Published online Apr 26, 2021. doi: 10.12998/wjcc.v9.i12.2739
Peer-review started: November 11, 2020
First decision: December 31, 2020
Revised: January 13, 2021
Accepted: February 24, 2021
Article in press: February 24, 2021
Published online: April 26, 2021
Processing time: 154 Days and 15.8 Hours
Superficial CD34-positive fibroblastoma (SCPFT) is a newly discovered mesenchymal tumor characterized by high polymorphism, low mitotic rate, and diffuse CD34-positive reactions.
To further determine the clinicopathological features of SCPFT.
We retrospectively analyzed the clinicopathological data, immunohistochemistry results, and differential diagnoses of four patients with SCPFT and performed a literature review. Relevant fusion genes were also detected.
The tumors were all located in the lower extremities and presented as slow-growing painless masses located in the dermis and subcutaneous tissue. Microscopically, the tumors were composed of spindle-shaped to epithelioid cells with scattered abnormal and pleomorphic nuclei on a fibrous or fibromyxoid background. Necrosis was not found in the tumor tissues, and mitotic figures were rare. Immunohistochemically, the tumor cells were strongly positive for vimentin and CD34, and CKpan showed focal positivity in two tumors. All four patients were followed (13-57 mo, mean 35 mo), and one patient experienced local recurrence.
SCPFT is a newly discovered borderline mesenchymal tumor that can locally recur or even metastasize. Familiarity with its clinicopathological features will help avoid confusion with skin mesenchymal tumors with similar features.
Core Tip: Superficial CD34-positive fibroblastoma is a newly discovered mesenchymal tumor characterized by high polymorphism, low mitotic rate, and diffuse CD34-positive reactions. Microscopically, the tumors were composed of spindle-shaped to epithelioid cells with scattered bizarre and pleomorphic nuclei on a fibrous or fibromyxoid background.
- Citation: Ding L, Xu WJ, Tao XY, Zhang L, Cai ZG. Clinicopathological features of superficial CD34-positive fibroblastic tumor. World J Clin Cases 2021; 9(12): 2739-2750
- URL: https://www.wjgnet.com/2307-8960/full/v9/i12/2739.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i12.2739
Superficial CD34-positive fibroblastoma (SCPFT) is a rare lowgrade mesenchymal tumor first described by Carter et al[1] in 2014 and histopathologically characterized by fasciculated spindle cells mixed with pleomorphic epithelioid cells, low proliferation index, and diffuse immunohistochemical staining for CD34. SCPFT predominantly involves the superficial soft tissue of the lower extremity in adults without significant sex differences. At present, less than 50 cases have been reported in the literature[1-10]. Due to the fact that SCPFT has histopathological characteristics that are similar to those of other fibroblastic and myofibroblastic tumors, the diagnostic criteria for this type of tumor are not well understood by pathologists at present. To help pathologists better understand SCPFT, we report four cases with complete data, including clinical findings, histopathology, immunophenotype, differential diagnoses, and treatment, and also performed a literature review.
The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Bengbu Medical College. All patients provided written informed consent. A retrospective analysis was conducted on four patients who had pathologically proven SCPFTs at First Affiliated Hospital of Bengbu Medical College from 2015 to 2018. The clinical information and follow-up data were directly obtained from the hospital records and patients themselves. The routine hematoxylin–eosin staining and immunohistochemical sections were obtained from the Department of Pathology archives and reviewed by two experienced soft-tissue pathologists.
The specimens were fixed with 10% neutral formalin, routinely dehydrated, embedded in paraffin, sectioned, and stained with hematoxylin–eosin. Immuno-histochemical staining on all samples was performed using the EliVision two-step method and DAB staining. The primary antibodies used included those against CD34 (catalog No. Kit-0004), SMA (Kit-0006), CK (Pan) (RAB-0050), EMA (Kit-0011), S-100 protein (Kit-0007), HMB-45 (MAB-0098), desmin (Kit-0023), CD68 (Kit-0026), Ki-67 (MAB-0672), MDM2 (MAB-0116), CDK4 (RMA-0771), ALK (MAB-0848), STAT6 (RAB-0729), CD99 (MAB-0059), and p53 (MAB-0674). All of these antibodies were purchased from Fuzhou Maixin Biotechnology Co., Ltd. Phosphate buffer saline was used instead of the primary antibody as a negative control per the manufacturer’s instructions.
ALK and PDGFB gene rearrangements were analyzed in all four cases by fluorescence in situ hybridization (FISH). The probes used included the ZytoLight SPEC PDGFB Dual Color Break Apart Probe (Prod. No.: Z-2119-50, Bremerhaven, Germany) and ZytoLight SPEC ALK Dual Color Break Apart Probe (Prod. No.: Z-2124-50, Bremerhaven, Germany). FISH analysis was performed according to the manufacturer-provided protocol. Two researchers scored 100 cells in each tumor tissue in a double-blind manner. ALK gene rearrangement and PDGFB gene rearrangement were considered positive when at least 10% of the tumor cells showed a red-green separation signal.
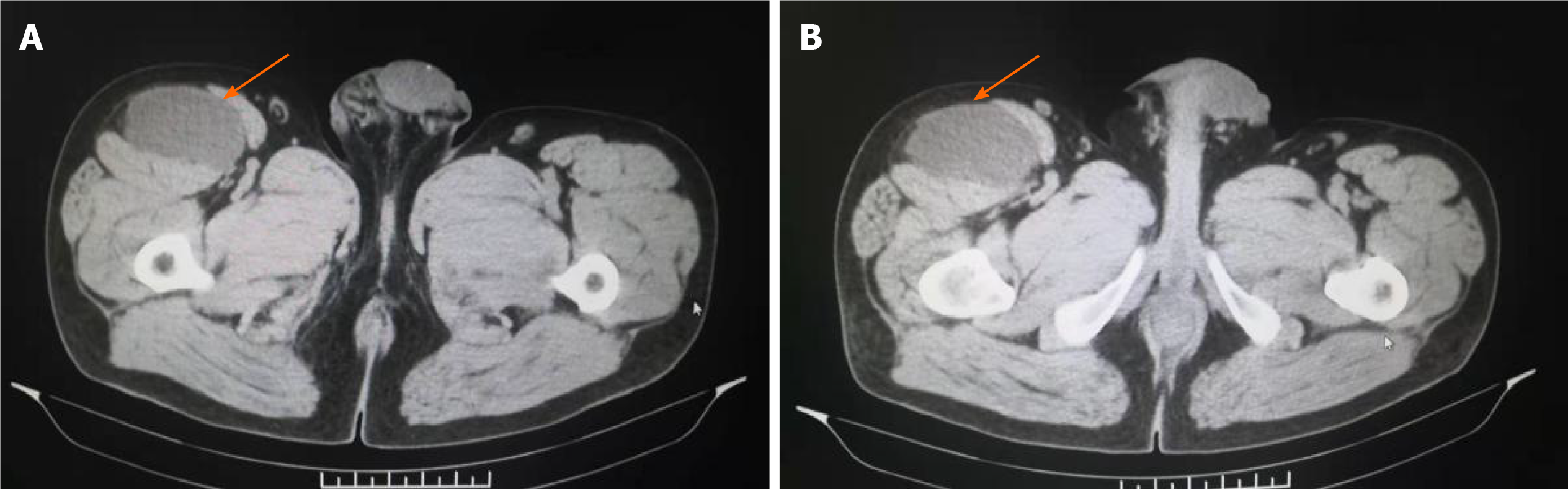
The clinical data of the patients are listed in Table 1. The patients reported in this study included three males and one female aged 27-51 years, with a median age of 41 years. The course of the disease ranged from 12 d to 3 mo. Four cases involved the soft tissue of the lower extremities, with masses occurring separately in the upper part of the right thigh, right lateral malleolus, left calf front, and left proximal thigh. These patients presented with the main clinical symptom of a painless, enlarged mass. In three of the four cases, the tumor tissue was mainly located in the dermis and subcutis. In one case (case 4), the tumor was located in the subcutaneous and intramuscular deep fascia, with a clear tumor boundary. We also retrieved the imaging data of all patients. For case 4, computed tomography showed that the tumor was located in the space between the sartorius muscle and tensor fascia lata muscle in front of the right thigh and exhibited a uniform density and clear boundary with surrounding muscles (Figure 1A and B). The surgical record indicated that the tumors were hard in texture, exhibited a clear boundary, were mainly located in the deep fascia, and exhibited local infiltration at the medial incision margin.
| Case | Gender/Age | Main symptoms | Location | Specimen type | Size (cm)1 | Distant metastasis | Treatment | Follow-up (mo) |
| 1 | F/35 | Painless mass | Upper part of the right thigh | Resection | 2.5 | No | Complete excision | 57; NR |
| 2 | F/27 | Painless mass | Right lateral malleolus | Resection | 3.0 | No | Complete excision | 28; NR |
| 3 | M/47 | Mass of right lower limb with pain | Left calf front | Resection | 5.0 | No | Expanded excision | 13; NR |
| 4 | M/51 | Progressive enlargement with tenderness | Left proximal thigh | Resection | 6.0 | No | Expanded excision | 6; R |
Three cases presented with a well-defined single nodular mass, and one case had a tumor that was ill-defined and interlaced with the surrounding adipose tissue. The tumor diameters ranged from 2.5-6.0 cm, and the section of the nodule was pale yellow in color and slightly hard in texture with no apparent hemorrhage or necrosis.
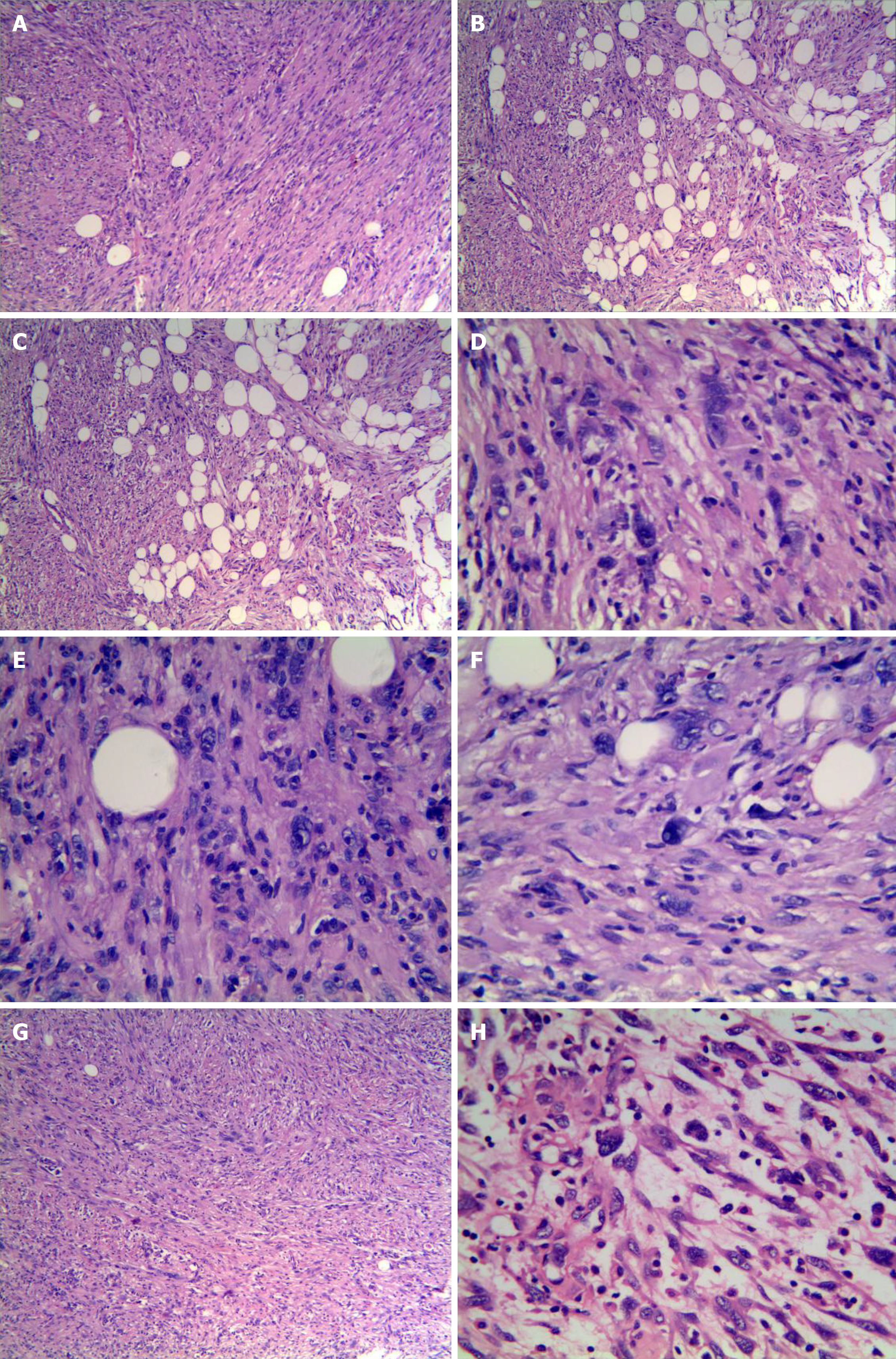
The tumors were composed of irregular spindle to ovalshaped cells with eosinophilic and granular cytoplasm arranged in fascicles and sheets, with some areas showing a vaguely woven pattern and focal infiltration into the surrounding adipose tissue (Figure 2A and B). The tumor cells in the local area exhibited epithelioid features arranged in sheets and nests (Figure 2C). A few scattered tumor cells were markedly polymorphic with hyperchromatic, abnormal, and pleomorphic nuclei that frequently displayed intranuclear pseudoinclusions (Figure 2D). A glassy or foam-like cytoplasm was found in the focal tumor cells. The nucleoli of some of the tumor cells were single and distinct, but mitosis was rarely observed (< 1/50 HFP). No areas of atypical mitosis or tumor necrosis were noted (Figure 2E). Tumor stroma with mucinous degeneration was not observed in the focal area (Figure 2F). Furthermore, thin-walled fissured vessels, a small number of scattered plasma cells, and lymphocyte infiltration were observed. In some areas, the spindle cells were relatively uniform and closely arranged with mild myxodegeneration in the stroma, and a small amount of lymphocyte infiltration was observed. These features are similar to inflammatory myofibroblastic tumors. In other areas, the stroma showed obvious mucoid degeneration with more inflammatory cell infiltration (Figure 2G), involving scattered large nuclear atypia cells with clear nucleoli, which is similar to myxoinflammatory fibroblastic sarcoma (Figure 2H).
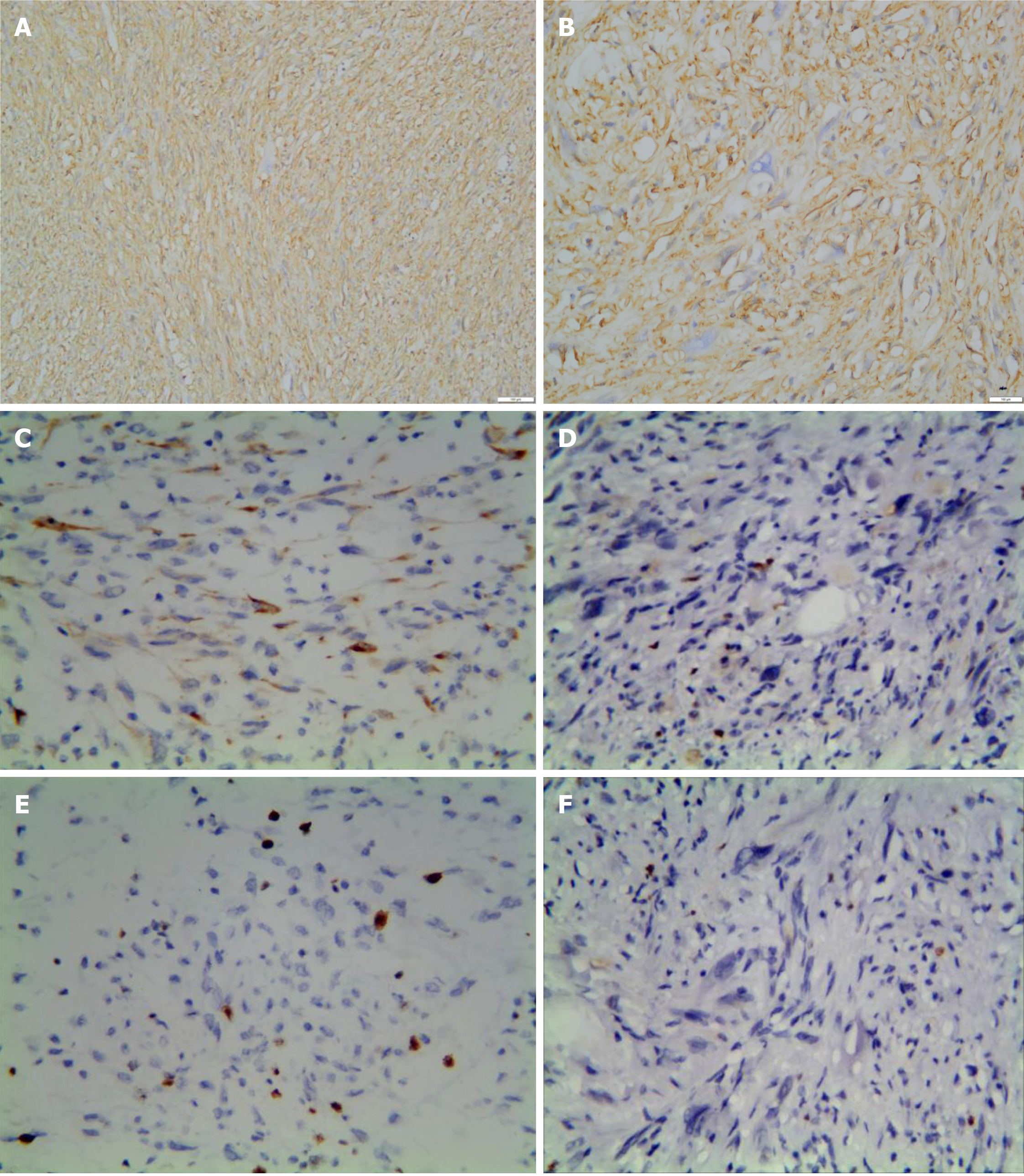
The immunohistochemical results are summarized in Table 2. The tumor cells were diffusely and strongly positive for CD34 (Figure 3A and B). CKpan showed focal positivity in two tumors (Figure 3C). Case 2 was weakly positive for CD99. The Ki-67 proliferation index was 1%-2%, and the proliferative index of case 3 locally reached 5% (Figure 3D-E). SMA, EMA, S-100 protein, HMB45, MDM-2, CDK-4, ALK, STAT-6, p53 (Figure 3F), CD68, and desmin were all negative.
| Marker | CD34 | CK | SMA | EMA | S100 | HMB45 | MDM-2 | CDK-4 | ALK | STAT-6 | p53 | CD99 | CD68 | Des | Ki-67 |
| Case 1 | + | -/+ | - | - | - | - | - | - | - | - | - | - | - | - | 1% |
| Case 2 | + | - | - | - | - | - | - | - | - | - | - | ± | - | - | 2% |
| Case 3 | + | -/+ | - | - | - | - | - | - | - | - | - | - | - | - | 5% |
| Case 4 | + | - | - | - | - | - | - | - | - | - | - | - | - | - | 2% |
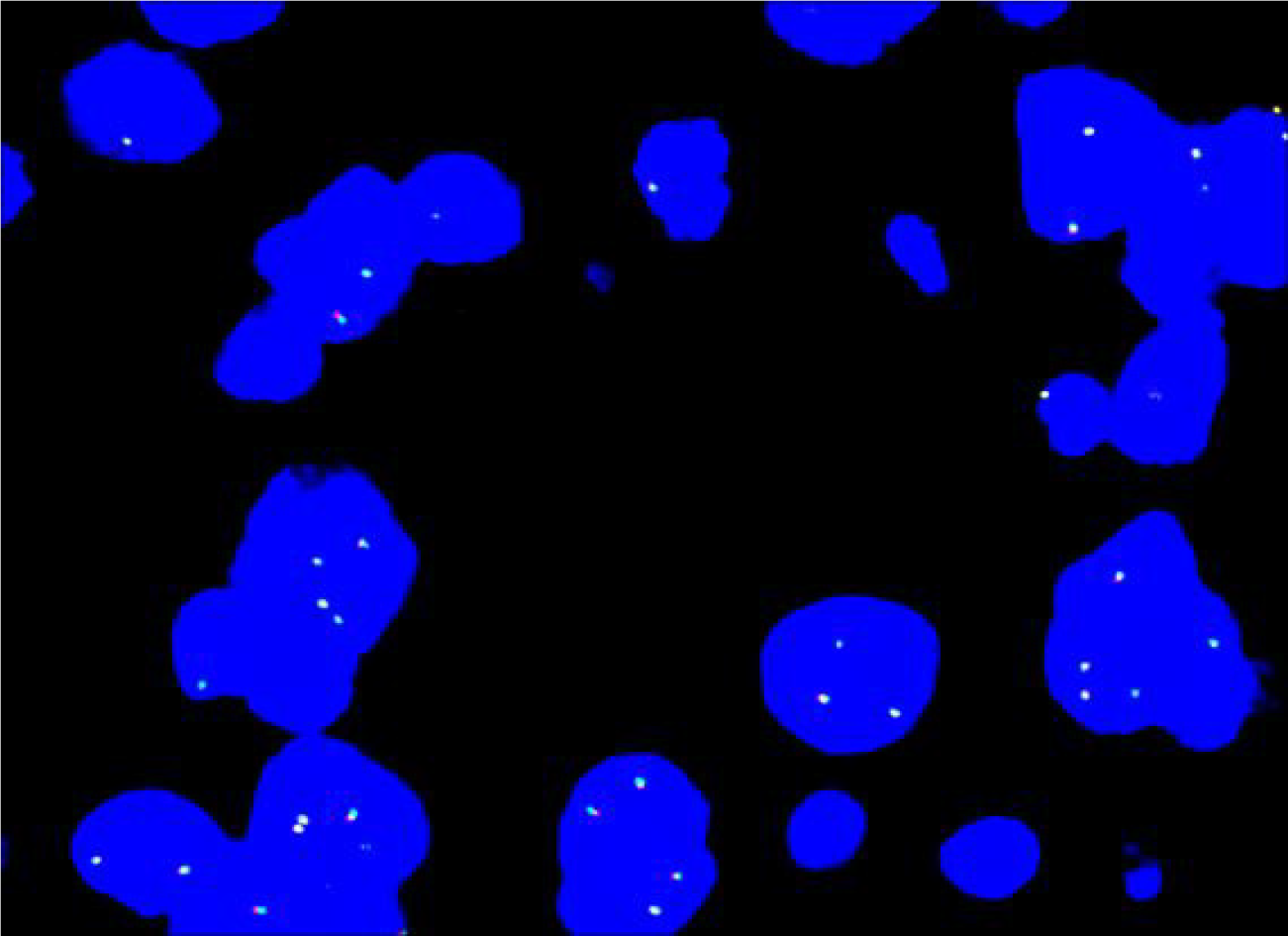
Based on FISH analysis, the four tumors were negative for ALK rearrangements (Figure 4), and PDGFB rearrangements were not detected.
All four patients underwent surgical resection in the follow-up period (range 13-57 mo). Three cases were deemed free of disease, and only one case suffered local recurrence 6 mo after resection. Simultaneously, imaging did not show any evidence of distant or lymph node metastasis.
SCPFT is a rare fibroblastic differentiated mesenchymal tumor that belongs to the family of borderline lesions. In 2014, it was first reported that SCPFT exhibits unique histomorphological and immunophenotypic characteristics[1]. Microscopically, the tumors typically showed expansive growth with varying degrees of peripheral fat infiltration or dermal involvement. Medium-sized spindle to epithelioid cells were arranged in bundles and sheets with obvious nuclear atypia and pleomorphism, but mitosis was rare (< 1/50HPF). Spindle cells had abundant granular cytoplasm with no obvious nucleoli. Epithelioid polymorphic cells had coarse granular chromatin and prominent nucleoli with granular or transparent cytoplasm, and pseudoinclusions were occasionally visible in the nucleus. No necrosis or atypical mitosis was observed. Branched capillaries often appeared, especially in the bundle hyperplasia area of the tumor cells. A small number of scattered mast cells and lymphocyte infiltration were observed in the tumor interstitium. Immunohistochemical staining showed that CD34 was strongly expressed in all cases, and approximately 60% of the tumors expressed creatine kinase locally (AE1/AE3). Other markers included SMA and ALK, which label inflammatory myofibroblastic tumors; MDM-2 and CDK-4, which are used for the diagnosis of pleomorphic liposarcoma; S-100 protein and HMB45, which are used for the diagnosis of spindle cell malignant melanoma; STAT-6, which is used for the diagnosis of solitary fibrous tumors; and EMA, p53, CD68, and desmin, which are used for the diagnosis of angiomatoid fibrous histiocytoma. The tumors were negative for these markers. The proliferation index of Ki-67 was low, typically less than 2%[1-3].
In general, the histological and immunophenotypic features of our results were consistent with those reported in the literature. The difference is the highest Ki-67 index in case 3 of approximately 5%, which was also observed in some other cases[5,9]. Based on the histological and immunophenotypic characteristics of the tumors, Carter et al[1] hypothesized that these tumors are fibroblastic tumors. Recently, ultrastructural analysis has provided evidence of the differentiation of fibroblasts[4,5].
Molecular genetic studies of SCPFT have revealed no specific TGFBR3 or MGEA5 gene rearrangements, such as those that are found in myxomatous inflammatory fibroblastic sarcoma[1,2]. Thus, we used FISH to detect the expression of ALK and PDGFB genes, which are relatively specific gene markers for inflammatory myofibroblastic tumors and dermatofibrosarcoma protuberans, respectively. All of our four cases were subject to FISH analysis of ALK and PDGFB rearrangements, but rearrangements were not detected. These findings also demonstrate that this type of tumor is an independent entity that is different from inflammatory myofibroblastic tumors and dermatofibrosarcoma protuberans. Due to the limited number of cases, the cytogenetic characteristics of SCPFT have not been determined, and only one report found t(2;5)(q31;q31) chromosomal translocations[5].
Clinically, the age and lesion locations were similar to those reported in the literature in three of our four cases. These studies reported that most lesions were located in the subcutaneous tissue and superficial fascia of the extremities and rarely involved the deep muscles; in addition, no significant sex differences were noted. In case 4, the tumor location was deep and mainly located between the deep fascia and the muscles. The tumors typically present as painless, slow-growing masses in patients with a variety of medical histories. Imaging examinations, including computed tomography and magnetic resonance imaging, exhibited no specific abnormalities, which is similar to the findings noted for other superficial soft tissue tumors. These techniques revealed wellmarginated tumors without calcification in subcutaneous adipose tissue. However, part of the tumor margins appeared to slightly infiltrate the subcutaneous deep fascia. These tumors exhibited homogenous low signal intensity on T1-weighted images and high signal intensity on T2-weighted images with small lobulated structures[5]. The tumor diameters varied from 1.5 to 10 cm (average 4.1 cm). Sections of the tumors were typically solid, grayish-yellow to grayish-brown in color, and glossy.
When the lesion incision margins are negative, complete excision is preferred for patients with SCPFT; otherwise, enlarged resection is performed[2,10]. In addition, two cases of successful Mohs micrographic surgery have been reported[11,12]. The four patients in our group were followed for 13-57 mo. Only one of these patients had short-term local recurrence, and no additional recurrence was noted after extensive resection. These findings suggest that the biological behavior of the tumor is relatively inert. Short-term local recurrence occurred in case 4. Fortunately, all patients were classified as free of disease after intensive resection. In contrast, in the study by Carter et al[1], 12 out of their 13 cases demonstrated no evidence of recurrence after an average follow-up of 24 mo, and only one patient had regional lymph node metastasis at 7 years after marginal resection of the primary tumor. No special manifestations were noted after another follow-up period of 20 mo.
SCPFT may share some morphological features with other superficial soft tissue tumors, and it is easily misdiagnosed as pleomorphic dermal sarcoma, dermato-fibrosarcoma protuberans, plaque-like CD34-positive dermal fibroma, myxoinflammatory fibroblastic sarcoma, superficial solitary fibrous tumors, inflammatory myofibroblastoma, superficial myxofibrosarcoma, fibrous histiocytoma, epithelioid sarcomas, and PRDM10-rearranged soft tissue tumors. Pleomorphic dermal sarcoma is differentiated from SCPFT by numerous mitotic figures, infiltrative tumor margins, and the lack of diffuse CD34 positivity[13]. Dermatofibrosarcoma protuberans is typically characterized by a focally storiform arrangement, a region of high-grade sarcomatoid transformation with predominant mitotic images, necrosis, relatively decreased expression of CD34, and an increased Ki-67 proliferation index[14]. Plaque-like CD34-positive dermal fibroma (medallion-like dermal dendrocyte hamartoma) distinctively shows cellular band-like fibroblastic proliferation and CD34 positivity. However, the former is common in the papillary and adjacent upper reticular dermis, and the latter lacks deep-stained abnormal nuclei[15]. For myxoinflammatory fibroblastic sarcoma (acral myxoinflammatory fibroblastic sarcoma), the degree of atypical tumor cells is low in inflammatory and mucoid areas, and viral inclusion body-like giant cells can be observed. Myxoinflammatory fibroblastic sarcoma is often characterized by TGFBR3/MGEA5 gene rearrangements[16] and lacks diffuse CD34 expression. Superficial solitary fibrous tumors are more common in women and most often involve the head. The typical histologic features include irregular fascicles of spindled cells, staghorn-like blood vessels, and variable amounts of collagen. The tumor cells are positive for STAT6[17]. Inflammatory myofibroblastic tumor cells are arranged in bundles or whorls, which express myogenic markers but are negative for CD34 expression[18]. In addition, in some regions, irregular, polygonal, or exotic cells containing eosinophilic or basophilic endosomes are noted. The significant difference between CD34-positive superficial myxofibrosarcoma and SCPFT is the alternate distribution of mucus and cell areas and pseudolipoma-like cells with more mitotic figures[19]. Although scattered large pleomorphic fibroblastic cells with scarce mitotic figures may also be seen in an atypical benign fibrous histiocytoma, robust expression of CD34 is not observed[20]. Epithelioid sarcomas are mainly composed of epithelioid cells with some degree of atypia, mitosis, and necrosis. INI1 immunolabeling shows no loss of expression, which is a difference between SCPFT and epithelioid sarcoma[21]. A new tumor type, namely, PRDM10-rearranged soft tissue tumors, is a rare neoplasm characterized by pleomorphic morphology and a low mitotic count. Its morphologic spectrum overlaps with that of SCPFT, and more cases are needed to further distinguish them at the genetic level[22].
In summary, SCPFT is considered an independent tumor entity in the relevant literature; the World Health Organization has not yet formally named this tumor[23]. Similar tumors may be labeled as "low-grade spindle cell sarcoma or low-grade malignant fibroblastic tumors" in the course of routine diagnostic practice. The lesions in the reported cases lack the variety of spindle cell tumors located in superficial sites, express CD34, and are not entirely superficial. Thus, we believe that it is more appropriate to consider SCPFT as a polymorphic CD34-positive fibroblastic tumor. Therefore, it is of substantial practical importance to better understand the occurrence, histomorphology, and biological behavior of this tumor. Our findings might further contribute to knowledge of SCPFT, which may aid in its diagnosis, treatment, and prognosis.
Superficial CD34-positive fibroblastic tumor (SCPFT) is a newly discovered mesenchymal tumor that is mainly composed of fibroblasts. Given the lack of established diagnostic criteria, SCPFT is associated with a high misdiagnosis rate
We are writing this paper to further describe the histopathological characteristics and genetic characteristics of this entity so that pathologists can more accurately diagnose the disease.
The main purpose of this study was to further elucidate the characteristics of SCPFT; its genetic characteristics are of great concern to pathologists.
We retrospectively analyzed the clinicopathological, immunohistochemical, and fluorescence in situ hybridization characteristics of four SCPFT patients treated at our institution.
In general, these tumors are mostly single well-defined nodules. Microscopically, the tumors were composed of irregular spindle to oval-shaped cells with eosinophilic and granular cytoplasm. A few scattered tumor cells were markedly polymorphic with hyperchromatic, abnormal, and pleomorphic nuclei that frequently displayed intranuclear pseudoinclusions. Hematoxylin–eosin staining of some tumors with interstitial mucoid degeneration was similar to that of mucinous fibroblastic sarcoma. Immunohistochemical staining showed that CD34 was strongly expressed in all cases, and approximately 60% of the tumors expressed CK locally (AE1/AE3). ALK and PDGFB gene rearrangements were analyzed in all four cases by fluorescence in situ hybridization. The four tumors were negative for ALK rearrangements, and PDGFB rearrangements were not detected.
Our findings may further contribute to the recognition of SCPFTs, including the clinical context in which they arise; it is important to avoid confusion with other pleomorphic soft tissue tumors, particularly neoplasms in the group of pleomorphic sarcomas, which are typically aggressive tumors, as that could lead to unnecessary overtreatment.
In follow-up work, more cases will be collected for comparative studies of the clinical and pathological aspects. An in-depth analysis will be conducted at the genetic level through second-generation sequencing to confirm the uniqueness of this entity and provide the basis for precise clinical treatment.
The authors are grateful to Professor Zenong Cheng at the Department of Pathology (Bengbu Medical College) for excellent technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Srinivasamurthy BC S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Li JH
| 1. | Carter JM, Weiss SW, Linos K, DiCaudo DJ, Folpe AL. Superficial CD34-positive fibroblastic tumor: report of 18 cases of a distinctive low-grade mesenchymal neoplasm of intermediate (borderline) malignancy. Mod Pathol. 2014;27:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 2. | Li W, Molnar SL, Mott M, White E, De Las Casas LE. Superficial CD34-positive fibroblastic tumor: Cytologic features, tissue correlation, ancillary studies, and differential diagnosis of a recently described soft tissue neoplasm. Diagn Cytopathol. 2016;44:926-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Lao IW, Yu L, Wang J. Superficial CD34-positive fibroblastic tumour: a clinicopathological and immunohistochemical study of an additional series. Histopathology. 2017;70:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Hendry SA, Wong DD, Papadimitriou J, Robbins P, Wood BA. Superficial CD34-positive fibroblastic tumour: report of two new cases. Pathology. 2015;47:479-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Yamaga K, Fujita A, Osaki M, Kuwamoto S, Ishiguro N, Yamamoto T, Nagashima H. Detailed analysis of a superficial CD34-positive fibroblastic tumor: A case report and review of the literature. Oncol Lett. 2017;14:3395-3400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Batur S, Ozcan K, Ozcan G, Tosun I, Comunoglu N. Superficial CD34 positive fibroblastic tumor: report of three cases and review of the literature. Int J Dermatol. 2019;58:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Hamada T, Katsuki N, Hosokawa Y, Ayano Y, Ikeda M. Additional case of superficial CD34-positive fibroblastic tumor in a Japanese patient. J Dermatol. 2019;46:e134-e136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Rekhi B, Banerjee D, Gala K, Gulia A. Superficial CD34-positive fibroblastic tumor in the forearm of a middle-aged patient: A newly described, rare soft-tissue tumor. Indian J Pathol Microbiol. 2018;61:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Wada N, Ito T, Uchi H, Nakahara T, Tsuji G, Yamada Y, Oda Y, Furue M. Superficial CD34-positive fibroblastic tumor: A new case from Japan. J Dermatol. 2016;43:934-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Li H, Liu C, Zhang ZH, Fan QH. [Superficial CD34-positive fibroblastic tumour: a clinicopathologic analysis of 3 cases]. Zhonghua Bing Li Xue Za Zhi. 2019;48:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Florek AG, Amin SM, Guitart J, Krunic AL. Superficial CD34-Positive Fibroblastic Tumor Successfully Treated With Mohs Micrographic Surgery. Dermatol Surg. 2018;44:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Donaldson MR, Weber LA. Superficial CD34-Positive Fibroblastic Tumor Treated With Mohs Micrographic Surgery. Dermatol Surg. 2017;43:1489-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Soleymani T, Tyler Hollmig S. Conception and Management of a Poorly Understood Spectrum of Dermatologic Neoplasms: Atypical Fibroxanthoma, Pleomorphic Dermal Sarcoma, and Undifferentiated Pleomorphic Sarcoma. Curr Treat Options Oncol. 2017;18:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Palmerini E, Gambarotti M, Staals EL, Zanella L, Sieberova G, Longhi A, Cesari M, Bonarelli S, Picci P, Ruggieri P, Alberghini M, Ferrari S. Fibrosarcomatous changes and expression of CD34+ and apolipoprotein-D in dermatofibrosarcoma protuberans. Clin Sarcoma Res. 2012;2:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kutzner H, Mentzel T, Palmedo G, Hantschke M, Rütten A, Paredes BE, Schärer L, Guillen CS, Requena L. Plaque-like CD34-positive dermal fibroma ("medallion-like dermal dendrocyte hamartoma"): clinicopathologic, immunohistochemical, and molecular analysis of 5 cases emphasizing its distinction from superficial, plaque-like dermatofibrosarcoma protuberans. Am J Surg Pathol. 2010;34:190-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Jurcić V, Zidar A, Montiel MD, Frković-Grazio S, Nayler SJ, Cooper K, Suster S, Lamovec J. Myxoinflammatory fibroblastic sarcoma: a tumor not restricted to acral sites. Ann Diagn Pathol. 2002;6:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Feasel P, Al-Ibraheemi A, Fritchie K, Zreik RT, Wang WL, Demicco E, Saeb-Lima M, Goldblum JR, Rubin BP, McKenney JK, Ko JS, Billings SD. Superficial Solitary Fibrous Tumor: A Series of 26 Cases. Am J Surg Pathol. 2018;42:778-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Ding Y, Yang HY, Zhang D, Guo F, Wang JX, Li YP, Li YA. Diagnosis and treatment of inflammatory myofibroblastoma in children and adolescents. Chin Med J (Engl). 2019;132:1110-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Smith SC, Poznanski AA, Fullen DR, Ma L, McHugh JB, Lucas DR, Patel RM. CD34-positive superficial myxofibrosarcoma: a potential diagnostic pitfall. J Cutan Pathol. 2013;40:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Guillou L. [Mesenchymal tumors of the skin. Atypical fibrous histiocytoma]. Ann Pathol. 2009;29:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Thway K, Jones RL, Noujaim J, Fisher C. Epithelioid Sarcoma: Diagnostic Features and Genetics. Adv Anat Pathol. 2016;23:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Puls F, Pillay N, Fagman H, Palin-Masreliez A, Amary F, Hansson M, Kindblom LG, McCulloch TA, Meligonis G, Muc R, Rissler P, Sumathi VP, Tirabosco R, Hofvander J, Magnusson L, Nilsson J, Flanagan AM, Mertens F. PRDM10-rearranged Soft Tissue Tumor: A Clinicopathologic Study of 9 Cases. Am J Surg Pathol. 2019;43:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Fletcher CD. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology. 2014;64:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |