Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2312
Peer-review started: October 15, 2020
First decision: December 28, 2020
Revised: January 4, 2021
Accepted: February 19, 2021
Article in press: February 19, 2021
Published online: April 6, 2021
Processing time: 165 Days and 14.8 Hours
Mandibular advancement devices (MADs) are used to treat mild to moderate obstructive sleep apnea (OSA), but there is a risk that the underlying condition can worsen in the long-term. Therefore, this case report is based on biomimetic oral appliance therapy as an alternative to MADs, which was found to be beneficial in the treatment of a case with severe OSA.
An overnight sleep study was undertaken in a 50-year-old male with excessive daytime sleepiness that lead to a diagnosis of severe OSA as the apnea-hypopnea index (AHI) was found to be 32.8/h. Since the patient was unable to comply with continuous positive airway pressure therapy and declined surgical intervention, treatment with a MAD was initiated. Approximately 10 years later, another sleep study was performed with no MAD in the mouth, which revealed an AHI of 67.9/h. In view of the deterioration in sleep quality, the patient sought alternative treatment and elected on biomimetic oral appliance therapy, using a mandibular repositioning nighttime appliance (mRNA appliance®, Vivos Therapeutics, Inc., United States). After 10 mo, another sleep study was performed with no device in the patient’s mouth, which revealed an AHI of 11.8/h, a mean oxygen saturation of 94% and a mean oxygen desaturation index of 5.3% while sleeping. Finite-element analysis of the pre- and post-treatment study models of the upper jaw showed localized size increases of 15%-17% in the premolar regions and 15%-23% in the molar regions.
In adults with severe OSA that are unable to accept continuous positive airway pressure or surgical treatment, biomimetic oral appliance therapy may be preferable over MADs since biomimetic oral appliance therapy may be able to prevent worsening of sleep parameters by remodeling the nasomaxillary complex. Long-term follow up studies are required to verify these novel findings.
Core Tip: Mandibular advancement devices (MADs) are considered to be a first line therapy in adult cases of mild to moderate obstructive sleep apnea that are unable to comply with continuous positive airway pressure. However, both MADs and continuous positive airway pressure represent a lifetime of therapy and there is a risk that the underlying condition may worsen over time as the patient undergoes ageing. In contrast, biomimetic oral appliance therapy differs from MADs since it attempts to mimic normal craniofacial growth and development. The primary target of biomimetic oral appliance therapy is the nasomaxillary complex, which is non-surgically corrected even in adult cases followed by re-coordination with the mandible.
- Citation: Singh GD, Kherani S. Changes in sleep parameters following biomimetic oral appliance therapy: A case report. World J Clin Cases 2021; 9(10): 2312-2319
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2312.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2312
For the treatment of severe obstructive sleep apnea (OSA), the use of continuous positive airway pressure (CPAP) is recommended[1]. However, compliance with CPAP is poor[2] and oral appliances such as mandibular advancement devices (MADs) are often used for those adults who are unable to comply[3]. While MADs are initially successful, especially in the treatment of mild to moderate OSA, there is a risk that the underlying condition may worsen in the long-term[4] as noted in this particular case. In contrast, biomimetic oral appliance therapy (BOAT) is an alternative to MADs since the initial target tissue is the nasomaxillary complex, which is non-surgically corrected in adult cases of OSA. This novel approach has additional benefits as discussed in this case report.
A 60-year-old male presented to a dental office complaining of worsening daytime sleepiness.
For the treatment of OSA, MAD treatment had been initiated and he had complied with this treatment as prescribed for approximately 10 years. However, the patient had recurrent episodes of excessive daytime sleepiness and sought further clinical advice.
The patient had a previous medical diagnosis of severe OSA since an overnight sleep study undertaken in June 2008 had revealed an apnea-hypopnea index (AHI) of 32.8/h.
The patient was unable to comply with CPAP therapy and declined surgical intervention.
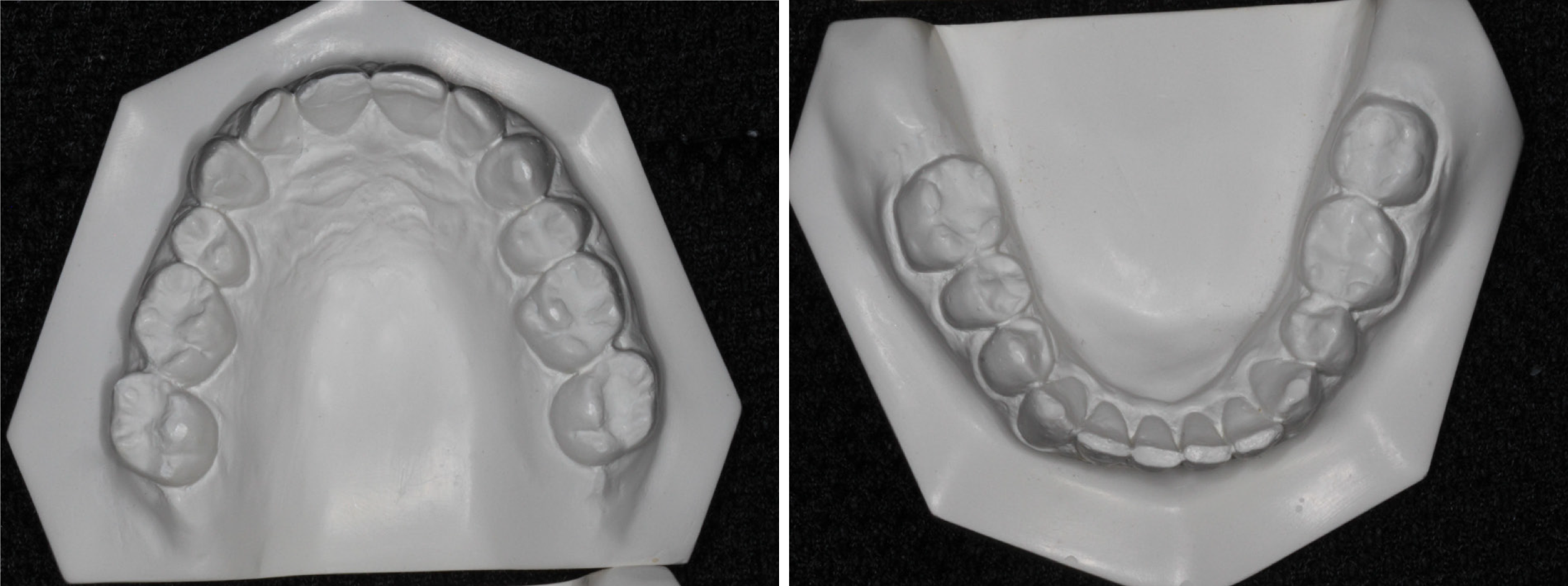
The patient’s body mass index was 25.7 kg/m2, indicating that obesity was not a pertinent consideration in this case. The neck circumference was 15 inches (38 cm) and the blood pressure was 131/96 mmHg, without any other pathological signs. Intra-oral examination revealed that the patient had good oral health with no signs of either dental decay or periodontal disease. The patient volunteered that four premolar teeth and four wisdom teeth had been removed for orthodontic reasons during his college years. On examination, it was noted that the upper and lower arches were reduced in size with mild crowding and wear facets of the anterior teeth (Figure 1). A Mallampati score of 3 indicated that the oropharyngeal inlet was reduced in aperture. The tongue was crenated but not restricted by the lingual frenum.
The initial home sleep apnea test (HSAT) taken in June 2008 (Watch PAT, United States) was available. It had recorded an AHI of 32.8/h, a respiratory disturbance index (RDI) of 34.3/h, a mean oxygen desaturation index of 13.8% and a mean oxygen saturation of 95%. There was 21.3% rapid eye movement (REM) sleep and a mean pulse of 76 bpm. Therefore, another HSAT was taken in February 2019 with no device in the patient’s mouth, which revealed an AHI of 67.9/h, an RDI of 68.4/h, a mean oxygen desaturation index of 52.6% and a mean oxygen saturation of 93%. There was 6.7% REM sleep and a mean pulse of 65 bpm.
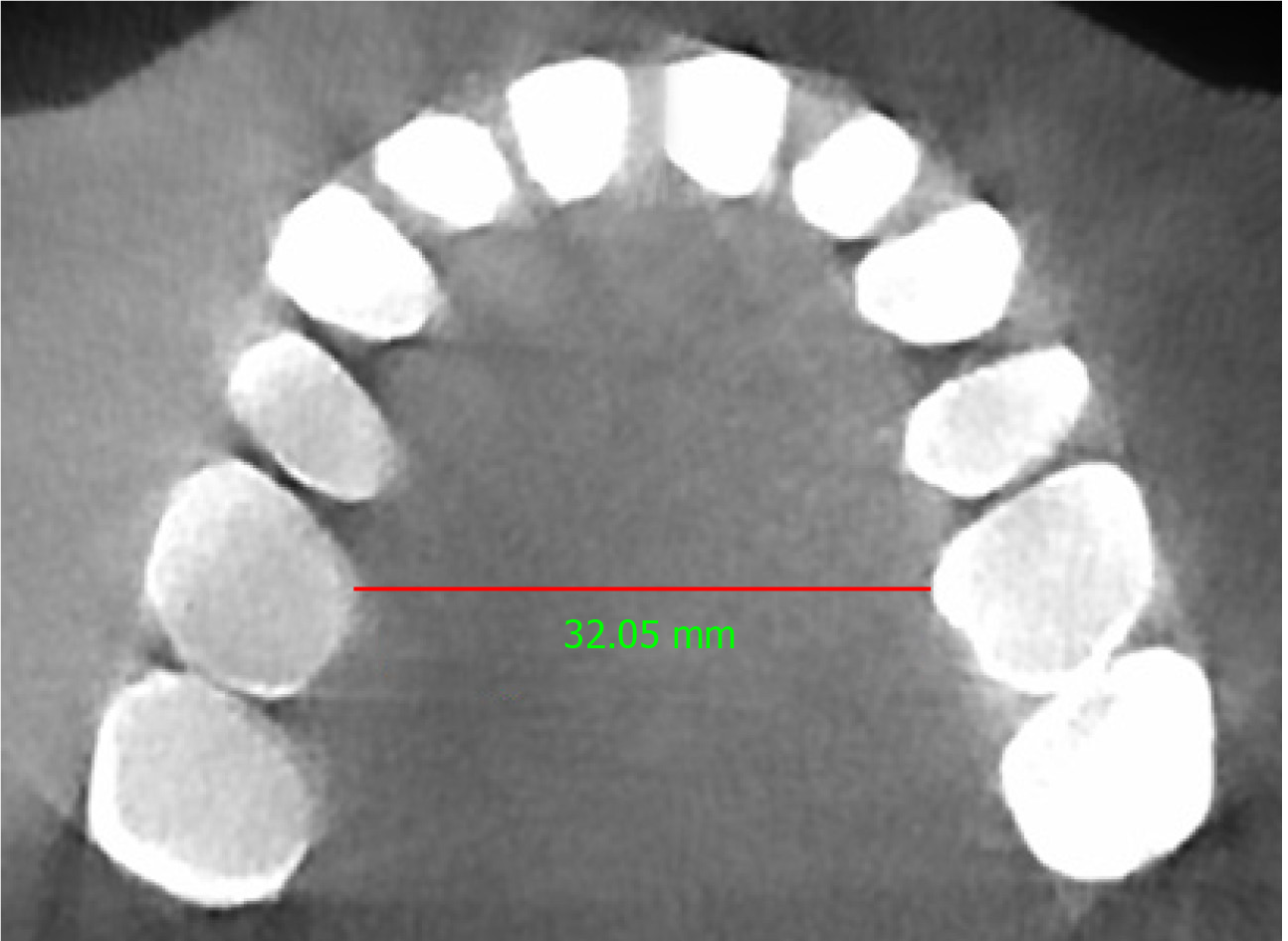
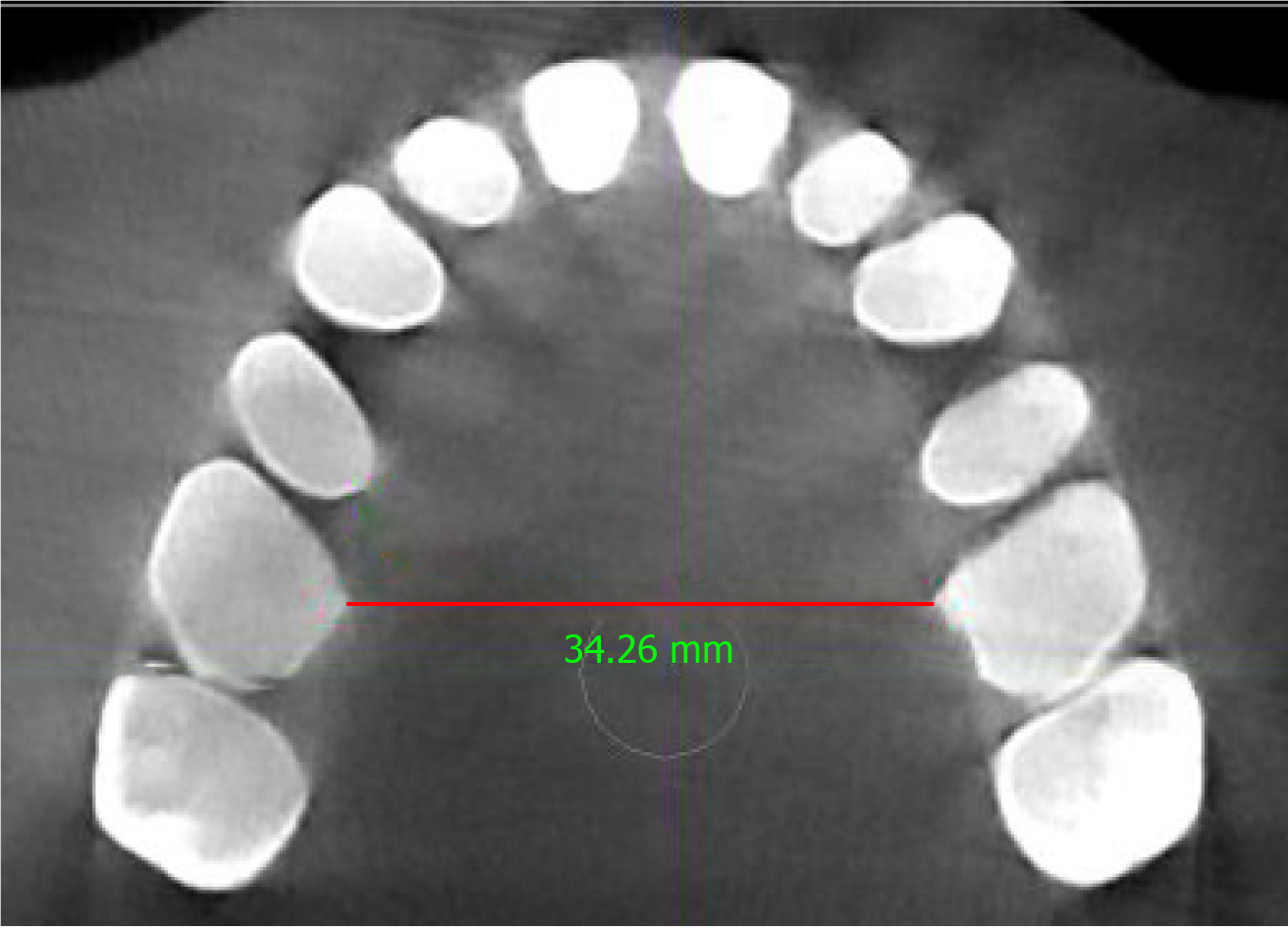
In this case, a pre-treatment 3D cone-beam computed tomography (CBCT) scan was available. The minimum transpalatal bone width measured at the cervical margin of the mesio-palatal cusps of the first molars on the CBCT scan was 32 mm (Figure 2). The nasal airway volume was 35.2 cm3 and the area of the posterior nasal aperture was 589.6 mm2.
A final diagnosis of severe OSA was made.
The patient declined CPAP and/or surgical treatment. Therefore, after obtaining informed consent, a neuromuscular bite registration was taken to achieve the physiologic rest position of the mandible[5]. This procedure involves the use of low-voltage transcutaneous electrical nerve stimulation to relax the muscles of mastication in the upright-sitting position to obtain the corrected jaw posture in the vertical axis. Subsequently, intra-oral upper and lower polyvinylsiloxane impressions were also obtained. After this, the upper study model was mounted using the hamular notch-incisive papilla plane method on a Stratos articulator (Ivoclar-Vivadent, Amherst, United States), and the lower model was mounted relative to the upper model, using the bite registration captured in the mandible’s physiologic rest position.
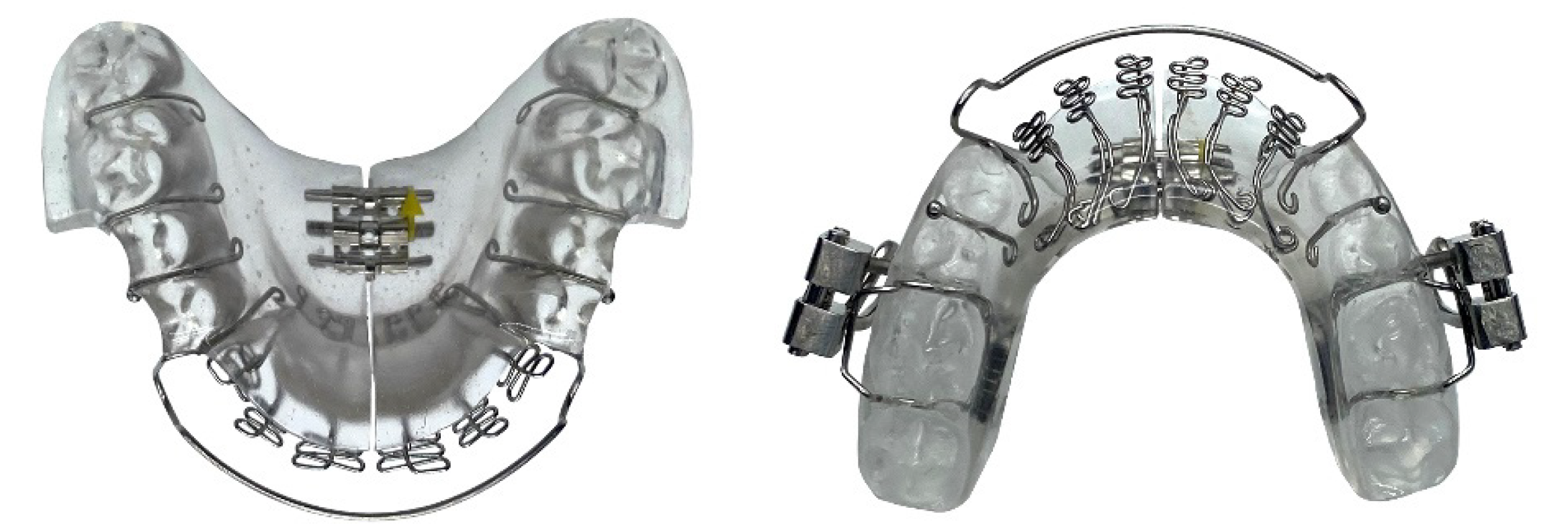
Next, a biomimetic oral appliance (mRNA appliance®, Vivos Therapeutics, Inc. United States) was prescribed since the patient declined another MAD. The biomimetic device was made following Food and Drug Administration (FDA) approved protocols and materials, by an FDA-registered laboratory. Note that BOAT aims to mimic natural growth and development of the maxillofacial complex, and the system is designed to correct craniofacial and upper airway issues in both children and adults. The device used in this study had: 6 anterior 3D axial springs; midline anterior/posterior 3-way screw; occlusal pads; retentive clasps, a labial bow and a screw-fin mechanism (Figure 3). The patient was instructed to wear the device during the late afternoon, early evening and at nighttime (for approximate 12-16 h in total), but not during the daytime and not while eating, partly in line with the circadian rhythm of tooth eruption[6], although this only occurs in children. Adjustments to the device were performed with 0.25 mm activation as required to optimize its efficacy. Only gentle pressures were transmitted to the teeth, and the functionality of the device was checked with the patient activating a mild force on biting. The appliance was adjusted by the clinician to fit the occlusion of the lower arch approx. every 4 wk when the patient reported for review. At each monthly follow-up, examination for the progress of midfacial development was recorded.
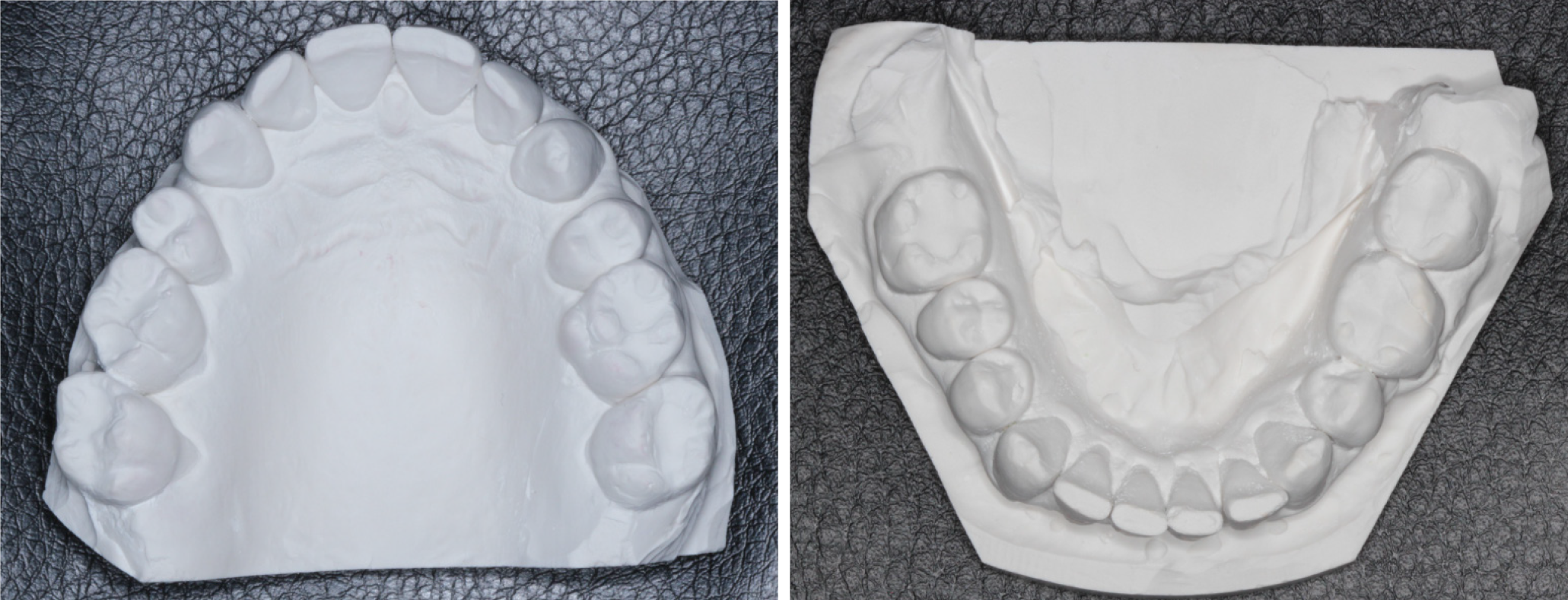
The patient reported subjective improvements in daytime sleepiness and a post-treatment HSAT was performed after 10 mo (December 2019) with no device in the patient’s mouth while sleeping. The HSAT recorded 6 h of sleep and revealed an AHI of 11.8/h, an RDI of 16.6/h, a mean oxygen desaturation index of 5.3%, and a mean oxygen saturation of 94%. These results are summarized in Table 1. There was 11.3% REM sleep and a mean pulse of 64 bpm. Since the patient’s body mass index remained stable, a post-treatment CBCT scan and study models were taken as before. The study models showed that the upper arch had increased in size with partial recapture of the premolar spaces (Figure 4). Similarly, the minimum transpalatal bone width measured on the CBCT scan had increased to approximate 34.2 mm (Figure 5); the nasal airway volume was now 36.1 cm3 in volume and the area of the posterior nasal aperture was 596.8 mm2.
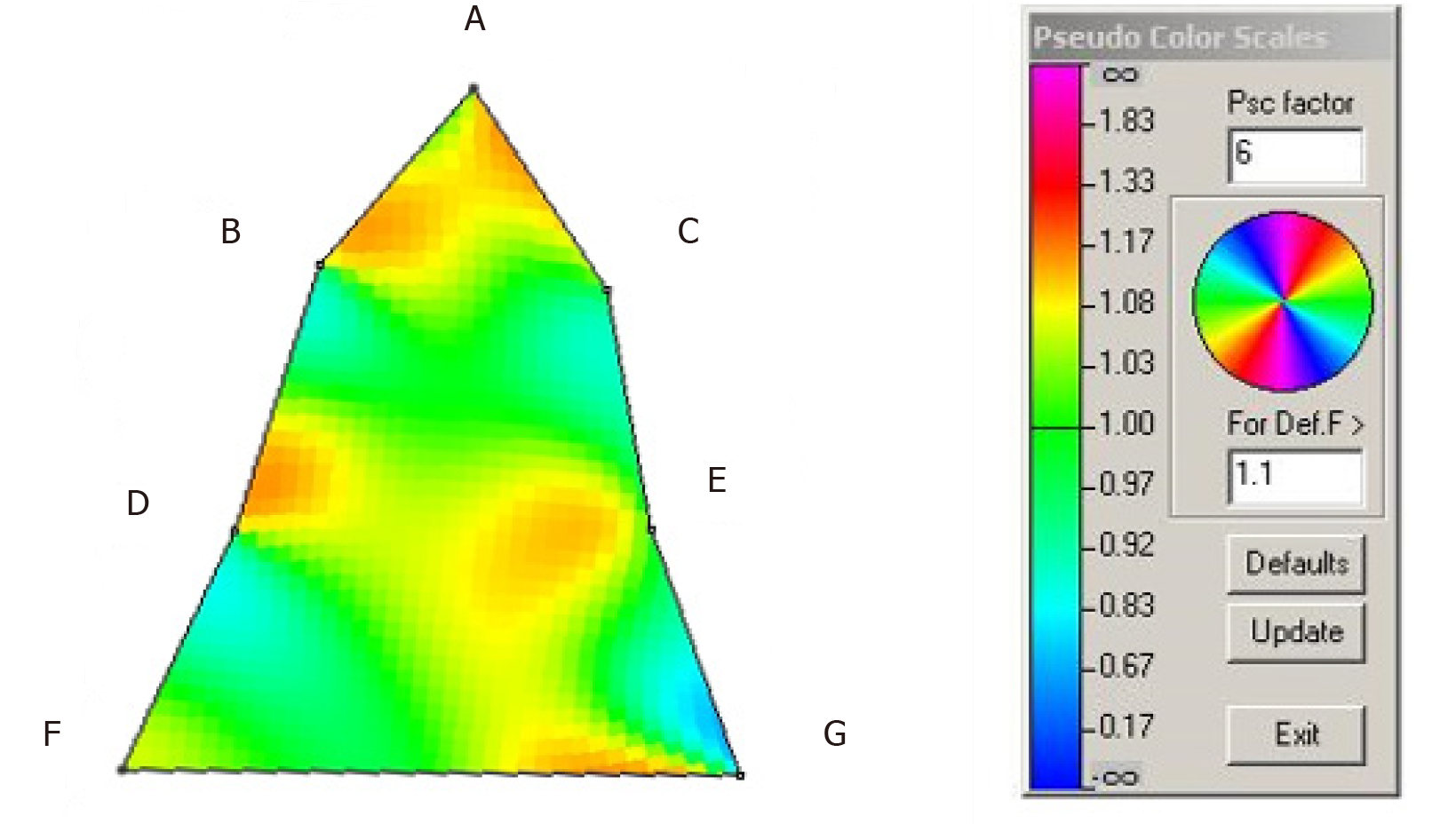
Finite-element analysis of the pre- and post-treatment upper arch (Figure 6) indicated localized size increases of 15%-20% in the incisor region; 15%-17% in the premolar regions and 15%-23% in the molar regions over 10 mo.
| Date | AHI (events/h) | ODI (%) | PaO2 (%) | RDI (events/h) |
| 06-2008 | 32.8 | 13.8 | 92 | 34.3 |
| 02-2019 | 67.9 | 52.6 | 91 | 68.4 |
| 12-2019 | 11.8 | 5.3 | 90 | 16.6 |
The treatment most preferred for the management of severe OSA with an AHI > 30/h is CPAP[1]. Despite these recommendations, low compliance with CPAP therapy is well established[2] and many patients seek alternative solutions. For these cases, several surgical procedures have been developed. For example, Liu et al[7] recently described the use of surgical implants combined with Le Fort I osteotomies for the successful treatment of mild to severe cases of OSA. In fact, several other surgical approaches, such as maxillo-mandibular advancement, have also been advocated[8]. However, even though a plethora of surgical procedures are available, many patients decline surgical intervention on the basis of surgical morbidity and prefer non-invasive approaches instead. Given this context of patient concerns, oral appliance therapy (typically MADs) have become the first line of treatment in cases of mild to moderate OSA, and even in severe cases, where no other viable alternatives are available. Nevertheless, MADs have their own limitations since some studies have shown long term deterioration of the underlying condition in cases treated with MADs[4]. This case report describes one such patient and sets the context for biomimetic oral appliance therapy.
Biomimetic oral appliance therapy differs from conventional MADs. While MADs are thought to mechanically enlarge the airway while being worn during sleep[9], BOAT aims to mimic natural craniofacial growth and development to induce putative upper airway remodeling that persists even after the device has been removed. It has been shown that BOAT increases the midfacial bone volume in adults non-surgically[10], which ostensibly permits nasal breathing through an enhanced nasal airway volume[11]. In fact, several studies have shown the efficacy of BOAT in mild, moderate and even severe cases of OSA[12]. However, a study reporting the effect of recapturing upper premolar spaces for the treatment of severe OSA has never been reported in the medical, dental or orthodontic literature to the best of our knowledge. The reason for this omission perhaps relates to the controversial role of teeth and OSA. For example, Guilleminault et al[13] are of the opinion that hypodontia, congenitally missing teeth and tooth extractions are associated with an increased risk of developing OSA. In contrast, Larsen et al[14] undertook an epidemiologic survey and reported that there was no difference in the prevalence of OSA in patients that had premolar teeth extracted for orthodontic reasons and those that did not. However, no sleep studies were undertaken in that survey and it’s possible that many cases of OSA remained undiagnosed in that study.
In view of the above contentions, this case report addresses several concerns. First, it describes the treatment of severe OSA in an adult that was unable to comply with CPAP but had also declined surgical intervention. Second, this particular case complied with MAD therapy for about 10 years not knowing that the underlying condition had worsened from an AHI of 32.8/h in June 2008 to an AHI of 67.9/h in February 2019. Third, this case illustrates that recapture of the upper premolar spaces, at least in part, appears to help in the amelioration of severe OSA. Figure 1 shows the patient’s upper study model with missing first premolars. This morphology was associated with a HSAT showing an AHI of 67.9/h. Post-treatment the AHI was reduced by 82.6% to 11.8/h after BOAT, which included the recapture of the upper premolar spaces (Figure 4). Finite-element analysis of the pre- and post-treatment upper arch (Figure 6) indicated localized size increases of 15%-17% in the premolar regions. These findings are in accord with the Spatial Matrix Hypothesis, which teaches that developmental compensation occurs to permit compromised function, precipitating conditions such as OSA. In the craniofacial region, decompensation is required through treatment with appropriate spatial signaling (such as the recapture of premolar spaces) to re-establish genomic pattern formation for optimal form and function, and it appears that this was achieved in this particular case using biomimetic oral appliance therapy.
In adults that are unable to accept continuous positive airway pressure or surgical treatment for severe obstructive sleep apnea, biomimetic oral appliance therapy may be preferable over mandibular advancement devices as it appears that biomimetic oral appliance therapy might be able to prevent worsening of sleep parameters by remodeling the nasomaxillary complex. However, long-term follow up studies are required to verify the novel findings presented in this particular case report.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Umbro I S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2019;15:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 461] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 2. | Li HY, Lee LA, Tsai MS, Chen NH, Chuang LP, Fang TJ, Shen SC, Cheng WN. How to manage continuous positive airway pressure (CPAP) failure -hybrid surgery and integrated treatment. Auris Nasus Larynx. 2020;47:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Medical Advisory Secretariat. Oral appliances for obstructive sleep apnea: an evidence-based analysis. Ont Health Technol Assess Ser. 2009;9:1-51. [PubMed] |
| 4. | Marklund M. Long-term efficacy of an oral appliance in early treated patients with obstructive sleep apnea. Sleep Breath. 2016;20:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Cooper BC, Cooper DL, Lucente FE. Electromyography of masticatory muscles in craniomandibular disorders. Laryngoscope. 1991;101:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Proffit WR, Frazier-Bowers SA. Mechanism and control of tooth eruption: overview and clinical implications. Orthod Craniofac Res. 2009;12:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Liu SY, Guilleminault C, Huon LK, Yoon A. Distraction Osteogenesis Maxillary Expansion (DOME) for Adult Obstructive Sleep Apnea Patients with High Arched Palate. Otolaryngol Head Neck Surg. 2017;157:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Faria AC, Xavier SP, Silva SN Jr, Trawitzki LV, de Mello-Filho FV. Cephalometric analysis of modifications of the pharynx due to maxillo-mandibular advancement surgery in patients with obstructive sleep apnea. Int J Oral Maxillofac Surg. 2013;42:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Strollo PJ Jr, Rogers RM. Obstructive sleep apnea. N Engl J Med. 1996;334:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 448] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Singh GD, Heit T, Preble D. Changes in 3D midfacial parameters after biomimetic oral appliance therapy in adults. J Ind Orthod Soc. 2014;48:104-108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Singh GD, Heit T, Preble D, Chandrashekhar R. Changes in 3D nasal cavity volume after biomimetic oral appliance therapy in adults. Cranio. 2016;34:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Singh GD, Griffin TM, Cress SE. Biomimetic oral appliance therapy in adults with severe obstructive sleep apnea. J Sleep Disord Ther. 2016;5:1-5. |
| 13. | Guilleminault C, Abad VC, Chiu HY, Peters B, Quo S. Missing teeth and pediatric obstructive sleep apnea. Sleep Breath. 2016;20:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Larsen AJ, Rindal DB, Hatch JP, Kane S, Asche SE, Carvalho C, Rugh J. Evidence Supports No Relationship between Obstructive Sleep Apnea and Premolar Extraction: An Electronic Health Records Review. J Clin Sleep Med. 2015;11:1443-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |