Published online Apr 26, 2021. doi: 10.12998/wjcc.v9.i12.2854
Peer-review started: November 19, 2020
First decision: January 7, 2021
Revised: January 13, 2021
Accepted: February 23, 2021
Article in press: February 23, 2021
Published online: April 26, 2021
Processing time: 146 Days and 16.3 Hours
Behcet’s disease (BD) is a chronic disease characterized by oral and vulvar ulcers as well as eye and skin damage and involves multiple systems. It presents as an alternating process of repeated attacks and remissions. Esophageal venous rupture and bleeding caused by BD is rarely reported at home and abroad. This paper reports a case of bleeding from oesophageal varices caused by BD, aiming to provide an additional dimension for considering the cause of bleeding from esophageal varices in the future.
A 38-year-old female patient was admitted due to a gradual increase in shortness of breath and chest tightness after the activity, and was admitted to our hospital for treatment. After admission, relevant examinations showed that the patient had multiple blood clots. Four days after admission, she suddenly experienced massive hematemesis. Emergency esophagogastroduodenoscopy revealed bleeding from esophageal and gastric varices. The patient had no history of viral hepatitis or drinking habits, and no history of special genetic diseases or congenital vascular diseases. There is no obvious abnormality in liver function. After reviewing the medical history, it was found that the patient had recurred oral ulcers since childhood, ulcers were visible in the perineum during menstruation, and there was an intermittent red nodular rash and uveitis. The current skin acupuncture reaction is positive, combined with the evaluation of the external hospital and our hospital, the main diagnosis is BD. She received methylprednisolone, cyclophosphamide, immunomodulation, acid suppression, gastric protection, and anticoagulation and anti-infection treatments, and was discharged from the hospital. During the 1-year follow-up period, the patient did not vomit blood again.
This case highlights bleeding from esophageal varices caused by BD, aiming to provide an additional dimension concerning the cause of bleeding from esophageal varices in the future.
Core Tip: Esophageal venous rupture and bleeding caused by Behcet’s disease is rarely reported at home and abroad. This article reports a case of portal hypertension caused by Behcet’s disease and rupture of esophageal varices, which can provide some help for clinical diagnosis and treatment.
- Citation: Xie WX, Jiang HT, Shi GQ, Yang LN, Wang H. Behcet’s disease manifesting as esophageal variceal bleeding: A case report. World J Clin Cases 2021; 9(12): 2854-2861
- URL: https://www.wjgnet.com/2307-8960/full/v9/i12/2854.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i12.2854
Behcet’s disease (BD) is a chronic disease characterized by oral and vulvar ulcers as well as eye and skin damage and involves multiple systems. It presents as an alternating process of repeated attacks and remissions. It is a type of vasculitis. It can cause venous and arterial blockage and aneurysm formation; when combined with gastrointestinal ulcers, bleeding, perforation, etc., it is considered the gastrointestinal type, but it is often missed or misdiagnosed due to the lack of specificity. BD causes rupture of esophageal varices, and hemorrhage is rarely been reported at home or abroad. This paper reports a case of bleeding from esophageal varices caused by BD, aiming to provide an additional dimension concerning the cause of bleeding from esophageal varices in the future.
A 38-year-old female patient had chest tightness and shortness of breath after repeated activities for 3 mo, which then worsened for 7 d.
The patient’s symptoms started as chest tightness and shortness of breath for 3 mo after repeated activities that were then aggravated for 7 d.
More than 1 year ago, the patient went to another hospital due to a “left neck mass and abdominal discomfort” and underwent related examinations including esophagogastroduodenoscopy, which revealed “pseudoaneurysm and portal hypertensive gastropathy”, but the cause was not identified. A left carotid artery stent was implanted, and aspirin and clopidogrel were taken regularly after the operation. She went to another hospital for “lower limb thrombosis” 7 mo ago and underwent “right inguinal saphenous vein transplantation” (specificity unknown). Three months ago, due to hemorrhage of large blood vessels in the abdominal cavity, she underwent "exploratory laparotomy", and a balloon was inserted (specifically unknown). She denied a history of bad habits of smoking and alcohol consumption, hepatitis, or family genetic diseases.
The patient’s personal history and family history are nothing special.
The patient's vital signs were within the normal range, with clear mind, body check-up cooperation, and mild facial edema. The jugular vein was irritated. Double lung breathing sound was clear, unheard, and wet. The heart rhythm was homogeneous, the heart bound was normal, and the third and fourth ribs of the left edge of the sternum were heard. An about 10 cm surgical scar in the middle of the abdomen and an about 3 cm surgical scar in the bilateral groin were seen. The patient had a flat abdomen with slight compression pain, no obvious backbeat pain, no weakening or enhancement of intestinal sound (3 points per minute), and no liver touch. She had mild edema of both lower limbs.
Blood analysis revealed the following: Hemoglobin, 104 g/L; platelets, 57 × 109 g/L; D-dimer, 1.8 μg/mL; platelet antibody test, positive; and C-reactive protein, 92.5 mg/L. Hepatitis B and C virus tests and antinuclear antibody spectrum showed no abnormalities. The patient's liver function, kidney function, coagulation function, blood sugar, blood lipids, and other tests showed no obvious abnormalities.
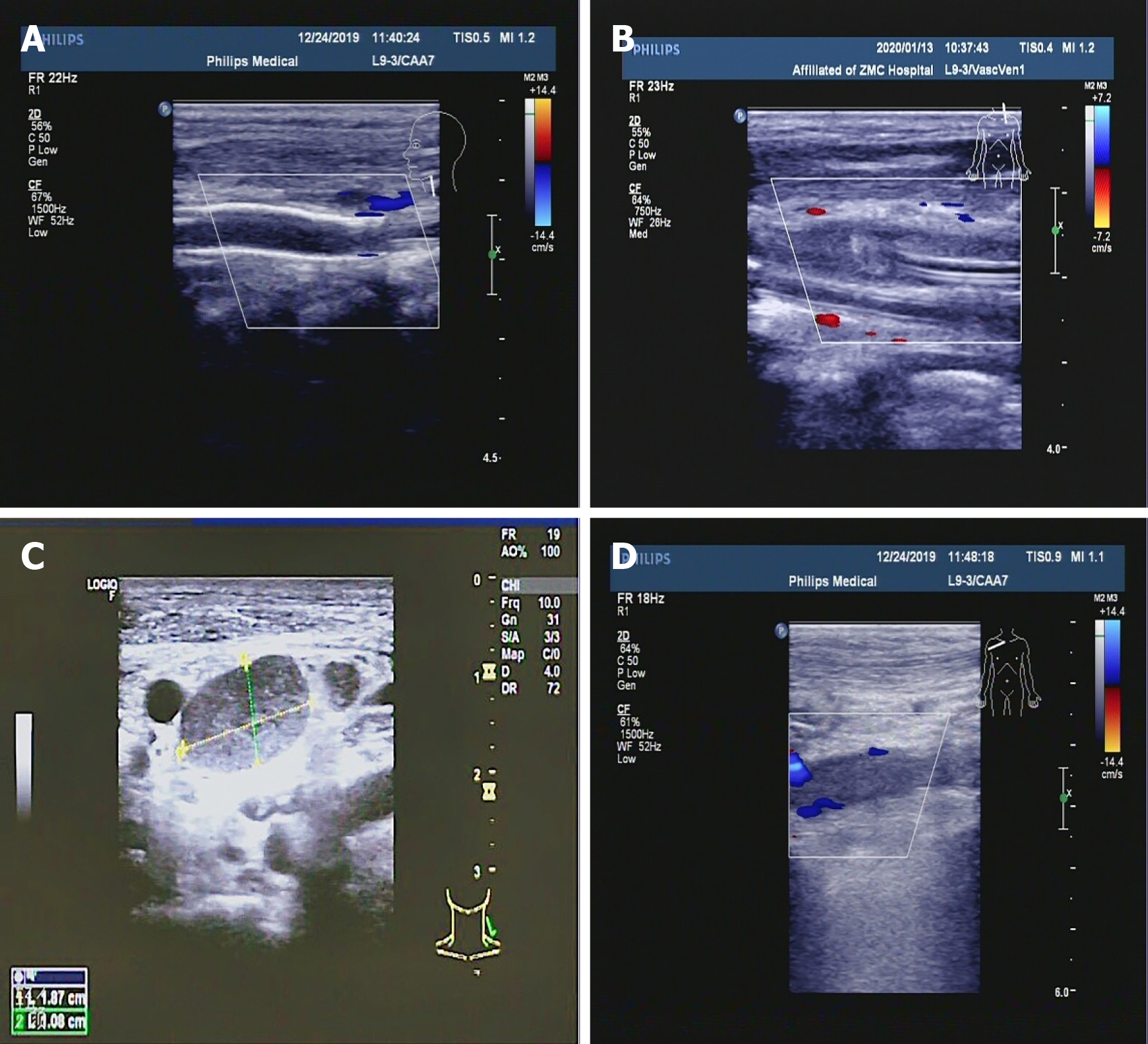
Electrocardiography revealed sinus tachycardia and incomplete right bundle branch block. Chest computed tomography (CT) revealed a small amount of pneumonia and fibrosis in the lower lobes of the lungs, as well as some atelectasis in the lower lobes of the lungs. Heart color Doppler ultrasound at rest demonstrated moderate to severe tricuspid regurgitation; thickening and contracture of the anterior tricuspid valve; mild stenosis of the tricuspid valve; enlarged right ventricle and right atrium; thickened ventricular wall; and abnormal muscle bundles. Bilateral jugular artery and vein color Doppler ultrasound revealed postoperative left carotid artery stent occlusion; left common carotid artery near-mid subtotal occlusion; and right internal jugular vein near heart thrombosis and partial recanalization. Bilateral upper extremity artery and vein color Doppler ultrasound showed that the right subclavian vein, axillary vein, and axillary vein near the heart thrombosis were partially recanalized, and the remaining blood vessels were not abnormal. Arteriovenous color Doppler ultrasound of both lower extremities revealed thrombosis of the left common femoral artery, bilateral common femoral veins, and proximal femoral vein and proximal deep femoral vein thrombosis and partial recanalization. CT of the whole abdomen revealed splenomegaly, as well as a small amount of fluid in the abdominal cavity. The inferior vena cava filter was present. Abdominal angiography showed embolism of the portal vein, splenic vein, and superior mesenteric vein, formation of collateral circulation, and esophageal and gastric fundus varices. After the inferior vena cava filter was implanted, the lumen was significantly narrowed or occluded. Dual-source CT of the pulmonary artery showed that there was no obvious abnormality on pulmonary artery angiography. Limb angiography demonstrated that the aneurysm at the beginning of the left common iliac artery at the lower end of the abdominal aorta was enlarged, the left external iliac artery was narrowed and dilated to varying degrees, and emboli formed at the distal end of the left external iliac artery and the beginning of the femoral artery. The femoral artery of the right external iliac artery and the deep femoral artery were occluded.
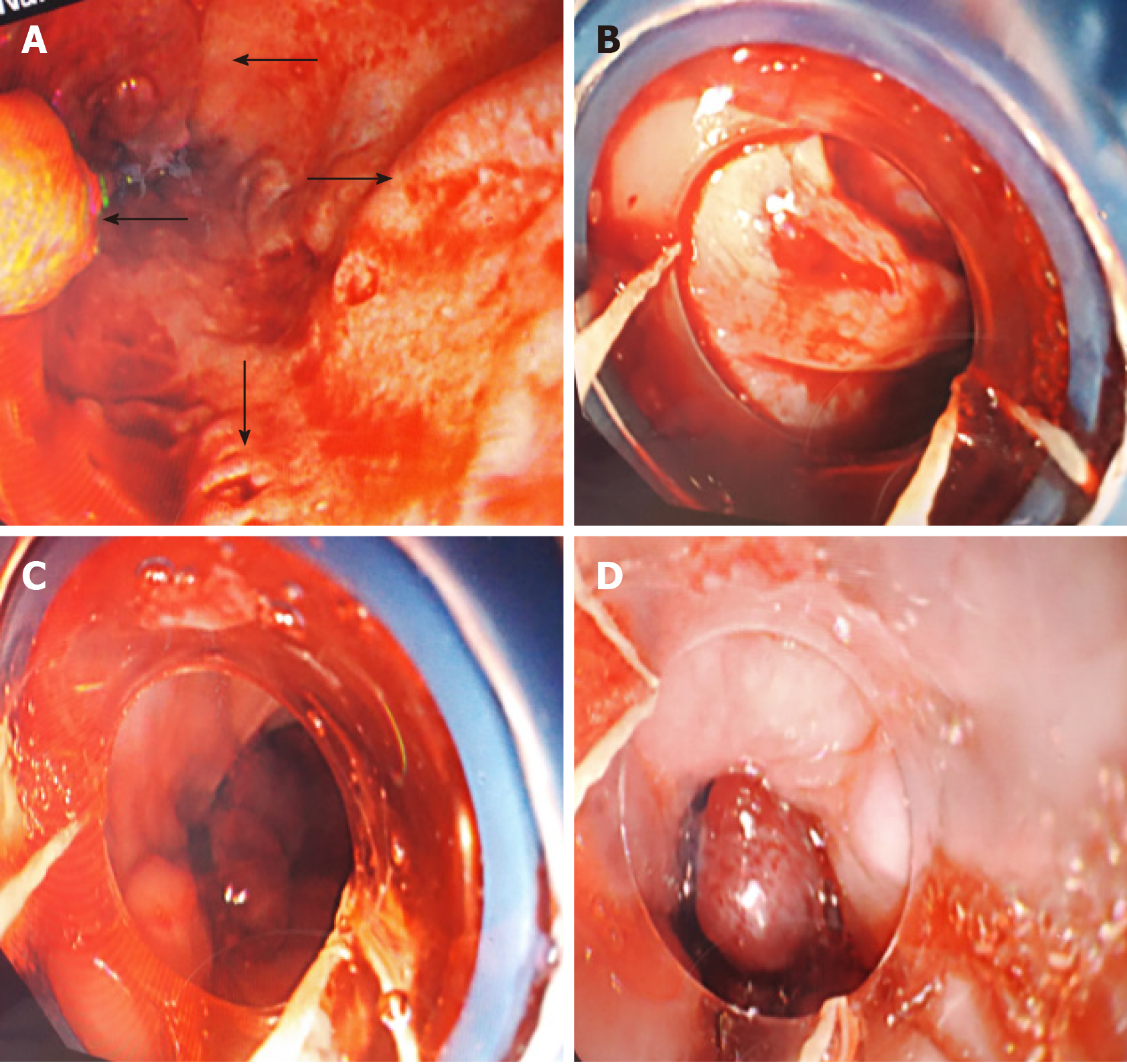
After 4 d of hospitalization, the patient suddenly vomited blood with a volume of approximately 300 mL. She underwent emergency esophagogastroduodenoscopy to stop the bleeding from ruptured esophageal varices. Four severe esophageal varices were seen during the operation, and rupture was seen (Figure 1). The bleeding stopped after surgery. A large amount of fresh blood and blood clots were seen in the stomach, and the observation was unclear. There were no varicose veins or bleeding found in the fundus of the stomach near the cardia (Figure 2).
The history of the disease indicated that oral ulcers, a nodular red skin rash, ocular uveitis, and ulcers can be seen in the perineum during menstruation. The skin acupuncture reaction was positive, and combined with the external hospital examination and our hospital examination, the main diagnosis was BD.
Combining multiple aneurysm-like changes throughout the body, multiple venous collateral circulation, and heart disease changes throughout the body, the imaging doctor first considered "BD"; the nephrologist inquired the medical history and found that the patient had oral ulcers in the past. Pussy ulcers and eye damage included uveitis and frequent herpes on lower limbs; combined with a positive acupuncture test, BD can be clearly diagnosed. The hematologist also agreed to diagnose the disease and recommended anticoagulation treatment; the gastroenterologist believed that the patient’s gastrointestinal bleeding was a secondary disease, and the primary disease should be actively treated first.
The final diagnosis of the presented case was BD.
After treatment with methylprednisolone, cyclophosphamide, acid suppression, stomach protection, anticoagulants, anti-infection treatment, etc., the patient was discharged from the hospital.
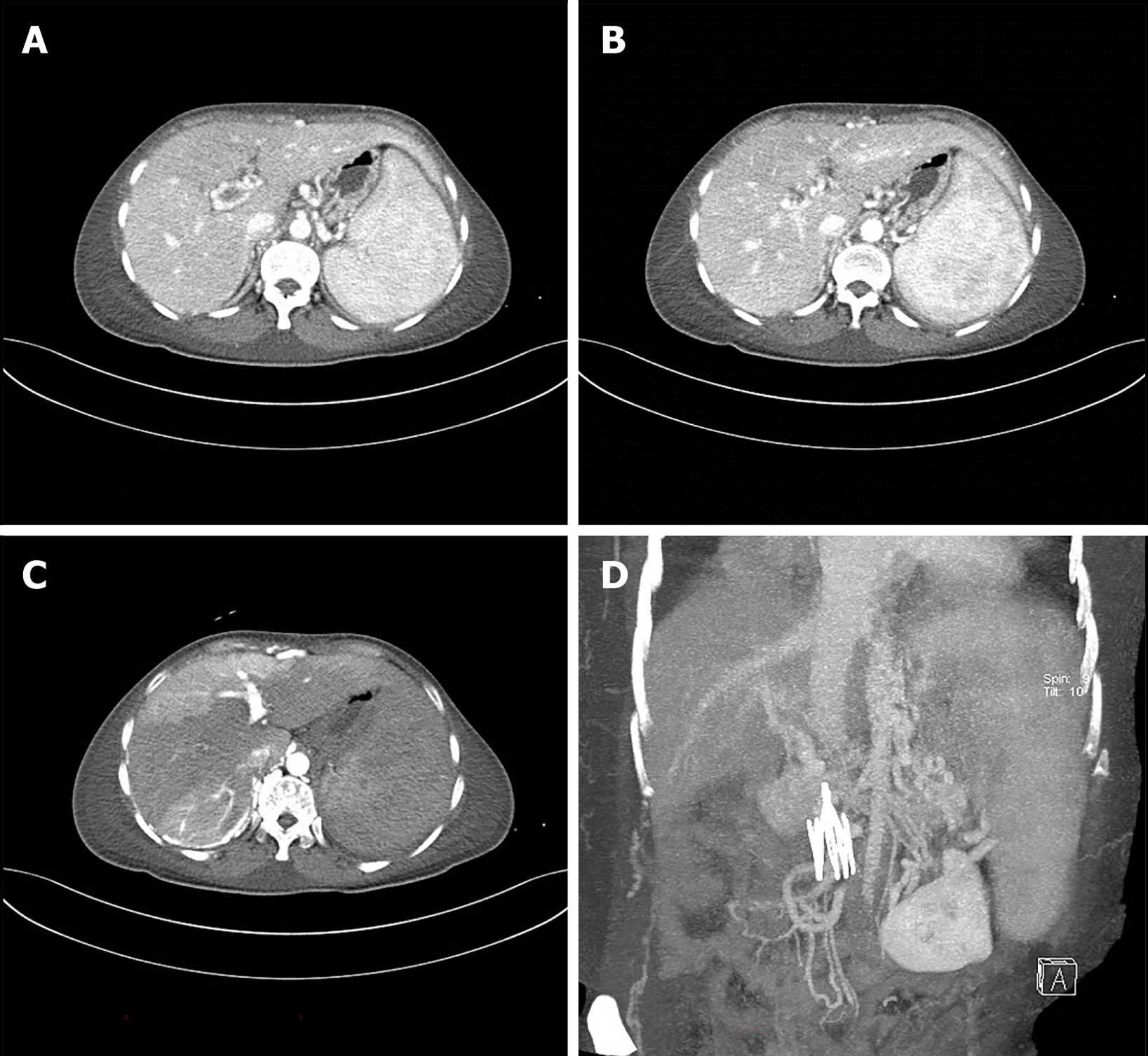
This patient is a young woman with a chronic course of disease and multiple thromboses throughout the body. Portal hypertensive gastropathy was found in the past but was not taken seriously. Hematemesis occurred during hospitalization. She underwent emergency band ligation hemostasis for bleeding from esophageal varices under esophagogastroduodenoscopy. CT of the abdomen showed a clear liver contour, no localized nodular hyperplasia, portal vein, splenic vein, and superior mesenteric vein thrombosis, collateral circulation formation, and esophageal and gastric fundus varices. The liver was not perfused uniformly due to portal vein thrombosis, and there was no obstructive disease of the inferior vena cava (Figure 3). Portal hypertension (PH) was considered to be caused by portal vein thrombosis, which led to rupture and bleeding of esophageal varices. The postoperative medical history was followed, and there were repeated oral and vulvar ulcers and ocular uveitis, multiple blood clots throughout the body, and positive acupuncture reactions. According to the diagnostic criteria for BD[1], the diagnosis was “BD”. The patient was in critical condition, so no liver biopsy was performed. She was treated with methylprednisolone, cyclophosphamide, immune adjustment, acid suppression, stomach protection, anticoagulation, and anti-infection treatment and was discharged from the hospital. I was planning to review the esophagogastroduodenoscopy one month later, but the patient refused to undergo esophagogastroduodenoscopy due to poor cardiac function. After 1 year of follow-up, she did not vomit blood again. Due to the patient's multiple thromboses, general condition, and poor cardiac function, the gastroscopy and abdominal CT results were not reviewed; she visited the doctor for edema, abdominal distension, and anorexia many times. The mean echo was filled, the umbilical vein was open, and this indicates PH and the formation of lateral branch circulation. The patient is now regularly using infliximab, methylprednisolone, pantoprazole, rivaroxaban, and methoxazole to control the disease.
BD is a systemic immune system disease characterized by repeated oral and vulvar ulcers as well as eye and skin damage and is a type of vasculitis. Most women in China with this disease are predominantly between the ages of 16 and 40[2]. The cause is unknown and may be related to genetic factors and pathogen infection. BD has strong regional specificity and ethnic differences, with a high incidence in China, Japan, the Middle East, and the Mediterranean region[3], and it is also known as the "silk road disease". It is a rare disease. According to surveys[4], in North American and European countries, 1 out of every 15000 to 500000 people suffers from BD. The pathological manifestations are inflammatory cell infiltration around the blood vessels. In severe cases, there is necrosis of the vascular wall. Large, medium, small, and micro vessels (arteries and veins) can be affected, with luminal stenosis and aneurysmal changes. The disease is divided into gastrointestinal type, vascular type, nerve type, etc., according to the damage to internal organs. The vascular type refers to those with involvement of large and middle arteries and/or veins; the nerve type refers to those with central or peripheral nerve involvement; and the gastrointestinal type refers to those with gastrointestinal ulcers, bleeding, perforation, etc. In my country, women account for the majority of cases, but male patients with uveitis and visceral involvement are 3 to 4 times more prevalent than female patients. BD with cardiac lesion involvement is relatively rare, the incidence of which is reported to be 0.5%-8.1%[5], mainly involving valves, the myocardium, the conduction system, coronary arteries, the pericardium, wall thrombus, aneurysms, and so on.
The gastrointestinal type appears in many patients with episodes. According to the frequency of symptoms, abdominal pain is the most common symptom, and right lower abdominal pain is common, accompanied by local tenderness and rebound pain, followed by nausea, vomiting, abdominal distension, anorexia, diarrhea, and dysphagia. The basic pathology of the digestive tract is multiple ulcers, which can be seen in any part from the esophagus to the descending colon, and the incidence can be as high as 50%. Severe cases involve complications such as ulcer bleeding, intestinal paralysis, intestinal perforation, peritonitis, fistula formation, esophageal stenosis, and even death. Ulcers caused by intestinal BD can appear in the whole digestive tract, and the typical part of the common occurrence is the ileocecal part[6,7]. Endoscopic ulcers are scattered, present as multiple or single lesions, are mostly lateral, and are often located on the opposite side of the mesentery. Ulcer margins are more regular, diffuse invasion is rare, and the morphology can be roughly divided into three types: Volcanic type, map type, and aphthous ulcer type[8]. However, due to the impact of various factors, such as the stage of the lesion, the severity of the biopsy, the depth of the biopsy, the specimen preparation process, and other factors, many lesions do not show the typical pathological manifestations but show nonspecific chronic inflammatory changes, which are related to inflammatory bowel diseases (Crohn's disease and ulcerative colitis), and intestinal tuberculosis is difficult to distinguish. According to the endoscopic manifestations, although the two diseases have their own characteristics, such as longitudinal ulcers and fissure-like, paving stone-like changes in Crohn's disease, BD is often confined to the ileocecal part of single or multiple ulcers, and ulcers can be crater, map-like and mouth sore-like[9].
Studies have found that[10] vascular types involving large blood vessels mainly include venous obstruction, arterial obstruction, and aneurysm formation, and the proportion of involvement is 5.6% to 6.3%. In China, men with BD are more likely to develop macrovascular disease. Chinese case data suggest that BD has a large vascular disease component of approximately 12.8%, with a male-to-female ratio of 3.86:1 and an average age of onset of 29.5 ± 11.3 years; 70.6% of cases show venous involvement. In venous disease, deep vein thrombosis of the lower limbs is the most common feature, followed by thrombosis of the inferior vena cava, superior vena cava, and cerebral venous sinus[11]. Vascular lesions mostly occur within 1 year of the initial symptoms[12]. Studies have shown that damage to endothelial function may be the main cause of thrombosis in patients with BD. Endothelial damage leads to decreased vascular smoothness and blood flow stasis. The production of anti-endothelial cell antibodies further aggravates vascular damage. At the same time, the dysfunction of vascular endothelial cells leads to an increase in the secretion of various tissue factors and adhesion molecules and induces platelet activation and aggregation, which is also a factor in patients with BD[13].
This patient was a woman who was admitted to the hospital due to chest tightness and shortness of breath. The symptomatic correction of heart failure was not effective. The imaging data showed multiple thromboses throughout the body. The medical history showed repeated occurrence of oral and genital ulcers since childhood. The acupuncture test was positive. According to the ICBD[4] criteria, this case involved oral and genital lesions and positive combined acupuncture tests, with a score greater than 3, so the clear diagnosis was "BD". Sudden hematemesis during hospitalization and emergency gastroscopy showed rupture of esophageal varices, and abdominal CT showed PH. The patient had no basis for hepatitis or cirrhosis, no drinking habits, and no congenital vascular malformations or exclusion of external pressure caused by PH. PH was clearly defined and caused by portal vein thrombosis, which was caused by BD. Tavakkoli et al[14] once reported a case of "downhill" esophageal variceal bleeding caused by Behcet's superior vena cava syndrome.
PH is a clinical disease that is a comprehensive clinical manifestation of portal venous blood circulation caused by various causes of portal venous blood circulation. All types can cause portal vein blood flow disorder and/or increase blood flow and can lead to PH. Liver cirrhosis is the main cause, followed by schistosomiasis (developing countries), portal and splenic vein thrombosis, Budd-Chiari syndrome, and, less commonly, pre-sinus or post-sinus obstructive diseases. Studies have found that the factors of venous thrombosis include blood hypercoagulability, hemodynamic changes, and vascular endothelial injury. The above factors can occur separately or simultaneously due to different causes[15]. Studies have shown that portal vein thrombosis is caused by a variety of causes and risk factors, of which cirrhosis is the most common cause[16]. Other causes include the following: (1) Portal vein thrombosis after splenectomy or splenic embolization. Surgical splenectomy or splenic artery embolization are commonly used to improve hypersplenism, but splenectomy may lead to severe portal vein thrombosis[17]. The diameter of the splenic vein can be an important predictor of postoperative PVT formation[18,19]; and (2) portal vein thrombosis is related to non-chronic liver disease and non-malignant tumors, and non-neoplastic, non-chronic liver disease causing portal vein thrombosis is mostly related to systemic procoagulant factors and local risk factors[15]. This patient's PH was caused by portal vein thrombosis, which was caused by BD. Liver function was basically normal. It was not related to portal vein thrombosis after splenectomy or splenic embolization, nor was it related to portal vein thrombosis after splenectomy or tumor-related portal vein thrombosis. Vasculitis causes portal vein thrombosis, which causes PH and further leads to bleeding from esophageal varices. It is rarely reported that BD causes esophageal variceal rupture and bleeding. Analysis of the diagnosis and treatment process of this patient can provide one more possibility for the etiology of PH.
BD can involve multiple blood vessels throughout the body and may only manifest as oral or vulvar ulcers in the early stage; thus, it is easily ignored, and the optimal time for diagnosis and treatment can be missed. Bleeding due to esophageal variceal rupture caused by BD is rare and easy to ignore and misdiagnose in clinical work. Therefore, for unexplained portal hypertensive gastropathy and esophageal varices with aneurysms or multiple vascular lesions, we should be vigilant in determining whether BD is present. Once diagnosed, immunotherapy is the main focus for achieving early detection, early diagnosis, and early treatment.
I would like to express my sincere gratitude to the doctors who helped the patient and the people who helped me in the process of writing this article.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang CH, Pelletier AL S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet. 1990;335:1078-1080. [PubMed] |
| 2. | Zhang D, Zhang Y, Wang Z, Zhang X, Sheng M. Target radiofrequency combined with collagenase chemonucleolysis in the treatment of lumbar intervertebral disc herniation. Int J Clin Exp Med. 2015;8:526-532. [PubMed] |
| 3. | Sakane T, Takeno M, Suzuki N, Inaba G. Behçet's disease. N Engl J Med. 1999;341:1284-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1225] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 4. | Davatchi F, Sadeghi Abdollahi B, Chams-Davatchi C, Shahram F, Shams H, Nadji A, Faezi T, Akhlaghi M, Ghodsi Z, Mohtasham N, Ashofteh F. The saga of diagnostic/classification criteria in Behcet's disease. Int J Rheum Dis. 2015;18:594-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Zhang Z, He F, Shi Y. Behcet's disease seen in China: analysis of 334 cases. Rheumatol Int. 2013;33:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Fujiwara S, Shimizu I, Ishikawa M, Uehara K, Yamamoto H, Okazaki M, Horie T, Iuchi A, Ito S. Intestinal Behcet's disease with esophageal ulcers and colonic longitudinal ulcers. World J Gastroenterol. 2006;12:2622-2624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Hisamatsu T, Naganuma M, Matsuoka K, Kanai T. Diagnosis and management of intestinal Behçet's disease. Clin J Gastroenterol. 2014;7:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Wu QJ, Zhang FC, Zhang X. Adamantiades-Behcet's disease-complicated gastroenteropathy. World J Gastroenterol. 2012;18:609-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Lee CR, Kim WH, Cho YS, Kim MH, Kim JH, Park IS, Bang D. Colonoscopic findings in intestinal Behçet's disease. Inflamm Bowel Dis. 2001;7:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Chae EJ, Do KH, Seo JB, Park SH, Kang JW, Jang YM, Lee JS, Song JW, Song KS, Lee JH, Kim AY, Lim TH. Radiologic and clinical findings of Behçet disease: comprehensive review of multisystemic involvement. Radiographics. 2008;28:e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Fei Y, Li X, Lin S, Song X, Wu Q, Zhu Y, Gao X, Zhang W, Zhao Y, Zeng X, Zhang F. Major vascular involvement in Behçet's disease: a retrospective study of 796 patients. Clin Rheumatol. 2013;32:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Desbois AC, Wechsler B, Cluzel P, Helft G, Boutin D, Piette JC, Cacoub P, Saadoun D. [Cardiovascular involvement in Behçet's disease]. Rev Med Interne. 2014;35:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Emmi G, Silvestri E, Squatrito D, Amedei A, Niccolai E, D'Elios MM, Della Bella C, Grassi A, Becatti M, Fiorillo C, Emmi L, Vaglio A, Prisco D. Thrombosis in vasculitis: from pathogenesis to treatment. Thromb J. 2015;13:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Tavakkoli H, Asadi M, Haghighi M, Esmaeili A. Therapeutic approach to "downhill" esophageal varices bleeding due to superior vena cava syndrome in Behcet's disease: a case report. BMC Gastroenterol. 2006;6:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Battistelli S, Coratti F, Gori T. Porto-spleno-mesenteric venous thrombosis. Int Angiol. 2011;30:1-11. [PubMed] |
| 16. | Okuda K, Ohnishi K, Kimura K, Matsutani S, Sumida M, Goto N, Musha H, Takashi M, Suzuki N, Shinagawa T. Incidence of portal vein thrombosis in liver cirrhosis. An angiographic study in 708 patients. Gastroenterology. 1985;89:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 246] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Qi X, Han G, Ye C, Zhang Y, Dai J, Peng Y, Deng H, Li J, Hou F, Ning Z, Zhao J, Zhang X, Wang R, Guo X. Splenectomy Causes 10-Fold Increased Risk of Portal Venous System Thrombosis in Liver Cirrhosis Patients. Med Sci Monit. 2016;22:2528-2550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Kuroki T, Kitasato A, Tokunaga T, Takeshita H, Taniguchi K, Maeda S, Fujioka H. Predictors of portal and splenic vein thrombosis after laparoscopic splenectomy: a retrospective analysis of a single-center experience. Surg Today. 2018;48:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Danno K, Ikeda M, Sekimoto M, Sugimoto T, Takemasa I, Yamamoto H, Doki Y, Monden M, Mori M. Diameter of splenic vein is a risk factor for portal or splenic vein thrombosis after laparoscopic splenectomy. Surgery. 2009;145:457-64; discussion 465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |