Published online Apr 26, 2021. doi: 10.12998/wjcc.v9.i12.2816
Peer-review started: September 14, 2020
First decision: December 24, 2020
Revised: January 2, 2021
Accepted: February 26, 2021
Article in press: February 26, 2021
Published online: April 26, 2021
Processing time: 213 Days and 1.3 Hours
Coronavirus disease 2019 (COVID-19) has spread around the globe. On February 28, 2020, the World Health Organization adjusted the risk of spread and impact of COVID-19 to “very high” at the global level. Studies have mainly focused on the etiology, epidemiology, and treatment of COVID-19 to limit further spread and the negative impact of the disease, while less attention has been devoted to the follow-up and reexamination of patients who recovered from COVID-19 or were released from quarantine.
This study reports two cases where patients who had negative reverse transcription-polymerase chain reaction (RT-PCR) test results and met the criteria for discharge subsequently had positive RT-PCR test results. The clinical manifestations and computed tomography (CT) findings of these patients were examined. The conversion of RT-PCR test results in these two patients may be related to false-negative and false-positive outcomes of the test. CT images helped track improvement of pulmonary lesions.
The timing of discharge of COVID-19 patients should be determined by comprehensive analysis of CT images and RT-PCR test results.
Core Tip: Current research on coronavirus disease 2019 (COVID-19) mainly focuses on limiting further spread and the negative impact of the disease, while less attention has been devoted to the follow-up and reexamination of patients who have recovered from COVID-19. We present two cases of COVID-19 that recovered from the disease but tested positive again after discharge. This reminds us to be vigilant about false-negative and false-positive reverse transcription-polymerase chain reaction results. Once the patient is discharged, we should focus on his/her follow-up, isolation, and retesting.
- Citation: Huang KX, He C, Yang YL, Huang D, Jiang ZX, Li BG, Liu H. Positive reverse transcription-polymerase chain reaction assay results in patients recovered from COVID-19: Report of two cases. World J Clin Cases 2021; 9(12): 2816-2822
- URL: https://www.wjgnet.com/2307-8960/full/v9/i12/2816.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i12.2816
Since the coronavirus disease 2019 (COVID-19) outbreak, the World Health Organization (WHO) raised the risk level of the global spread of COVID-19 to “very high” on February 28, 2020. As of December 13, 2020, more than 70 million confirmed cases have been reported worldwide. COVID-19 has resulted in more deaths than severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome combined, despite having a lower-case fatality rate[1].
Published studies have mainly focused on the etiology, epidemiology, and treatment of COVID-19 to limit further spread and the negative impact of the disease, while less attention has been devoted to the follow-up and reexamination of patients who recovered from COVID-19 or were released from quarantine. We herein report two patients whose reverse transcription-polymerase chain reaction (RT-PCR) assay results were positive after they had recovered from COVID-19.
Case 1: On January 23, 2020, a 58-year-old woman went to Guiyang Public Health Center for a medical evaluation because of fever (Figure 1).
Case 2: A 16-year-old male adolescent developed a fever (his highest body temperature was 39 °C), so he presented for medical care (Figure 1). Chest CT showed bilateral patchy opacities, especially in the right lower lobe.
Case 1: The patient with a history of hypertension who works and lives in Wuhan, Hubei Province returned to her hometown in Guizhou Province on January 22, 2020. Two days prior to arriving in Guizhou, she had developed a cough.
Case 2: The patient lived in Guizhou Province. During the period of 5 d before January 15, 2020, he lived with two relatives from Yichang, Hubei Province. The patient developed an intermittent productive cough on January 27, 2020, accompanied by fatigue and loss of appetite.
Case 1: Physical examination revealed a mild fever (37.5 °C).
Case 2: Physical examination revealed a moderate fever (39 °C).
Case 1: Laboratory examination showed a normal leukocyte count (5.8 × 109/L), normal percentage of lymphocytes (25.9%), elevated C-reactive protein (15.0 mg/L), increased erythrocyte sedimentation rate (60 mm/h), and decreased CD4/CD8 ratio (0.76). The first throat swab RT-PCR assay for SARS coronavirus 2 (SARS-CoV-2) was negative, and the second throat swab was positive.
Case 2: The laboratory examination indicated an increased leukocyte count (11.6 × 109/L), decreased percentage of lymphocytes (14.4%), increased percentage of neutrophils (81.2%), elevated C-reactive protein (5 mg/L), and increased erythrocyte sedimentation rate (36 mm/h). Mycoplasma pneumoniae antibody [immunoglobulin M (IgM) was positive, and respiratory syncytial virus antibody (IgM) was positive. His throat swab RT-PCR assay for SARS-CoV-2 was positive on February 2, 2020.
UCT 510 (United-Imaging; Shanghai, China) was used for all CT scans. Both patients were scanned from the thoracic inlet to the level of the costophrenic angle in the supine position. The scanning parameters were set as follows: Tube voltage, 120 kVp; automatic tube current; modulation matrix, 512 × 512; slice thickness, 8 mm; field of view, 313 mm × 313 mm; spacing between slices, 8 mm; window center, -400 HU; and window width, 1400 HU.
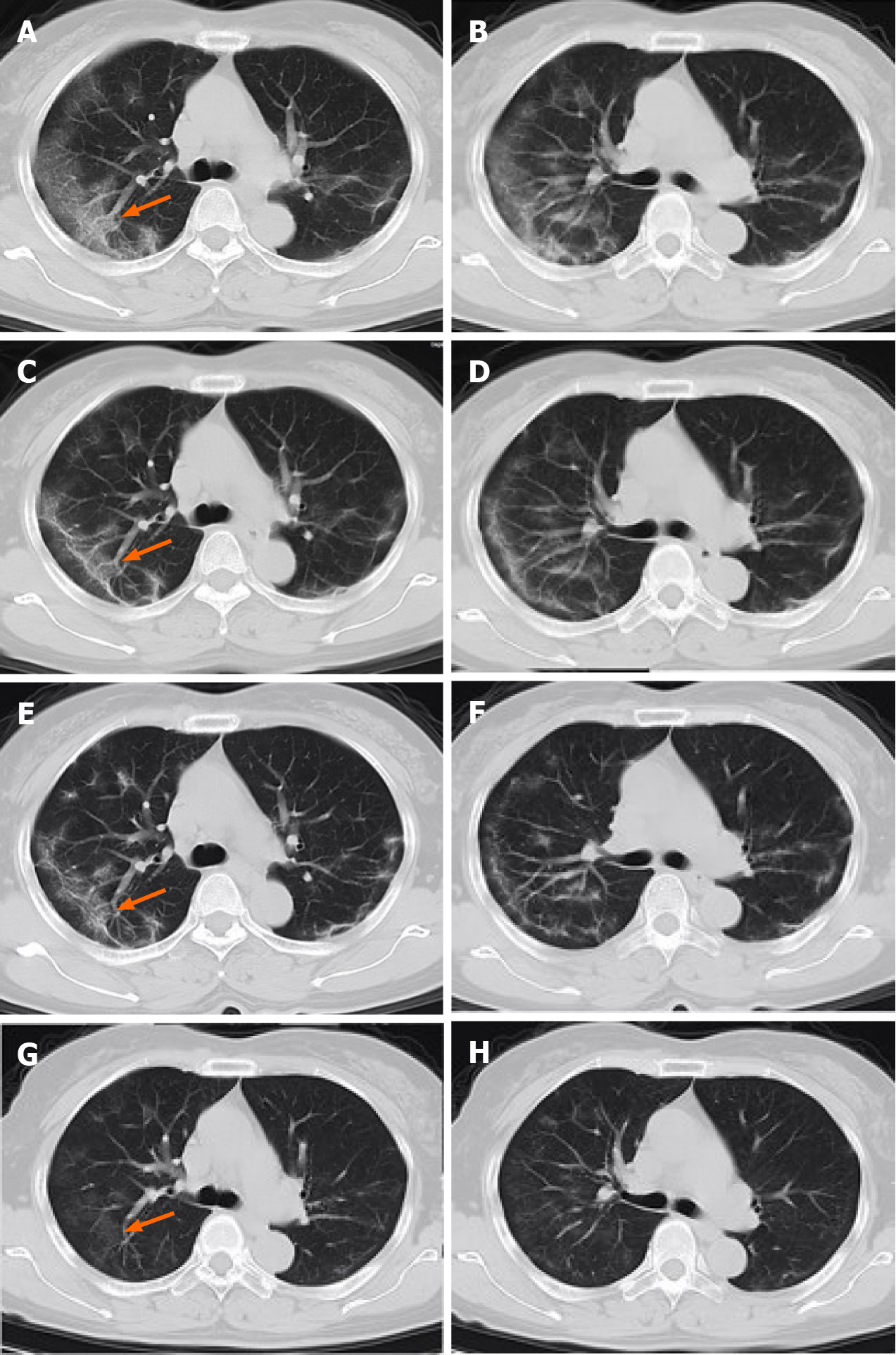
Case 1: Ten days after symptom onset, CT showed patchy bilateral subpleural ground-glass opacities.
Case 2: Chest CT showed bilateral patchy opacities, especially in the right lower lobe.
Cases 1 and 2: The final diagnosis was moderate type SARS-CoV-2, identified by RT-PCR of transtracheal aspiration samples.
Case 1: The patient’s condition was evaluated daily for the duration of the hospitalization. The patient was treated with conventional treatments such as oxygen inhalation, antibiotics (moxifloxacin), antivirals (lopinavir and interferon), and an immune modulator (thymosin), while receiving systemic therapy with traditional Chinese medicine.
Case 2: The patient’s condition was evaluated daily for the duration of the hospitalization. He was treated with conventional antiviral therapy including antivirals (lopinavir and interferon), an immune modulator (thymosin), and methylprednisolone. Moreover, the patient also received traditional Chinese medicine treatment.
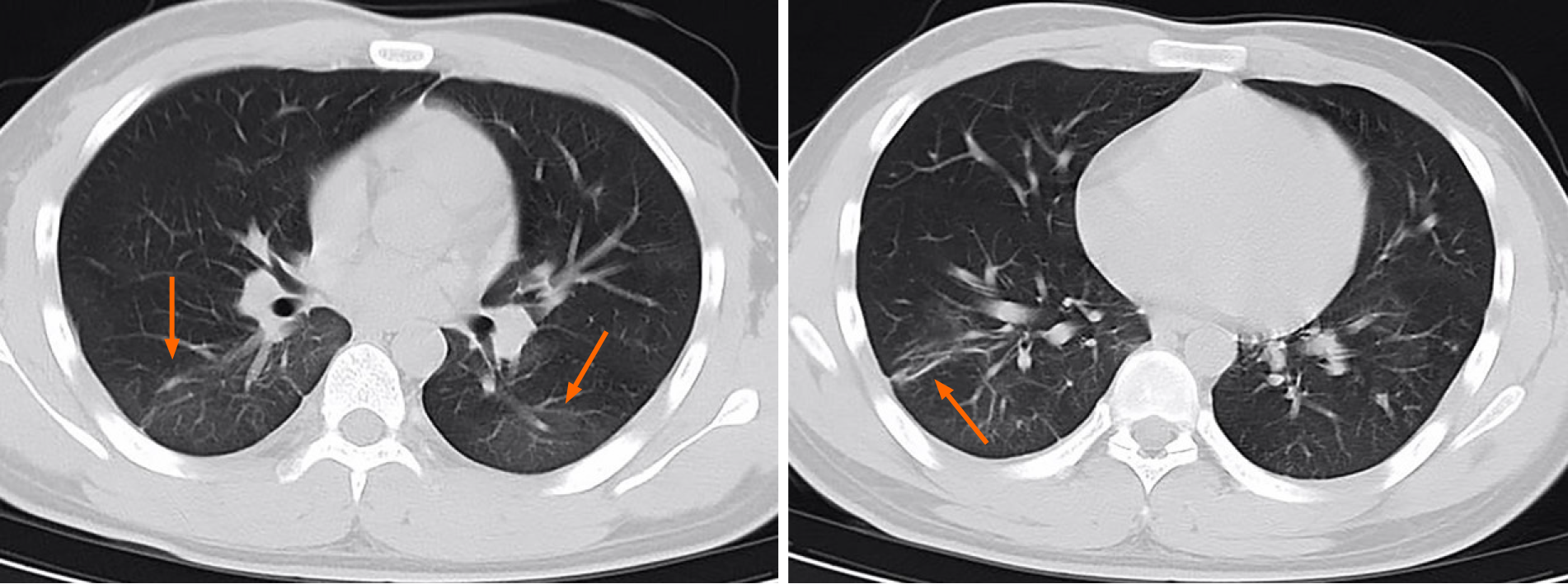
Case 1: During hospitalization, the patient’s body temperature normalized, but she developed episodes of vomiting. After antiviral and supportive treatment, the patient was discharged from the hospital. Before discharge, two RT-PCR tests with samples taken 24 h apart were negative, and a chest CT scan showed a decrease in the size and extent of pulmonary opacities (Figure 2). The patient remained asymptomatic during the subsequent 14 d of isolation and observation at a rehabilitation center. After the isolation period, she had a repeat RT-PCR assay that was positive. Therefore, she was re-admitted to the hospital where she had two additional RT-PCR tests over the next week, one negative and one positive. Her body temperature stayed normal. The laboratory tests showed a normal leukocyte count (4.4 × 109/L), normal percentage of lymphocytes (36.5%), and normal percentage of neutrophils (55%). Chest CT showed near resolution of linear and ground-glass opacities (GGO) in both lungs (Figure 3).
Case 2: The patient’s symptoms gradually improved during hospitalization. Two throat swab RT-PCR assays performed 24 h apart were negative. Chest CT showed decreased pulmonary opacities. He was discharged on February 11, 2020. His throat swab test was positive after 14 d of isolation and observation following discharge, despite a lack of clinical symptoms. He was re-admitted to the hospital where he had three repeat RT-PCR tests performed over the next 8 d, one positive and two negative (Figure 1). Laboratory examination showed a normal leukocyte count (4.7 × 109/L), normal C-reactive protein (2 mg/L), and normal erythrocyte sedimentation rate (5 mm/h).
Both patients met the criteria for recovery of COVID-19, so they were allowed to be discharged from the hospital; nevertheless, both had positive RT-PCR test results 14 d later. According to the COVID-19 diagnosis and treatment plan (trial version 6) published by the National Health Commission of China, patients should meet the following criteria simultaneously for hospital discharge: (1) Normal body temperature lasting more than 3 d; (2) resolved respiratory symptoms; (3) pulmonary imaging showing significant improvement in acute exudative lesions; and (4) two consecutive negative nucleic acid tests for respiratory pathogens (sampling interval of at least 1 d). These results raise additional questions such as imperfect sampling, finite sensitivity of virus detection, intermittency of virus shedding, and reinfection or recurrence. The current criteria for hospital discharge or discontinuation of quarantine might need to be reevaluated[2].
The accuracy of the RT-PCR assay was dependent on both the adequacy of the collected sample and the accuracy of the test kit. A study on the correlation of chest CT and RT-PCR tests showed that the positive rates of RT-PCR assays and chest CTs were 59% and 88%, respectively, for patients with suspected COVID-19[3]. The sample for RT-PCR can be collected by various methods including nasopharyngeal or throat swabs or bronchoscopy. Throat swab collection is more convenient than other methods and was used to collect samples prior to discharge in both patients. It has been speculated that the main target tissue of COVID-19 is pulmonary alveoli[4]. Therefore, the concentration of the virus in the throat may not be sufficient for detection[5]. Another study confirmed the presence of SARS-CoV-2 RNA in extra-pulmonary sites including blood and anal swabs, and the concentration of viral RNA in the anal swab was higher than that in the blood in some patients, suggesting that the virus might replicate in the digestive tract[6]. When the throat swabs became negative, the virus might still have been present at extra-pulmonary sites. Moreover, several patients infected with SARS-CoV-2 only presented with gastrointestinal symptoms including diarrhea, persistent nausea, and vomiting[7]. We observed similar gastrointestinal symptoms with respect to case 1. On the other hand, a more recent study reports the persistence of the virus for more than 60 d with a median duration of RT-PCR positivity of more than 30 d[8]. Therefore, we surmise that it is possible that the RT-PCR tests prior to discharge were all false negatives. RT-PCR assays are highly sensitive; however, sensitive detection is associated with false-positive results[9]. Both patients had one subsequent positive RT-PCR test after rehospitalization. The possibility of two false-positive results in the tests was low but the durability of the SARS-CoV-2 antibody response is not completely understood at this point, so such a possibility cannot be excluded. Some studies have reported that viral shedding has been observed in some cases for up to 3 mo. This might be an important cause of false-positive RT-PCR results[10]. On the other hand, the fact that we detected SARS-CoV-2 RNA in the upper respiratory tract secretions and other bodily fluids does not necessarily imply that the virus was viable.
Some guidelines from the Centers for Disease Control and Prevention did acknowledge that, in view of the occurrence of false-positive test results, relying on negative RT-PCR results to clear a patient from transmission-based precautions would not be reliable. They extended the timeframe for stopping preventive measures for discharged patients[11]. This requisite also implies that more negative RT-PCR results may be needed for patients to be discharged or to be released from isolation. However, it remains to be determined if patients with a viral concentration too low for detection by throat swab pose a threat to transmit the disease once discharged into the community. These findings warrant further studies into the potential of viral persistence in the bodily fluid specimens of discharged patients. Being able to identify those with false-positive RT-PCR test results and those who are asymptomatic[5] will be meaningful for further COVID-19 research.
The WHO has recommended adopting chest X-rays for the diagnosis and therapeutic planning of the patients with suspected or confirmed COVID-19 based on the following factors: (1) CT imaging normally has a lower specificity compared to RT-PCR; (2) chest radiography is associated with lower radiation doses than CT scanner; (3) it is easier to repeat X-rays sequentially for monitoring disease progression; and (4) the portable equipment at the point of care can be easily used to increase efficiency. However, according to common practices, literature reports, and Cochrane review, we found that chest X-rays have no advantages over the CT chest imaging, given that in the initial assessment of COVID-19, most studies focused on detecting pneumonia rather than viral pneumonia by X-rays[12,13]. In addition, CT is more sensitive and more commonly used than X-rays, with a 94% sensitivity[14] for COVID-19 diagnosis. Furthermore, in our daily practice, an additional CT machine has also been arranged to examine patients with a high fever and patients suspected of having COVID-19. Some medical institutions have even taken urgent actions to develop mobile CT scanners for dedicated use. These arrangements help to reduce the risk of cross-infections from patients undergoing imaging examinations.
The CT image of the first patient during initial hospitalization showed that acute GGO and consolidation improved and evolved into a linear band-like appearance (Figure 2). The CT changes met the discharge requirements. When the patient was re-hospitalized, CT demonstrated further resolution of pulmonary opacities, and there were no new lesions or residual fibrosis found in the lung tissue (Figure 2)[15]. Similarly, the CT images of patient 2 also showed near complete absence of CT abnormalities when he was re-hospitalized (Figure 3). Previous studies have shown that while chest CT signs of improvement began at approximately 14 d after the onset of initial symptoms, the absorption stage lasted for more than 26 d[16]. From the CT images, we can observe the continuous process of gradual absorption of pulmonary lesions, and there were no new lesions. Low-dose CT screening has steadily gained popularity for routine physical examination, and gradual resolution of pulmonary lesions can be observed on serial CT images.
In conclusion, the recovery of patients with COVID-19 tends to be a lengthy process. Gradual resolution of pulmonary lesions can be observed on serial CT images. Given the reported false-negative and false-positive rates of RT-PCR, further refinement of discharge criteria might be necessary. More than two negative RT-PCR test results prior to termination of isolation can potentially reduce the risk of a subsequent positive testing and viral transmission in the community. Therefore, prior to discharge, test samples should be collected from multiple sites, and the decision to discharge should be made by performing a comprehensive analysis of the CT images and RT-PCR test results. Once the patient is discharged, further rehabilitation, isolation, and medical observation should follow.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vela D S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 368] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 2. | Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, Xu H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020;323:1502-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 827] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 3. | Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296:E32-E40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3614] [Cited by in RCA: 3283] [Article Influence: 656.6] [Reference Citation Analysis (0)] |
| 4. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5774] [Article Influence: 1154.8] [Reference Citation Analysis (2)] |
| 5. | Huang K, Zhang J, Wu W, Huang D, He C, Yang Y, Zeng X, Jiang Z, Li B, Liu H. A retrospective analysis of the epidemiology, clinical manifestations, and imaging characteristics of familial cluster-onset COVID-19. Ann Transl Med. 2020;8:747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai X, Li L, He R, Tan Y, Gao M, Tang G, Zhao L, Wang J, Fan Q, Wen C, Tong Y, Tang Y, Hu F, Li F, Tang X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9:469-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 7. | Song Y, Liu P, Shi XL, Chu YL, Zhang J, Xia J, Gao XZ, Qu T, Wang MY. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69:1143-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 8. | Zhou B, She J, Wang Y, Ma X. Duration of Viral Shedding of Discharged Patients With Severe COVID-19. Clin Infect Dis. 2020;71:2240-2242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Mattison K, Grudeski E, Auk B, Brassard J, Charest H, Dust K, Gubbay J, Hatchette TF, Houde A, Jean J, Jones T, Lee BE, Mamiya H, McDonald R, Mykytczuk O, Pang X, Petrich A, Plante D, Ritchie G, Wong J, Booth TF. Analytical performance of norovirus real-time RT-PCR detection protocols in Canadian laboratories. J Clin Virol. 2011;50:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Meyerowitz EA, Richterman A, Bogoch II, Low N, Cevik M. Towards an accurate and systematic characterisation of persistently asymptomatic infection with SARS-CoV-2. Lancet Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 11. | CDC. Discontinuation of Transmission-Based Precautions and Disposition of Patients with COVID-19 in Healthcare Settings (Interim Guidance). August 10, 2020. [cited 20 October 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html. |
| 12. | Guan HX, Xiong Y, Shen NQ, Fan YQ, Shao JB, Li HJ, Li XM, Hu DY, Zhu WZ, Jin ZY. Clinical and thin-section CT features of patients with the COVID-19 in Wuhan (in Chinese). Fangshexue Shijian. 2020;35:125-130. [DOI] [Full Text] |
| 13. | Chen J, Wu L, Zhang J, Zhang L, Gong D, Zhao Y, Chen Q, Huang S, Yang M, Yang X, Hu S, Wang Y, Hu X, Zheng B, Zhang K, Wu H, Dong Z, Xu Y, Zhu Y, Chen X, Zhang M, Yu L, Cheng F, Yu H. Deep learning-based model for detecting 2019 novel coronavirus pneumonia on high-resolution computed tomography. Sci Rep. 2020;10:19196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 14. | Kim H, Hong H, Yoon SH. Diagnostic Performance of CT and Reverse Transcriptase Polymerase Chain Reaction for Coronavirus Disease 2019: A Meta-Analysis. Radiology. 2020;296:E145-E155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 365] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 15. | Kligerman SJ, Franks TJ, Galvin JR. From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics. 2013;33:1951-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295:715-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1617] [Cited by in RCA: 1756] [Article Influence: 351.2] [Reference Citation Analysis (0)] |