Published online Apr 26, 2021. doi: 10.12998/wjcc.v9.i12.2721
Peer-review started: November 20, 2020
First decision: February 12, 2021
Revised: February 19, 2021
Accepted: March 4, 2021
Article in press: March 4, 2021
Published online: April 26, 2021
Processing time: 145 Days and 23.4 Hours
Circulating tumor cells (CTCs) can be clustered into three subtypes according to epithelial-mesenchymal transition (EMT) markers: CTCs with epithelial markers (E-CTCs), CTCs with mesenchymal markers (M-CTCs), and CTCs with both markers (E&M-CTCs). CTC detection has clinical implications in the diagnosis of lung cancer (LC).
To clarify the diagnostic value of CTCs categorized by EMT markers in LC.
The study included 106 patients with lung adenocarcinoma, including 42 ground-glass opacities (GGO) and 64 solid lesions, who underwent surgery between July 2015 and December 2019. Eleven patients with benign tumors and seventeen healthy controls were included. CTCs in peripheral blood and associated EMT markers were detected preoperatively using the CanPatrolTM technique. The diagnostic power of CTCs for discriminating LC cases from controls was analyzed by the receiver operating characteristic (ROC) curve. The CytoploRare technique was used in 20 cases and 18 controls for validation, and Kappa values were calculated to evaluate consistency between techniques.
Of the 106 LC cases, 94 (89.6%) had at least one CTC. CTCs were detectable in 35 (83.3%) of 42 GGO cases. Total CTCs and E&M-CTCs were significantly more frequent in LC cases than in benign or healthy controls. The proportion of M-CTCs plus E&M-CTCs increased gradually from healthy controls, to benign controls, to LC cases. The area under the ROC curve of total CTCs and E&M-CTCs was > 0.8 and > 10.75, respectively. The combined sensitivity of total-CTCs and E&M-CTCs was 85.85% for LC patients (80.95% for GGO patients) and the specificity was 78.57%. The Kappa value was 0.415, indicating relative consistency between CanPatrolTM and CytoploRare.
CTC detection is valuable for distinguishing LC from controls, and particularly E&M-CTC detection warrants further study.
Core Tip: Circulating tumor cells (CTCs) with epithelial markers (E-CTCs), CTCs with mesenchymal markers (M-CTCs), and CTCs with both markers (E&M-CTCs) are three subtypes of CTCs. Detection of E&M-CTCs have good diagnostic value for distinguishing lung cancer from controls.
- Citation: Jiang SS, Mao CG, Feng YG, Jiang B, Tao SL, Tan QY, Deng B. Circulating tumor cells with epithelial-mesenchymal transition markers as potential biomarkers for the diagnosis of lung cancer. World J Clin Cases 2021; 9(12): 2721-2730
- URL: https://www.wjgnet.com/2307-8960/full/v9/i12/2721.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i12.2721
Circulating tumor cells (CTCs) destroy the integrity of the base membrane, detach from the primary site, and spread through the peripheral blood, and they have the potential to form distant metastases in the appropriate microenvironment[1].
The epithelial-to-mesenchymal transition (EMT), which is characterized by the upregulation of mesenchymal markers (e.g., cytokeratin [CK] 8, 18, and 19 and epithelial cell adhesion molecule [EpCAM]) and downregulation of epithelial markers (e.g., vimentin and Twist), can lead to the transformation of epithelial cells into mesenchymal cells[2]. This process can also promote tumor progression, metastasis, and chemotherapy resistance[3]. CTCs include a variety of subtypes and are divided into groups according to the expression of EMT markers[4]. Recent studies suggest that restricted EMT can generate hybrid CTCs with both epithelial and mesenchymal markers, as well as enhance their survival and carcinogenic abilities[5]. The EMT process in these CTCs may lead to cancer metastasis and poor prognosis[3,6]. CTCs are used as biomarkers for the diagnosis of lung cancer (LC), pancreatic cancer, and gastric cancer[7-9]. A meta-analysis found that the sensitivity and specificity (95% confidence interval [CI]) of CTCs for the diagnosis of LC is 75% (95%CI: 54-88) and 92% (95%CI: 82-97), respectively[8]. A pilot study from our group found that CTCs can be detected in early-stage lung adenocarcinoma (LUAD)[10].
Herein, we clarified the diagnostic value of CTCs categorized by the expression of EMT markers in LC.
The study protocol was reviewed and approved by the Ethics Committee of Daping Hospital, Army Medical University 2019, No. 183, and all patients who agreed to participate in the study signed an informed consent.
The study was performed between July 2015 and December 2019 and included 106 patients with LUAD. Computed tomography (CT) performed before thoracoscopic surgery indicated that 42 patients had ground glass opacity (GGO) and 64 had solid lesions. A total of 28 controls were recruited, including 11 benign tumor cases (benign controls) and 17 healthy controls who underwent physical and radiographical examination to exclude the possibility of an occult tumor. Postoperative pathological results were reviewed by professional pathologists. Subjects with bronchiectasis, viral hepatitis, or ischemic cardiac or cerebrovascular disease were excluded from the study. Demographic-clinical data of patients and controls are shown in Table 1.
| Subjects | Gender (n) | Mean age in yr | Pathologic features (n) | Stage (n) | Size in cm |
| Lung cancer patients (n = 106) | Male (47), Female (59) | 59 | AAH (1) | Ia (84) | 2.03 ± 1.06 |
| AIS (10) | Ib (10) | ||||
| MIA (11) | IIa (2) | ||||
| IAC (83) | IIb (7) | ||||
| III (3) | |||||
| Benign controls (n = 11) | Male (4), Female (7) | 49 | IPT (6)1 | NA | |
| Tuberculoma (2) | |||||
| Hamartoma (1) | |||||
| Fibroma (1) | |||||
| Pleural tumor (1) | |||||
| Healthy controls (n = 17) | Male (10), Female (7) | 31 | NA | NA | NA |
At 1 or 2 d prior to surgery, 10 mL peripheral blood was collected from the median cubital vein of patients and controls and transferred into a sample preservation tube containing ammonium chloride dissolution buffer (Surexam Biotechnology, Guangzhou, China). The samples were incubated for 30 min at room temperature. CTCs and associated EMT markers were detected using CanPatrolTM as follows[1,11]. (1) Red blood cells were lysed and removed using red blood cell lysis buffer, and cluster of differentiation-positive (CD45+) leukocytes were depleted from blood samples using magnetic beads; (2) CTCs were enriched using a calibrated 8 μm pore membrane filter; and (3) RNA in situ hybridization (ISH) was used to identify CTCs using the branched DNA signal amplification assay and EMT markers such as CK 8, 18, and 19, EpCAM, vimentin, and Twist were detected according to a recently published protocol[12].
Peripheral blood (3 mL) was collected before surgery and stored in a vacuum tube containing ethylenediamine tetraacetic acid. Red blood cell lysis buffer was used to lyse erythrocytes, and magnetic beads were used to deplete CD45+ leukocytes. The folate receptor, which is only expressed on tumor cells, and a synthesized oligonucleotide were used to label the CTCs. The conjugate was annealed and extended using a reverse transcriptase primer prior to amplification. The enriched CTCs were stained by immunofluorescence. Cells expressing the folate ligand and cytokeratin, which were stained with the nuclear stain 4′,6-diamidino- 2-phenylindole, were defined as folate receptor positive (FR+) CTCs. These procedures were completed within 1 h[13,14]. The threshold for positivity was an FR+ CTC value ≥ 8.7 FU/3 mL[13,14].
The Student-Newman-Keuls test was used to analyze the variance among groups. Positive rates were compared using the χ2 test, and comparisons between two groups were performed using the Kappa Concordance Test. Cutoff values were determined using receiver operating characteristic (ROC) curves. Statistical analyses were performed using SPSS Version 23.0 software for Windows (SPSS, Inc., Chicago, IL, United States). A value of P < 0.05 (two-sided) was considered statistically significant.
Sensitivity = True positive/(true positive + false negative), Specificity = True negative/(true negative + false positive).
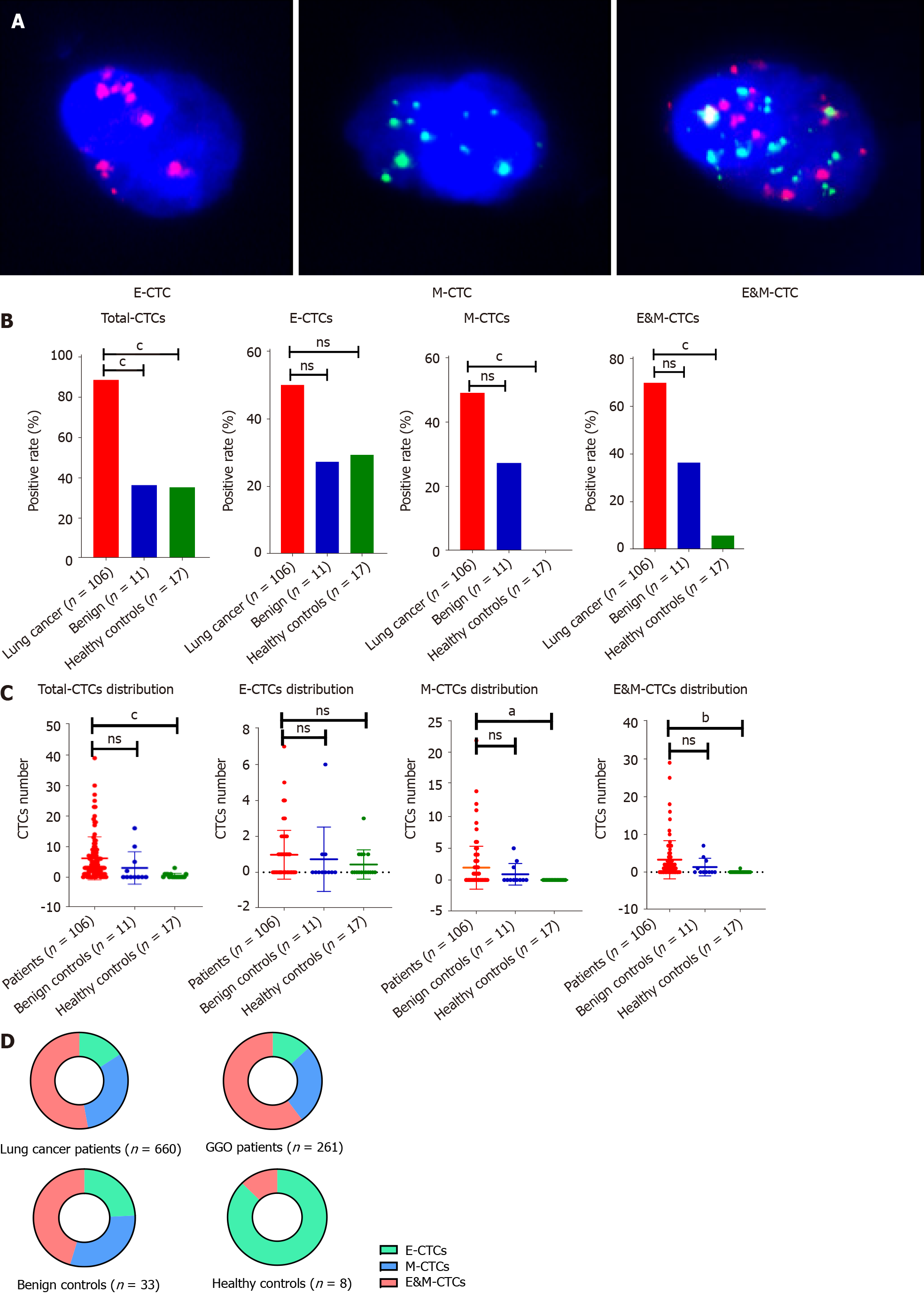
As shown in Figure 1A, epithelial (E) and mesenchymal (M), and E&M markers were detected in CTCs. Therefore, CTCs were divided into three subtypes according to the associated markers, i.e. E-CTCs, M-CTCs and E&M-CTCs.
Of the 106 LC cases, 94 (88.6%) had at least one CTC, whereas CTCs were only detectable in 4 of 11 benign controls and 6 of 17 healthy controls. The detection rate of CTCs was significantly higher in LC cases than in benign or healthy controls (Figure 1B). E-CTCs were detected in 53 (53/106) LC cases, 3 benign controls (3/11), and 5 healthy controls (5/17) (Figure 1B). There was no significant difference in the incidence of E-CTCs among LC cases, benign cases, and healthy controls. The incidence of M-CTCs was significantly higher in LC cases than in healthy controls (52/106 vs 0/17; P < 0.05), whereas there was no significant difference between LC cases and benign controls (52/106 vs 3/11). The incidence of E&M-CTCs was significantly higher in LC cases than in benign controls (74/106 vs 4/11; P < 0.05) and in healthy controls (74/106 vs 1/17; P < 0.01). CTCs were detectable in 35 (83.3%) of 42 GGO cases including E-CTCs in 20 cases, M-CTCs in 19 cases, and E&M-CTCs in 28 cases. There was no significant correlation between CTC number and pathological and demographic features such as tumor size, age, and gender (data not shown).
The number of CTCs, M-CTCs, and E&M-CTCs was significantly higher in LC cases than in healthy controls (P < 0.05), whereas there was no difference between LC cases and benign controls (Figure 1C). The proportions of M-CTCs plus E&M-CTCs increased gradually from healthy controls, to benign controls, to LC cases (LC vs benign controls: P = 0.405; benign controls vs healthy controls: P = 0.086; LC vs healthy controls: P < 0.05) (Figure 1D). The proportion of M-CTCs plus E&M-CTCs in GGO cases was similar to that in other LC cases.
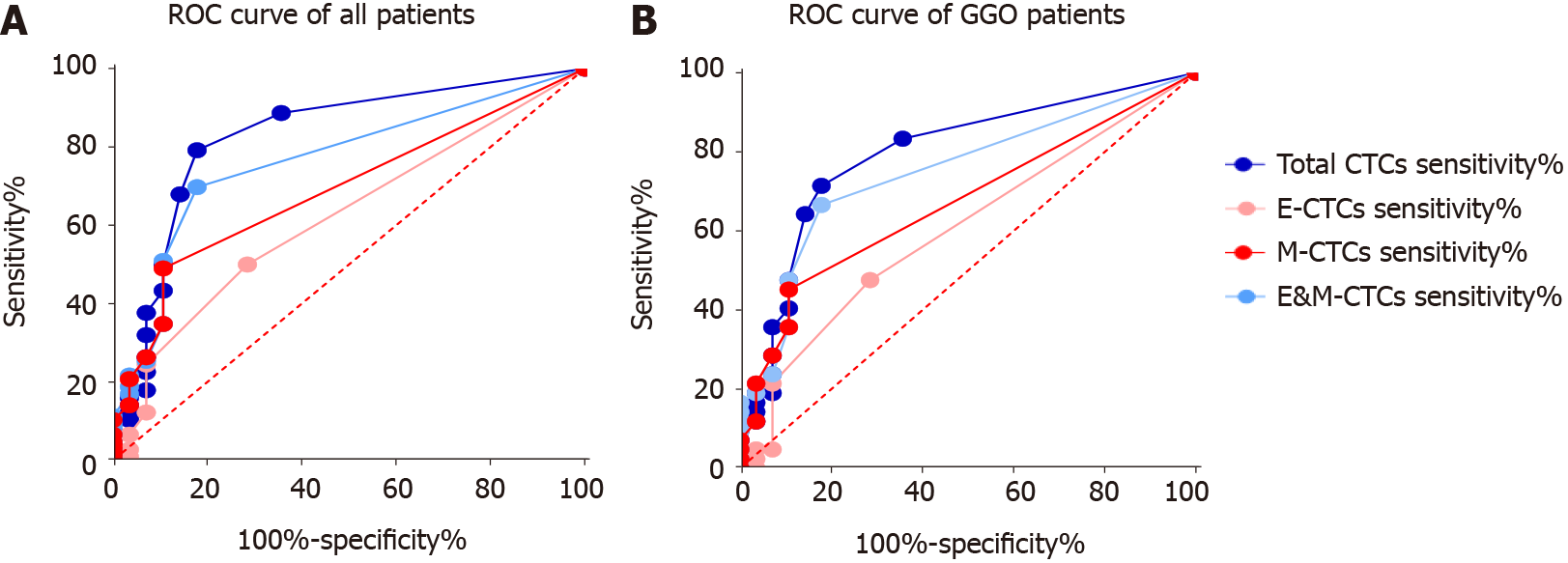
As shown in Figure 2 and Table 2, the area under the ROC curve (AUC) of total CTCs and E&M-CTCs was > 0.8 and > 0.75, respectively, indicating their potential diagnostic value for discriminating LC cases from controls. Compared to GGO vs controls, the AUC of total CTCs or E&M-CTCs remained > 0.75.
| Subjects | All lung cancer patients, n = 106 | Lung cancer patients presenting as GGO, n = 42 | |||||
| AUC | P value | Sensitivity | AUC | P value | Sensitivity | Specificity | |
| Total CTCs | 0.828 | < 0.0001 | 79.24% | 0.7963 | < 0.0001 | 71.42% | 82.14% |
| E-CTCs | 0.6193 | 0.0527 | 50.00% | 0.6029 | 0.1469 | 47.62% | 71.43% |
| M-CTCs | 0.6892 | 0.0021 | 49.05% | 0.6722 | 0.0152 | 42.85% | 89.29% |
| E&M-CTCs | 0.7648 | < 0.0001 | 69.80% | 0.7504 | 0.0004 | 66.67% | 82.14% |
| Total CTCs and E&M-CTCs | 85.85% | 80.95% | 78.57% | ||||
The most effective cut-off for positivity according to the AUC was determined to be total CTCs ≥ 2/5 mL and E&M-CTCs ≥ 1/5 mL (Table 2). These cut-off values were used to determine the sensitivity of total CTCs and E&M-CTCs for diagnosing LUAD, which showed that their sensitivity was higher than that of E-CTCs and M-CTCs (Table 2). After defining either total CTCs ≥ 2/5 mL or E&M-CTCs ≥ 1/5 mL for positivity, the sensitivity was 85.85% for LC patients and 80.95% for GGO patients, and the specificity was 78.57%.
The CanpatrolTM method was validated using CytoploRare in 20 LC cases, 7 benign cases, and 11 healthy controls. As shown in Table 3, the Kappa values reached 0.415, indicating that the detection rate of total CTCs and E&M-CTCs was comparable to that of FR+ CTCs (detected by CytoploRare). Although M-CTCs also had a P value of < 0.05 compared with FR+ CTCs, this index was excluded because of the low Kappa value (data not shown).
| Total CTCs detected by CanpatrolTM | E&M-CTCs detected by CytoploRare | ||||
| Positive, n | Negative, n | Positive, n | Negative, n | ||
| FR-CTCs | Positive, n | 16 | 4 | 17 | 6 |
| Negative, n | 7 | 11 | 6 | 9 | |
| Kappa/P value | 0.415/0.010 | 0.339/0.037 | |||
Detection and measurement of CTCs is a noninvasive liquid biopsy method that can provide reliable information on the diagnostic, prognostic, and/or predictive biomarkers of different types of cancer compared with invasive diagnostic modalities[15]. CTC detection is performed in two steps: enrichment and identification. CTC enrichment is based on distinguishing tumor cell morphology and sorting cell surface markers. For example, CellSearch enriches CTCs by sorting the surface marker EpCAM using magnetic beads. ISET is a method of enriching cells by filtering cells through filter membranes based on cell size. Ficoll separation enriches cells by density gradient centrifugation based on different cell densities[16]. CTC identification is achieved using polymerase chain reaction (PCR), immunofluorescence, and ISH[17].
Most of the methods currently used in the clinic provide simultaneous enrichment and identification of CTCs. In this study, CanPatrolTM was used to enrich CTCs through an 8-micron filter membrane, and RNA ISH was used to identify and characterize the enriched cells based on branch DNA signal amplification technology. In addition, the expanded lanes in the CanPatrolTM system allow the detection of EMT markers such as EpCAM, CK 8, 18, and 19, vimentin, and Twist. The expression of these markers allows the classification of CTCs into different subtypes, namely, E-CTCs, M-CTCs, and E&M-CTCs[12]. A recent study[18] reported that the sensitivity and specificity of total CTCs detected by CanPatrol™ technology for the diagnosis of LC is 81.6% and 86.8%, respectively, which is consistent with the present results.
We used a widely used method, CytoploRare, to validate the CanPatrolTM results. The FR is a glycoprotein that is highly expressed on the cell surface and can be detected on lung and ovarian cancer cells[19]. Samples consisting of 3 mL peripheral blood were used to enrich FR+ CTCs; white blood cells were removed using immunomagnetic beads, and FR, a tumor-specific ligand, as well as a synthesized oligonucleotide were labeled on the cells. Quantitative PCR was used to analyze the conjugates. FR CTCs were defined as cells expressing folate ligands and cytokeratin with positive nuclear staining[13]. The sensitivity and specificity of this technology for the detection of FR+ CTCs is 81.94% and 73.08%, respectively[20]. Kappa values indicated a relatively similar detection efficacy for CanPatrolTM and CytoploRare, suggesting acceptable consistency. However, further studies with a larger sample size are necessary to confirm these results.
Another advantage of CanPatrolTM is that it can identify CTCs based on mesenchymal markers, whereas current CTCs monitoring methods, including CellSearch and MACS, are based on the detection of EpCAM, and thereby do not detect M-CTCs or E&M-CTCs. CTCs undergoing EMT should receive increased attention because they are characterized by higher aggressiveness and carcinogenicity[21,22]. Tumor cells are more likely to form E&M-CTCs than M-CTCs to obtain both mesenchymal and epithelial cell characteristics[5].
TelomeScan F35, which uses a telomerase-specific replication-selective adenovirus (OBP-1101) to detect CTCs using EMT markers, was used to show that CTCs with EMT-markers are effective for the early diagnosis of non-small cell lung cancer [mean sensitivity: 69.1% (59.6%, 40.0%, 85.7%, and 75.0% for stage I, II, III, and IV, respectively)][23].
In this study, we found that E&M-CTCs had a greater discriminative power for the diagnosis of LC than E-CTCs or M-CTCs. In addition, we showed that CTCs can be present in benign and healthy controls. However, E&M-CTCs were detected in one case in the control group that corresponded to a smoker. Similarly, CTCs can be recognized as a “sentinel” in cases associated with inflammatory diseases such as chronic obstructive pulmonary disease[24]. In patients with benign colon diseases, CTCs can also be detected with both the CellSearch system (11.3%) and the CK19-EPISPOT assay (18.9%), because inflammatory epithelial cells from these benign lesions may enter the peripheral blood[25]. In 232 patients with benign breast diseases, the CTC detection rate reached 15.95%[26]. Herein, we hypothesized that tumor cells or debris, which can be shed from the benign tumor into the peripheral blood, are captured and recognized as CTCs by CanPatrolTM. However, we cannot completely rule out that these CTCs in the controls were not malignant because CTCs may appear at a very early stage of cancer development, even if the lesion is undetectable by systemic physical and radiological examinations. Whether these “benign” tumors or diseases were in a premalignant stage requires long-term follow-up.
In this study, the proportions of M-CTCs and E&M-CTCs were highest in LC patients compared with benign and healthy controls, supporting the critical role of the EMT process in CTCs in promoting tumor invasion, migration, and metastatic growth.
Detection of E&M-CTCs is effective for distinguishing LC, including GGO and solid lesions, from controls, thus providing a new method for discriminating between malignant and benign pulmonary nodes in clinical practice. Because the study only included two benign cases presented as GGO, we were unable to determine the diagnostic value of E&M-CTCs for discriminating between malignant and benign GGO. Intriguingly, fibrosis can be found in either benign or malignant lung GGO[27,28]. However, very few published studies have focused on CTCs and GGO, not to mention CTCs and fibrosis. Furthermore, pathological reports from our hospital did not clearly describe the fibrosis condition of these GGO cases. Therefore, we are going to design further clinical trials regarding CTCs in GGO, which will consider the fibrosis condition and the expressions of relevant biomarkers, e.g., CXCL4 and LECT2[29,30]. Additional studies with a large sample size and a multicenter design are warranted in the near future.
In conclusion, after defining total CTCs ≥ 2/5 mL or E&M-CTCs ≥ 1/5 mL as the cut-off for positivity, the sensitivity and specificity for LC diagnosis were 85.85% and 78.57%, respectively. CTC detection is valuable for distinguishing LC from controls.
Circulating tumor cells (CTCs) can be clustered into three subtypes according to surface biomarkers. CTC detection has clinical implications in the diagnosis of lung cancer (LC).
CTCs categorized by epithelial-mesenchymal transition (EMT) markers have diagnostic value in LC and ground-glass opacities (GGO) patients.
To clarify the diagnostic value of CTCs categorized by the expression of EMT markers in LC and GGO patients
Of the 106 patients with lung adenocarcinoma comprising the study cohort, 42 had GGO and 64 had solid lesions; 11 patients with benign tumors and 17 healthy controls were included as controls. Total CTCs and CTCs with both markers (E&M-CTCs) of these patients were detected, the diagnostic value of CTCs categorized by the expression of EMT markers. CytoploRare technique was used in 20 cases and 18 controls for validation.
Total CTCs and E&M-CTCs were significantly more frequent in LC cases than in benign or healthy controls. The area under the receiver operating characteristic curve of total CTCs and E&M-CTCs was > 0.8 and > 0.75, respectively. The combined sensitivity of total-CTCs and E&M-CTCs was 85.85% for LC patients (80.95% for GGO patients) and the specificity was 78.57%.
CTC detection is valuable for distinguishing LC as well as GGO patients from controls.
Epithelial (E)-, mesenchymal (M)-, and E&M markers can be identified in CTC. Therefore, CTCs were clustered into three subtypes, as per the markers on CTC, i.e. E-CTC, M-CTC and E&M-CTC. Detection of E&M-CTC has good diagnostic value in distinguishing lung cancer, providing a new method to discriminate between malignant and benign pulmonary node in clinical practice.
We thank Dr. Xu-Rui Jin for reviewing the statistical methods of this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Brody AR, Pasini L, Ullah M S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Wu S, Liu Z, Liu S, Lin L, Yang W, Xu J. Enrichment and enumeration of circulating tumor cells by efficient depletion of leukocyte fractions. Clin Chem Lab Med. 2015;53:337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Cao Z, Livas T, Kyprianou N. Anoikis and EMT: Lethal "Liaisons" during Cancer Progression. Crit Rev Oncog. 2016;21:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Yin W, Han YM, Li ZL, Huang ZX, Huang L, Zhong XG. Clinical significance of perioperative EMT-CTC in rectal cancer patients receiving open/laparoscopic surgery. Neoplasma. 2020;67:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Zhao XH, Wang ZR, Chen CL, Di L, Bi ZF, Li ZH, Liu YM. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice. World J Gastroenterol. 2019;25:138-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Liu X, Li J, Cadilha BL, Markota A, Voigt C, Huang Z, Lin PP, Wang DD, Dai J, Kranz G, Krandick A, Libl D, Zitzelsberger H, Zagorski I, Braselmann H, Pan M, Zhu S, Huang Y, Niedermeyer S, Reichel CA, Uhl B, Briukhovetska D, Suárez J, Kobold S, Gires O, Wang H. Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source of metastasis. Sci Adv. 2019;5:eaav4275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 6. | Liu DG, Xue L, Li J, Yang Q, Peng JZ. Epithelial-mesenchymal transition and GALC expression of circulating tumor cells indicate metastasis and poor prognosis in non-small cell lung cancer. Cancer Biomark. 2018;22:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Zhou J, Ma X, Bi F, Liu M. Clinical significance of circulating tumor cells in gastric cancer patients. Oncotarget. 2017;8:25713-25720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Huang H, Shi Y, Huang J, Wang X, Zhang R, Chen H. Circulating tumor cells as a potential biomarker in diagnosis of lung cancer: a systematic review and meta-analysis. Clin Respir J. 2018;12:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Xie ZB, Yao L, Jin C, Fu DL. Circulating tumor cells in pancreatic cancer patients: efficacy in diagnosis and value in prognosis. Discov Med. 2016;22:121-128. [PubMed] |
| 10. | Jin XR, Zhu LY, Qian K, Feng YG, Zhou JH, Wang RW, Bai L, Deng B, Liang N, Tan QY. Circulating tumor cells in early stage lung adenocarcinoma: a case series report and literature review. Oncotarget. 2017;8:23130-23141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Liu YK, Hu BS, Li ZL, He X, Li Y, Lu LG. An improved strategy to detect the epithelial-mesenchymal transition process in circulating tumor cells in hepatocellular carcinoma patients. Hepatol Int. 2016;10:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Wu S, Liu S, Liu Z, Huang J, Pu X, Li J, Yang D, Deng H, Yang N, Xu J. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One. 2015;10:e0123976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 13. | Chen X, Zhou F, Li X, Yang G, Zhang L, Ren S, Zhao C, Deng Q, Li W, Gao G, Li A, Zhou C. Folate Receptor-Positive Circulating Tumor Cell Detected by LT-PCR-Based Method as a Diagnostic Biomarker for Non-Small-Cell Lung Cancer. J Thorac Oncol. 2015;10:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Yu Y, Chen Z, Dong J, Wei P, Hu R, Zhou C, Sun N, Luo M, Yang W, Yao R, Gao Y, Li J, Yang G, He W, He J. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol. 2013;6:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Deng CJ, Dai FQ, Qian K, Tan QY, Wang RW, Deng B, Zhou JH. Clinical updates of approaches for biopsy of pulmonary lesions based on systematic review. BMC Pulm Med. 2018;18:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Geeurickx E, Hendrix A. Targets, pitfalls and reference materials for liquid biopsy tests in cancer diagnostics. Mol Aspects Med. 2020;72:100828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 17. | Liu X, Zhang Z, Zhang B, Zheng Y, Zheng C, Liu B, Zheng S, Dong K, Dong R. Circulating tumor cells detection in neuroblastoma patients by EpCAM-independent enrichment and immunostaining-fluorescence in situ hybridization. EBioMedicine. 2018;35:244-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Li J, Liao Y, Ran Y, Wang G, Wu W, Qiu Y, Liu J, Wen N, Jing T, Wang H, Zhang S. Evaluation of sensitivity and specificity of CanPatrol™ technology for detection of circulating tumor cells in patients with non-small cell lung cancer. BMC Pulm Med. 2020;20:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Zhou Q, Geng Q, Wang L, Huang J, Liao M, Li Y, Ding Z, Yang S, Zhao H, Shen Q, Pan C, Lou J, Lu S, Chen C, Luo Q. Value of folate receptor-positive circulating tumour cells in the clinical management of indeterminate lung nodules: A non-invasive biomarker for predicting malignancy and tumour invasiveness. EBioMedicine. 2019;41:236-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Xue Y, Cong W, Xie S, Shu J, Feng G, Gao H. Folate-receptor-positive circulating tumor cells as an efficacious biomarker for the diagnosis of small pulmonary nodules. J Cancer Res Ther. 2018;14:1620-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1963] [Cited by in RCA: 1920] [Article Influence: 160.0] [Reference Citation Analysis (0)] |
| 22. | Effenberger KE, Schroeder C, Hanssen A, Wolter S, Eulenburg C, Tachezy M, Gebauer F, Izbicki JR, Pantel K, Bockhorn M. Improved Risk Stratification by Circulating Tumor Cell Counts in Pancreatic Cancer. Clin Cancer Res. 2018;24:2844-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Togo S, Katagiri N, Namba Y, Tulafu M, Nagahama K, Kadoya K, Takamochi K, Oh S, Suzuki K, Sakurai F, Mizuguchi H, Urata Y, Takahashi K. Sensitive detection of viable circulating tumor cells using a novel conditionally telomerase-selective replicating adenovirus in non-small cell lung cancer patients. Oncotarget. 2017;8:34884-34895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud JM, Padovani B, Mouroux J, Marquette CH, Hofman P. "Sentinel" circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9:e111597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 25. | Pantel K, Denève E, Nocca D, Coffy A, Vendrell JP, Maudelonde T, Riethdorf S, Alix-Panabières C. Circulating epithelial cells in patients with benign colon diseases. Clin Chem. 2012;58:936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 26. | Jin L, Zhao W, Zhang J, Chen W, Xie T, Wang L, Fan W, Xie S, Shen J, Zheng H, Hu W, Wei Q, Dong M, Wang Q, Liu Y. Evaluation of the diagnostic value of circulating tumor cells with CytoSorter® CTC capture system in patients with breast cancer. Cancer Med. 2020;9:1638-1647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Qin Y, Xu Y, Ma D, Tian Z, Huang C, Zhou X, He J, Liu L, Guo C, Wang G, Zhang J, Wang Y, Liu H. Clinical characteristics of resected solitary ground-glass opacities: Comparison between benign and malignant nodules. Thorac Cancer. 2020;11:2767-2774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Lv YG, Bao JH, Xu DU, Yan QH, Li YJ, Yuan DL, Ma JH. Characteristic analysis of pulmonary ground-glass lesions with the help of 64-slice CT technology. Eur Rev Med Pharmacol Sci. 2017;21:3212-3217. [PubMed] |
| 29. | van Bon L, Affandi AJ, Broen J, Christmann RB, Marijnissen RJ, Stawski L, Farina GA, Stifano G, Mathes AL, Cossu M, York M, Collins C, Wenink M, Huijbens R, Hesselstrand R, Saxne T, DiMarzio M, Wuttge D, Agarwal SK, Reveille JD, Assassi S, Mayes M, Deng Y, Drenth JP, de Graaf J, den Heijer M, Kallenberg CG, Bijl M, Loof A, van den Berg WB, Joosten LA, Smith V, de Keyser F, Scorza R, Lunardi C, van Riel PL, Vonk M, van Heerde W, Meller S, Homey B, Beretta L, Roest M, Trojanowska M, Lafyatis R, Radstake TR. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med. 2014;370:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 30. | Xu M, Xu HH, Lin Y, Sun X, Wang LJ, Fang ZP, Su XH, Liang XJ, Hu Y, Liu ZM, Cheng Y, Wei Y, Li J, Li L, Liu HJ, Cheng Z, Tang N, Peng C, Li T, Liu T, Qiao L, Wu D, Ding YQ, Zhou WJ. LECT2, a Ligand for Tie1, Plays a Crucial Role in Liver Fibrogenesis. Cell 2019; 178: 1478-1492. e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |