR imaging features in 20 patients. Neuroradiology. 2012;54:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Huh KH, Heo MS, Lee SS, Choi SC. Three new cases of salivary duct carcinoma in the palate: a radiologic investigation and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hosal AS, Fan C, Barnes L, Myers EN. Salivary duct carcinoma. Otolaryngol Head Neck Surg. 2003;129:720-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Choi EC, Moon WJ, Lim YC. Case report. Tuberculous cervical lymphadenitis mimicking metastatic lymph nodes from papillary thyroid carcinoma. Br J Radiol. 2009;82:e208-e211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Fan CY, Melhem MF, Hosal AS, Grandis JR, Barnes EL. Expression of androgen receptor, epidermal growth factor receptor, and transforming growth factor alpha in salivary duct carcinoma. Arch Otolaryngol Head Neck Surg. 2001;127:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. 2010;16:2266-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |