Published online Apr 26, 2021. doi: 10.12998/wjcc.v9.i12.2711
Peer-review started: December 19, 2020
First decision: January 10, 2021
Revised: January 16, 2021
Accepted: March 19, 2021
Article in press: March 19, 2021
Published online: April 26, 2021
Processing time: 116 Days and 12 Hours
In 75% of women with polycystic ovary syndrome (PCOS), insulin action is impaired. In obesity, visceral adipose tissue becomes dysfunctional: Chronic inflammation is favored over storage, contributing to the development of metabolic complications. PCOS, metabolic syndrome (MetSy) and non-alcoholic fatty liver disease (NAFLD) apparently share common pathogenic factors; these include abdominal adiposity, excess body weight and insulin resistance. Alterations in the gut microbiome have been noted in women with PCOS compared to controls; these may lead to deterioration of the intestinal barrier, increased gut mucosal permeability and immune system activation, hyperinsulinemia and glucose intolerance, which hamper normal ovarian function and follicular development (all being hallmarks of PCOS). It has been proposed that PCOS may entail higher susceptibility to coronavirus disease 2019 (COVID-19) via its associated comorbidities (NAFLD, obesity, MetSy and alterations in the gut microbiome). Studies have found an association between acute respiratory distress syndrome (seen in severe cases of COVID-19) and the intestinal microbiome. Furthermore, apparently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can gain entry to the gastrointestinal tract via locally-expressed angiotensin converting enzyme type 2 receptors. Excess body weight is associated with more severe COVID-19 and increased mortality. Although robust links between SARS-CoV-2 infection and PCOS/NAFLD/gut microbiome/metabolic consequences are yet to be confirmed, it seems that strategies for adapting the intestinal microbiome could help reduce the severity of COVID-19 in women with PCOS with or without NAFLD, MetSy or obesity.
Core Tip: Polycystic ovary syndrome (PCOS) may entail higher susceptibility to coronavirus disease 2019 (COVID-19). Furthermore, PCOS may also increase susceptibility to COVID-19 via its associated comorbidities (non-alcoholic fatty liver disease, metabolic syndrome and alterations in the gut microbiome). In order to determine whether the intestinal microbiome in women with PCOS has an effect on their risk of COVID-19 or if severe acute respiratory syndrome coronavirus 2 is the factor that changes the composition of their microbiome, more research will be needed. Strategies for adapting the intestinal microbiome could help reduce the severity of COVID-19 in women with PCOS.
- Citation: Ilias I, Goulas S, Zabuliene L. Polycystic ovary syndrome: Pathways and mechanisms for possible increased susceptibility to COVID-19. World J Clin Cases 2021; 9(12): 2711-2720
- URL: https://www.wjgnet.com/2307-8960/full/v9/i12/2711.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i12.2711
Polycystic ovary syndrome (PCOS) is a common and heterogeneous endocrine disorder that becomes symptomatic in adolescence and occurs in 5%-10% of women of reproductive age[1]; it is frequently associated with metabolic abnormalities. It is the most common cause of androgen hypersecretion and accounts for more than a third of menstrual disorders. PCOS is also the most common hormonal disorder that leads to hair loss, appearance of acne, seborrhoea and male pattern baldness (symptoms may be absent in patients with moderate hyperandrogenemia, as in most cases of PCOS[1]).
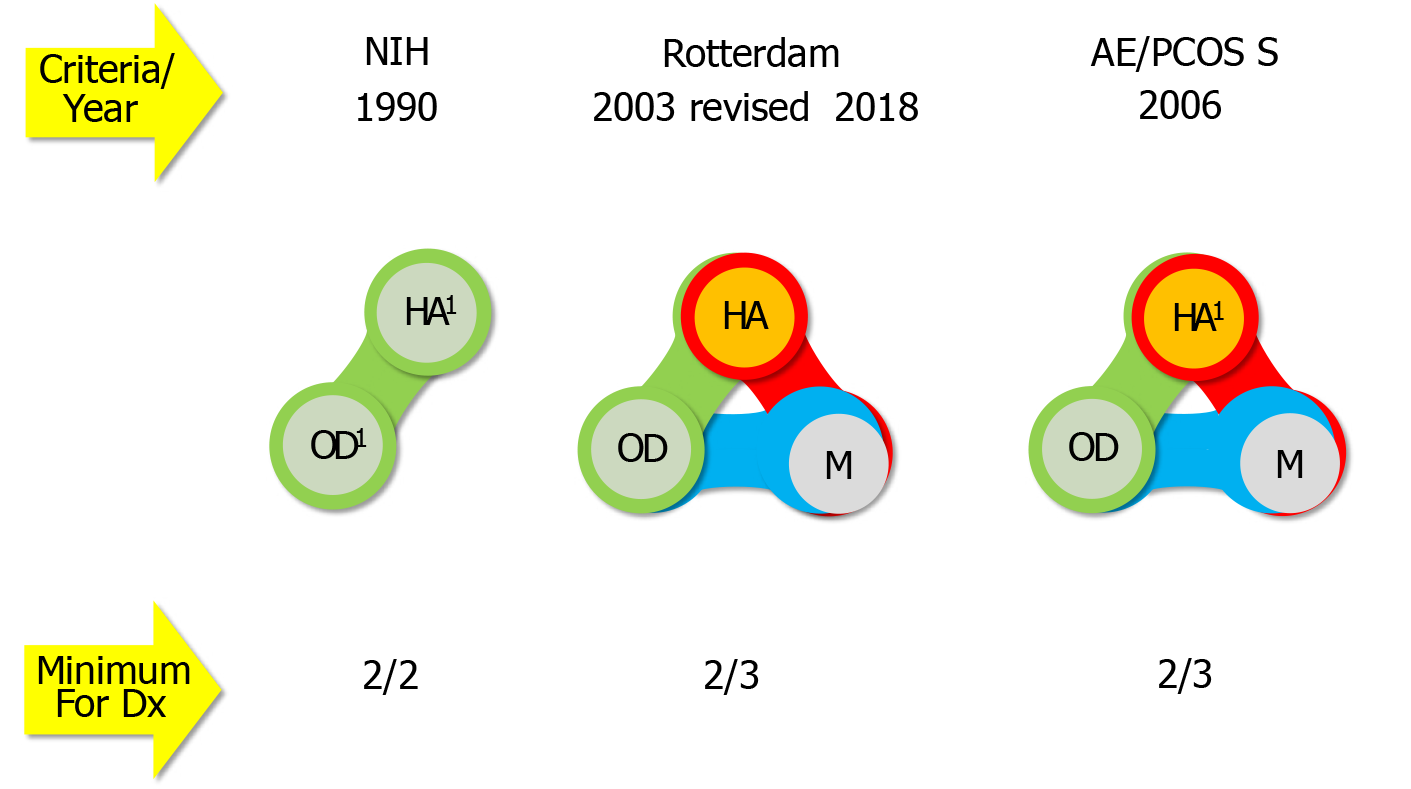
The definition of PCOS has shifted over the years, according to different diagnostic criteria, which define an array of disease phenotypes (Figure 1), taking into account signs of hyperandrogenemia and ovarian dysfunction and of ovarian morphology[1-4]. Currently, experts suggest the use of the Rotterdam criteria, as refined in 2018[5-7].
The current understanding of the pathogenesis of PCOS suggests that it is a complex polygenic disorder[8]. Various studies have studied candidate genes that can regulate the hypothalamic-pituitary-ovary axis as well as genes responsible for insulin resistance[9]. There is a familial predisposition for high levels of dehydroepiandro-sterone sulphate in siblings of women with PCOS, suggesting that this is a genetic characteristic[10]. Additionally, first-degree relatives of patients with PCOS carry an increased risk of cardiovascular disease, as do patients with PCOS[11-13]. Whether the molecules involved in low-grade inflammation are involved in the pathogenesis of hyperandrogenemia or, conversely, if excess androgens may-in some way-lead to the promotion of inflammation, is still a controversial issue. Although direct involvement of androgens in low-grade inflammation has not been shown, available evidence suggests that androgens may be indirectly involved in the development of low-grade inflammation, via an effect on adipose tissue and on resistance to insulin[14,15]. In fact, androgens stimulate adipocyte hypertrophy, affecting the expression of enzymes and proteins involved in lipid and carbohydrate metabolism, oxidative stress and differentiation of pre-adipocytes into mature adipocytes. Androgen excess can impair insulin action, either directly at the insulin receptor level or indirectly via changes effected at various tissues. In addition, androgens increase lipolysis, resulting in increased release of free fatty acids[16,17].
Research on the relationship between adiponectin and testosterone has given conflicting results. Similar results have been found for leptin. A strong negative relationship between circulating levels of ghrelin with androgens, especially androstenedione, has been found in women with PCOS[18]. The production of the latter steroid results from endogenously malfunctioning ovaries and adrenal glands. The high intra-ovarian concentration of androgens inhibits follicular maturation, leading to the appearance of polycystic ovaries. The usual source of excess androgens is due to functional ovarian hyperandrogenism, which is characterized by increased 17-hydroxyprogesterone after stimulation with luteinizing hormone releasing hormone or human chorionic gonadotropin. Endogenous dysfunction of ovarian cells appears to contribute to 50%-75% of cases of PCOS[19].
Obesity is present in about 50%-80% of women with PCOS, but this relationship also depends on environmental factors[20,21]. A lower prevalence of obesity in women with PCOS has been reported in populations where severe obesity is less prevalent, such as in Asians and in some Europeans[22,23]. Some argue that in the community there may not be more obese women with PCOS than obese women without PCOS[24]. With age, there is also increase in body mass index (BMI), waist circumference and of the waist to hip ratio[25,26]. The annualized conversion rate from normal glucose tolerance to impaired is higher in obese women with PCOS (16%) compared to the general obese population (1%-5%); the annualized conversion rate of obese women with PCOS from impaired glucose tolerance to diabetes at 2% is not different from the rate of the general obese population[27]. There is a synergistic effect of obesity on worsening glucose intolerance in women with PCOS. We have to note that regarding obesity, women with PCOS present a challenge for researchers, since BMI may not characterize them adequately[28,29]. Studies have evaluated the distribution of fat (subcutaneous and visceral fat) in obese women with PCOS using magnetic resonance imaging and dual energy X-ray with conflicting results. Women with PCOS have more central body fat distribution and increased waist/hip circumference compared to women without PCOS and similar BMI[11] (central obesity is a risk factor for pre-diabetes and cardiovascular disease). It is estimated that in about 75% of normal weight and overweight women with PCOS insulin action is impaired. Hyperinsulinemia and insulin resistance can induce both the endocrine and reproductive traits of PCOS, but the mechanisms that underlie this remain elusive[30]. Furthermore, in obesity, visceral adipose tissue becomes dysfunctional (with an increase in inflammatory molecules and a decrease in the expression of lipogenic enzymes); in this way–via various signaling pathways-chronic inflammation is favored over storage, contributing to the development of metabolic complications[31,32]. These obesity-associated signaling pathways and mechanisms are not fully delineated. Various adipose tissue genes are differentially expressed in subjects with obesity/insulin resistance; more in detail, in these subjects genes which are associated with lipid uptake and processing are less expressed compared to lean individuals[33,34]. Recent research indicates that in obesity, adipokine imbalance (low adiponectin and high leptin) modulates the activation of inflammasomes (receptors/sensors of the innate immune system that regulate caspase-1 activation and promote inflammation)[35]; thus the latter may be the connectors between excess adiposity and obesity-associated complications.
Various definitions by different authorities have been proposed for the definition of the cluster of metabolic disturbances that comprise the metabolic syndrome (MetSy); invariably they include central obesity, dyslipidemia, insulin resistance, and hypertension[36]. A diet rich in saturated fat and fructose may lead to non-alcoholic fatty liver disease[37,38] (NAFLD; indicating hepatic steatosis which is not attributed to alcohol or other specific etiologies and is found in at least 25% of the world population[39,40]), metabolic endotoxinemia and increased resistance to the action of insulin[36]. PCOS, MetSy and NAFLD apparently share common pathogenic factors; these include abdominal adiposity, excess body weight and insulin resistance[41,42]. Women with PCOS–particularly with hyperadrogenemia-have a two-fold to four-fold higher probability of having NAFLD compared to non-PCOS women[43,44]; 35%-70% of women with PCOS have NAFLD[44-46] and 60% have insulin resistance[45]. Insulin resistance, via activation–among others-of the carbohydrate response element binding protein and sterol response element binding protein 1c (both act as transcription factors), leads to intra-hepatic lipid accumulation[44].
Three main mechanisms have been put forth regarding the effect of intestinal microbiome on glucose intolerance/insulin resistance and type 2 diabetes: The promotion of metabolic inflammation, the modification of incretin secretion and the modification of hydroxybutyric acid production[47-49]. Parabacteroides merdae, Bacteroides fragilis, Catenibacterium and Kandleria genera and strains of Escherichia and Shigella are more abundant in women with PCOS compared to controls[50]; the presence of specific microbes in women with PCOS is positively correlated with BMI, high serum testosterone and elevated luteinizing hormone[51-54]. Women with PCOS have less hydroxybutyric acid-producing genera[55]. Low levels of interleukin 22 (IL-22) are noted in women with PCOS[56]. This interleukin helps to maintain the integrity of the gut epithelial barrier[57]. Thus, an altered gut microbiome may lead to deterioration of the intestinal barrier, increased gut mucosal permeability and passage into the circulation of lipopolysaccaride from Gram negative colonic bacteria. Lipopolysac-caride in the circulation (attached to the glycoprotein L. barbarum polysaccharides) binds to the CD14 toll-like receptor complex (TRL-4) on the surface of innate immune cells, leads to activation of a downstream signaling pathway and immune system activation[58]. The latter impedes insulin receptor function and leads to hyper-insulinemia and glucose intolerance, which hamper normal ovarian function and follicular development (all being hallmarks of PCOS)[58,59]. Modulation of the intestinal (and of the vaginal) microbiome has been put forth as holding therapeutic potential for PCOS[60].
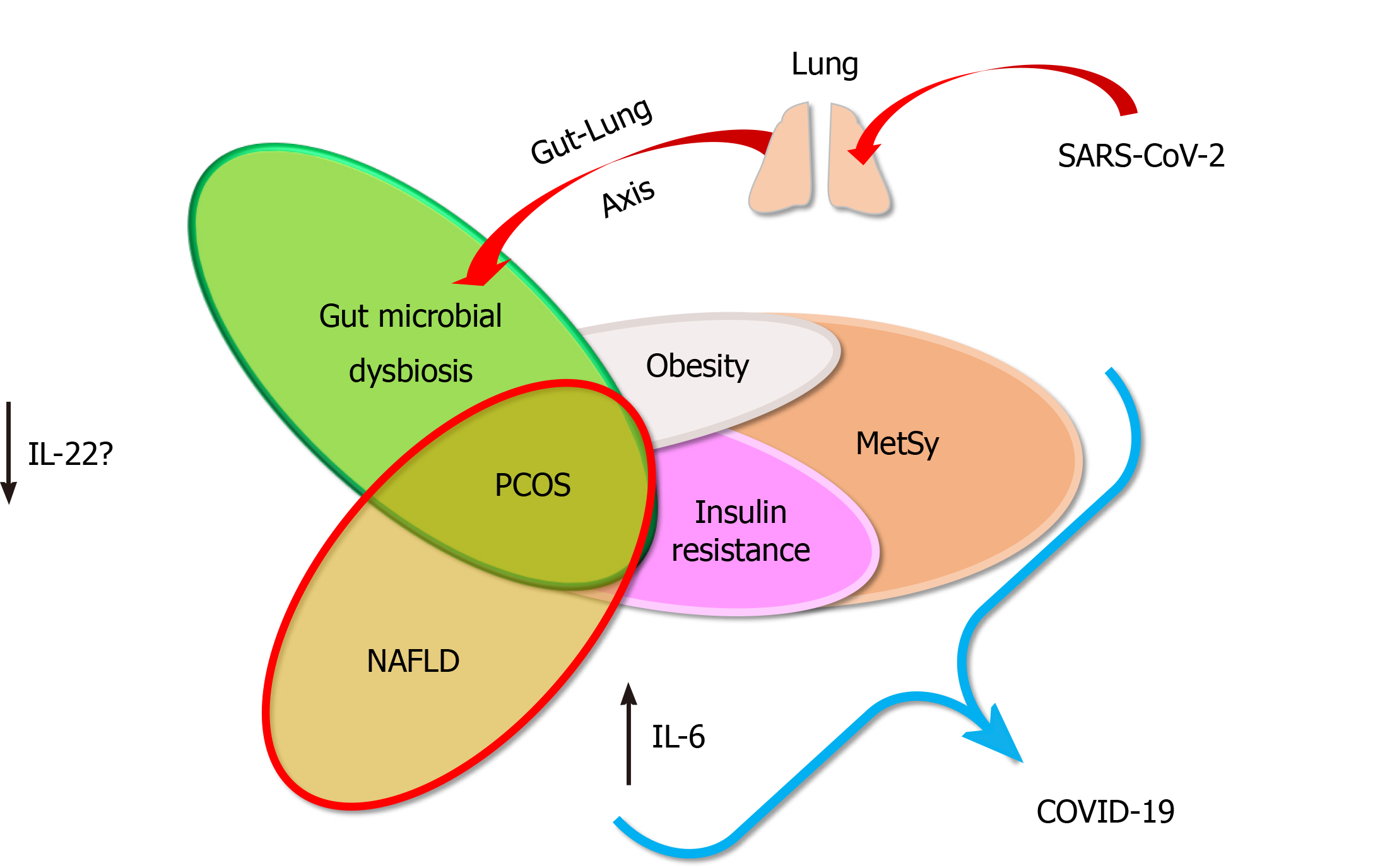
To gain cell entry, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses the host’s angiotensin-converting enzyme 2 (ACE2), in synergy with the host’s transmembrane protease, serine 2 (TMPRSS2); the latter’s expression is androgen-regulated[61,62]. It has been proposed that PCOS, given this condition’s hyperadrogen-emic environment, may entail higher susceptibility to coronavirus disease 2019 (COVID-19)[63-65]. Furthermore, PCOS may also increase susceptibility to COVID-19 via its associated comorbidities (NAFLD, obesity, MetSy and alterations in the gut microbiome) (Figure 2). Obese patients with advanced NAFLD have been shown to have increased hepatic mRNA expression of ACE2 and TMPRSS2, the critical molecules for SARS-CoV-2 cellular entry (gender-specific differences may exist in the expression of these molecules)[66].
The term gut-lung axis describes the interaction between the intestinal microbiome and the lungs[67]. This communication is, in fact, two-way[68]. Endotoxins and metabolites produced by bacteria in the gut (due to systemic inflammation, IL-6-induced vascular damage and increased intestinal permeability that may facilitate bacterial translocation) can move through the bloodstream and reach the lungs[69]. Similarly, pulmonary inflammation can have an effect on intestinal integrity. This raises the question of whether the SARS-CoV-2 virus can affect the intestinal microbiome[68]. In fact, several studies have shown that respiratory infections are associated with changes in the composition of the intestinal microbiome[70]. Some studies have found an association between acute respiratory distress syndrome (seen in severe cases of COVID-19) and the intestinal microbiome[67,69]. Furthermore, apparently, SARS-CoV-2 can gain entry to the gastrointestinal tract via locally-expressed ACE2 receptors[71].
Regardless of the definition of obesity (in western countries it is defined as a BMI higher than 30.0 kg/m2 or in China over 27.5 kg/m2), excess body weight is associated with more severe SARS-CoV-2 infection (COVID-19) and increased mortality[72,73]. The etiology for the latter is still obscure, although it is known that obesity is a state of low-grade inflammation, which COVID-19 pushes to extremes (with a characteristic “cytokine storm”[74]). Of note, obesity may lead not only to more adipose tissue accumulation but also to larger abdominal organ size[75]; we have speculated that larger abdominal organs may provide a larger tissue reservoir for the pervasive SARS-CoV-2 virus, since the latter has indeed been localized in abdominal organs[76].
The MetSy is characterized by hyperinsulinemia, which may be associated with facets of COVID-19[77], particularly regarding microvascular dysfunction[78-80], systemic hypercoagulability and extensive micro- and macrovascular thrombosis[81,82].
As indicated above, ACE2 offers entry to SARS-CoV-2 for cell infection[83]. This enzyme normally shows low expression in cholangiocytes and hepatocytes, but its expression increases–at least in cholangiocytes-in chronic liver disease and experimental diet-induced NAFLD[83]. The virus is pervasive and is localized in abdominal and extraabdominal organs, including the liver[84]. There are conflicting reports regarding NAFLD and COVID-19[85,86]. Some researchers have shown increased hospitalization[87], morbidity[88-91] and mortality from COVID-19 in patients with NAFLD, whereas other researchers have shown that NAFLD per se in hospitalized patients was not linked with worse prognosis[92] (but NAFLD-associated inflammatory parameters were associated with prognosis[86]).
Although robust links between SARS-CoV-2 infection and the chain of PCOS/NAFLD/gut microbiome/metabolic consequences have not been confirmed, there is evidence that merits further investigation. No research to date has been able to answer whether there is a cause-and-effect relationship. In order to determine whether the intestinal microbiome–particularly in women with PCOS with or without NAFLD, MetSy or obesity-affects the risk of COVID-19 or if SARS-CoV-2 is the factor that changes the composition of the microbiome, more research will be needed. Nevertheless, strategies for adapting the intestinal microbiome (probably in all patients) could help reduce the severity of COVID-19 in women with PCOS with or without NAFLD, MetSy or obesity.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good):
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Mohammadi M, Sun XD S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Mumusoglu S, Yildiz BO. Polycystic ovary syndrome phenotypes and prevalence: Differential impact of diagnostic criteria and clinical vs unselected population. Curr Opin Endocr Metab Res. 2020;12:66-71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Lucidi RS. Polycystic Ovarian Syndrome. Medscape 2019. [cited 25 October 2020]. Available from: https://emedicine.medscape.com/article/256806-overview#a1. |
| 3. | Neven ACH, Laven J, Teede HJ, Boyle JA. A Summary on Polycystic Ovary Syndrome: Diagnostic Criteria, Prevalence, Clinical Manifestations, and Management According to the Latest International Guidelines. Semin Reprod Med. 2018;36:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Condorelli RA, Calogero AE, Di Mauro M, Mongioi' LM, Cannarella R, Rosta G, La Vignera S. Androgen excess and metabolic disorders in women with PCOS: beyond the body mass index. J Endocrinol Invest. 2018;41:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Azziz R. Polycystic Ovary Syndrome. Obstet Gynecol. 2018;132:321-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 316] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 6. | Azziz R, Kintziger K, Li R, Laven J, Morin-Papunen L, Merkin SS, Teede H, Yildiz BO. Recommendations for epidemiologic and phenotypic research in polycystic ovary syndrome: an androgen excess and PCOS society resource. Hum Reprod. 2019;34:2254-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 419] [Cited by in RCA: 706] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 8. | Crespo RP, Bachega TASS, Mendonça BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab. 2018;62:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Gourbesville C, Kerlan V, Reznik Y. Le syndrome des ovaires polykystiques : quelles nouveautés en 2019 ? Ann Endocrinol (Paris). 2019;80 Suppl 1:S29-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Legro RS. Detection of insulin resistance and its treatment in adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2002;15 Suppl 5:1367-1378. [PubMed] |
| 11. | Yildir IC, Kutluturk F, Tasliyurt T, Yelken BM, Acu B, Beyhan M, Erkorkmaz U, Yilmaz A. Insulin resistance and cardiovascular risk factors in women with PCOS who have normal glucose tolerance test. Gynecol Endocrinol. 2013;29:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Goodarzi MO, Carmina E, Azziz R. DHEA, DHEAS and PCOS. J Steroid Biochem Mol Biol. 2015;145:213-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | Luque-Ramírez M, Escobar-Morreale HF. Adrenal Hyperandrogenism and Polycystic Ovary Syndrome. Curr Pharm Des. 2016;22:5588-5602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Dimitriadis GK, Kyrou I, Randeva HS. Polycystic Ovary Syndrome as a Proinflammatory State: The Role of Adipokines. Curr Pharm Des. 2016;22:5535-5546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Ezeh U, Chen IY, Chen YH, Azziz R. Adipocyte Insulin Resistance in PCOS: Relationship With GLUT-4 Expression and Whole-Body Glucose Disposal and β-Cell Function. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Baddela VS, Sharma A, Vanselow J. Non-esterified fatty acids in the ovary: friends or foes? Reprod Biol Endocrinol. 2020;18:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Newell-Fugate AE. The role of sex steroids in white adipose tissue adipocyte function. Reproduction. 2017;153:R133-R149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Repaci A, Gambineri A, Pasquali R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol. 2011;335:30-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 2010; 203: 201.e1-201. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Šimková M, Vítků J, Kolátorová L, Vrbíková J, Vosátková M, Včelák J, Dušková M. Endocrine disruptors, obesity, and cytokines - how relevant are they to PCOS? Physiol Res. 2020;69:S279-S293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Wekker V, van Dammen L, Koning A, Heida KY, Painter RC, Limpens J, Laven JSE, Roeters van Lennep JE, Roseboom TJ, Hoek A. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2020;26:942-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 212] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 22. | Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 23. | Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:618-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 555] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 24. | Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 25. | Chang SH, Beason TS, Hunleth JM, Colditz GA. A systematic review of body fat distribution and mortality in older people. Maturitas. 2012;72:175-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin BA, Zambon A, Barter P, Fruchart JC, Eckel RH, Matsuzawa Y, Després JP. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 984] [Article Influence: 196.8] [Reference Citation Analysis (0)] |
| 27. | Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236-3242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Goyal M, Dawood AS. Debates Regarding Lean Patients with Polycystic Ovary Syndrome: A Narrative Review. J Hum Reprod Sci. 2017;10:154-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Blundell JE, Dulloo AG, Salvador J, Frühbeck G; EASO SAB Working Group on BMI. Beyond BMI--phenotyping the obesities. Obes Facts. 2014;7:322-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 30. | Moghetti P, Tosi F. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest. 2021;44:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 31. | Poulain-Godefroy O, Lecoeur C, Pattou F, Frühbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1-R7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Catalán V, Gómez-Ambrosi J, Rodríguez A, Pérez-Hernández AI, Gurbindo J, Ramírez B, Méndez-Giménez L, Rotellar F, Valentí V, Moncada R, Martí P, Sola I, Silva C, Salvador J, Frühbeck G. Activation of noncanonical Wnt signaling through WNT5A in visceral adipose tissue of obese subjects is related to inflammation. J Clin Endocrinol Metab. 2014;99:E1407-E1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Catalán V, Gómez-Ambrosi J, Rotellar F, Silva C, Rodríguez A, Salvador J, Gil MJ, Cienfuegos JA, Frühbeck G. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res. 2007;39:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Clemente-Postigo M, Queipo-Ortuño MI, Fernandez-Garcia D, Gomez-Huelgas R, Tinahones FJ, Cardona F. Adipose Tissue Gene Expression of Factors Related to Lipid Processing in Obesity. PLOS ONE. 2011;6:e24783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Pham DV, Park PH. Recent insights on modulation of inflammasomes by adipokines: a critical event for the pathogenesis of obesity and metabolism-associated diseases. Arch Pharm Res. 2020;43:997-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 36. | Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129:4050-4057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 440] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 37. | Flores-Ramírez AG, Ibarra-Reynoso LDR, López-Lemus HL, Olvera-Juárez M, Luevano-Contreras C, Garay-Sevilla ME. Insulin-like growth factor binding protein-1, non-alcoholic fatty liver disease, and its relationship with fructose consumption in children with obesity. Rev Invest Clin. 2019;71:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Skenderian S, Park G, Jang C. Organismal Fructose Metabolism in Health and Non-Alcoholic Fatty Liver Disease. Biology (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263-8276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 543] [Cited by in RCA: 509] [Article Influence: 63.6] [Reference Citation Analysis (6)] |
| 40. | DiStefano JK, Shaibi GQ. The relationship between excessive dietary fructose consumption and paediatric fatty liver disease. Pediatr Obes. 2020;e12759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 41. | Softic S, Stanhope KL, Boucher J, Divanovic S, Lanaspa MA, Johnson RJ, Kahn CR. Fructose and hepatic insulin resistance. Crit Rev Clin Lab Sci. 2020;57:308-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 42. | Rocha AL, Oliveira FR, Azevedo RC, Silva VA, Peres TM, Candido AL, Gomes KB, Reis FM. Recent advances in the understanding and management of polycystic ovary syndrome. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 43. | Asfari MM, Sarmini MT, Baidoun F, Al-Khadra Y, Ezzaizi Y, Dasarathy S, McCullough A. Association of non-alcoholic fatty liver disease and polycystic ovarian syndrome. BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Lonardo A, Mantovani A, Lugari S, Targher G. NAFLD in Some Common Endocrine Diseases: Prevalence, Pathophysiology, and Principles of Diagnosis and Management. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 45. | Di Ciaula A, Christidis G, Krawczyk M, Lammert F, Portincasa P. Impact of Endocrine Disorders on the Liver. In: Portincasa P, Fruhbeck G, Nathoe HM. Endocrinology and Systemic Diseases. Cham, Switzerland: Springer Nature Switzerland AG, 2020: 157-177. |
| 46. | Di Ciaula A, Wang DQH, Sommers T, Lembo A, Portincasa P. Impact of Endocrine Disorders on Gastrointestinal Diseases. In: Portincasa P, Fruhbeck G, Nathoe HM. Endocrinology and Systemic Diseases. Cham, Switzerland: Springer Nature Switzerland AG, 2020: 179-225. |
| 47. | Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 48. | Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 646] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 49. | Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 507] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 50. | Insenser M, Murri M, Del Campo R, Martínez-García MÁ, Fernández-Durán E, Escobar-Morreale HF. Gut Microbiota and the Polycystic Ovary Syndrome: Influence of Sex, Sex Hormones, and Obesity. J Clin Endocrinol Metab. 2018;103:2552-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 51. | Chu W, Han Q, Xu J, Wang J, Sun Y, Li W, Chen ZJ, Du Y. Metagenomic analysis identified microbiome alterations and pathological association between intestinal microbiota and polycystic ovary syndrome. Fertil Steril 2020; 113: 1286-1298. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 52. | Zhou L, Ni Z, Yu J, Cheng W, Cai Z, Yu C. Correlation Between Fecal Metabolomics and Gut Microbiota in Obesity and Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2020;11:628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 53. | Jobira B, Frank DN, Pyle L, Silveira LJ, Kelsey MM, Garcia-Reyes Y, Robertson CE, Ir D, Nadeau KJ, Cree-Green M. Obese Adolescents With PCOS Have Altered Biodiversity and Relative Abundance in Gastrointestinal Microbiota. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 54. | Lindheim L, Bashir M, Münzker J, Trummer C, Zachhuber V, Leber B, Horvath A, Pieber TR, Gorkiewicz G, Stadlbauer V, Obermayer-Pietsch B. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS One. 2017;12:e0168390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 55. | Liang Y, Ming Q, Liang J, Zhang Y, Zhang H, Shen T. Gut microbiota dysbiosis in polycystic ovary syndrome: association with obesity - a preliminary report. Can J Physiol Pharmacol. 2020;98:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 56. | Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y, Liang X, Gonzalez FJ, Patterson AD, Liu H, Mu L, Zhou Z, Zhao Y, Li R, Liu P, Zhong C, Pang Y, Jiang C, Qiao J. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 489] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 57. | Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015;33:747-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 682] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 58. | He FF, Li YM. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res. 2020;13:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 59. | Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA)--a novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses. 2012;79:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 60. | Wang L, Zhou J, Gober HJ, Leung WT, Huang Z, Pan X, Li C, Zhang N, Wang L. Alterations in the intestinal microbiome associated with PCOS affect the clinical phenotype. Biomed Pharmacother. 2021;133:110958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 61. | Dong M, Zhang J, Ma X, Tan J, Chen L, Liu S, Xin Y, Zhuang L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131:110678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 62. | Foresta C, Rocca MS, Di Nisio A. Gender susceptibility to COVID-19: a review of the putative role of sex hormones and X chromosome. J Endocrinol Invest. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 63. | Moradi F, Enjezab B, Ghadiri-Anari A. The role of androgens in COVID-19. Diabetes Metab Syndr. 2020;14:2003-2006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Morgante G, Troìa L, De Leo V. Coronavirus Disease 2019 (SARS-CoV-2) and polycystic ovarian disease: Is there a higher risk for these women? J Steroid Biochem Mol Biol. 2021;205:105770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Kyrou I, Karteris E, Robbins T, Chatha K, Drenos F, Randeva HS. Polycystic ovary syndrome (PCOS) and COVID-19: an overlooked female patient population at potentially higher risk during the COVID-19 pandemic. BMC Med. 2020;18:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 66. | Fondevila MF, Mercado-Gómez M, Rodríguez A, Gonzalez-Rellan MJ, Iruzubieta P, Valentí V, Escalada J, Schwaninger M, Prevot V, Dieguez C, Crespo J, Frühbeck G, Martinez-Chantar ML, Nogueiras R. Obese patients with NASH have increased hepatic expression of SARS-CoV-2 critical entry points. J Hepatol. 2021;74:469-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 67. | Pugin J, Chevrolet JC. [The intestine-liver-lung axis in septic syndrome]. Schweiz Med Wochenschr. 1991;121:1538-1544. [PubMed] |
| 68. | Uzzan M, Corcos O, Martin JC, Treton X, Bouhnik Y. Why is SARS-CoV-2 infection more severe in obese men? Med Hypotheses. 2020;144:110023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Cardinale V, Capurso G, Ianiro G, Gasbarrini A, Arcidiacono PG, Alvaro D. Intestinal permeability changes with bacterial translocation as key events modulating systemic host immune response to SARS-CoV-2: A working hypothesis. Dig Liver Dis. 2020;52:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 70. | Scaldaferri F, Ianiro G, Privitera G, Lopetuso LR, Vetrone LM, Petito V, Pugliese D, Neri M, Cammarota G, Ringel Y, Costamagna G, Gasbarrini A, Boskoski I, Armuzzi A. The Thrilling Journey of SARS-CoV-2 into the Intestine: From Pathogenesis to Future Clinical Implications. Inflamm Bowel Dis. 2020;26:1306-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Galanopoulos M, Doukatas A, Gazouli M. Origin and genomic characteristics of SARS-CoV-2 and its interaction with angiotensin converting enzyme type 2 receptors, focusing on the gastrointestinal tract. World J Gastroenterol. 2020;26:6335-6345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (2)] |
| 72. | Michalakis K, Ilias I. SARS-CoV-2 infection and obesity: Common inflammatory and metabolic aspects. Diabetes Metab Syndr. 2020;14:469-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 73. | Michalakis K, Panagiotou G, Ilias I, Pazaitou-Panayiotou K. Obesity and COVID-19: A jigsaw puzzle with still missing pieces. Clin Obes. 2021;11:e12420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, Hirayama AV, Mastroiani F, Turtle CJ, Harhay MO, Legrand M, Deutschman CS. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 660] [Cited by in RCA: 629] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 75. | Grant H, Zhang Y, Li L, Wang Y, Kawamoto S, Pénisson S, Fouladi DF, Shayesteh S, Blanco A, Ghandili S, Zinreich E, Graves JS, Park S, Kern S, Hooper J, Yuille AL, Fishman EK, Chu L, Tomasetti C. Larger organ size caused by obesity is a mechanism for higher cancer risk. 2020 Preprint. bioRxiv. . [DOI] [Full Text] |
| 76. | Zabuliene L, Ilias I. Obesity, abdominal organ size and COVID-19 severity. Med Hypotheses. 2020;144:110279-110279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 77. | Cooper ID, Crofts CAP, DiNicolantonio JJ, Malhotra A, Elliott B, Kyriakidou Y, Brookler KH. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: rationale for clinical management. Open Heart. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 78. | Bansal R, Gubbi S, Muniyappa R. Metabolic Syndrome and COVID 19: Endocrine-Immune-Vascular Interactions Shapes Clinical Course. Endocrinology. 2020;161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 79. | Hayden MR. Endothelial activation and dysfunction in metabolic syndrome, type 2 diabetes and coronavirus disease 2019. J Int Med Res. 2020;48:300060520939746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Smith M, Honce R, Schultz-Cherry S. Metabolic Syndrome and Viral Pathogenesis: Lessons from Influenza and Coronaviruses. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 81. | Gąsecka A, Borovac JA, Guerreiro RA, Giustozzi M, Parker W, Caldeira D, Chiva-Blanch G. Thrombotic Complications in Patients with COVID-19: Pathophysiological Mechanisms, Diagnosis, and Treatment. Cardiovasc Drugs Ther. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 82. | Lupu L, Palmer A, Huber-Lang M. Inflammation, Thrombosis, and Destruction: The Three-Headed Cerberus of Trauma- and SARS-CoV-2-Induced ARDS. Front Immunol. 2020;11:584514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Hammoud SH, Wehbe Z, Abdelhady S, Kobeissy F, Eid AH, El-Yazbi AF. Dysregulation of Angiotensin Converting Enzyme 2 Expression and Function in Comorbid Disease Conditions Possibly Contributes to Coronavirus Infectious Disease 2019 Complication Severity. Mol Pharmacol. 2021;99:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383:590-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1254] [Cited by in RCA: 1411] [Article Influence: 282.2] [Reference Citation Analysis (0)] |
| 85. | Valenti L, Jamialahmadi O, Romeo S. Lack of genetic evidence that fatty liver disease predisposes to COVID-19. J Hepatol. 2020;73:709-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Forlano R, Mullish BH, Mukherjee SK, Nathwani R, Harlow C, Crook P, Judge R, Soubieres A, Middleton P, Daunt A, Perez-Guzman P, Selvapatt N, Lemoine M, Dhar A, Thursz MR, Nayagam S, Manousou P. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One. 2020;15:e0240400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 87. | Bramante C, Tignanelli CJ, Dutta N, Jones E, Tamariz L, Clark JM, Usher M, Metlon-Meaux G, Ikramuddin S. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 88. | Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 89. | Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 90. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (2)] |
| 91. | Sharma P, Kumar A. Metabolic dysfunction associated fatty liver disease increases risk of severe Covid-19. Diabetes Metab Syndr. 2020;14:825-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 92. | Lopez-Mendez I, Aquino-Matus J, Gall SM, Prieto-Nava JD, Juarez-Hernandez E, Uribe M, Castro-Narro G. Association of liver steatosis and fibrosis with clinical outcomes in patients with SARS-CoV-2 infection (COVID-19). Ann Hepatol. 2021;20:100271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |