Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2181
Peer-review started: December 11, 2020
First decision: December 30, 2020
Revised: January 13, 2021
Accepted: February 11, 2021
Article in press: February 11, 2021
Published online: April 6, 2021
Processing time: 108 Days and 23 Hours
The role of macrophages in rheumatoid arthritis (RA) and its mechanism have attracted much attention in RA pathogenesis. Macrophages accumulate in the synoviums of RA, and the proportion of M1 type pro-inflammatory macrophages is higher than that of M2 type anti-inflammatory macrophages, leading to the secretion of inflammatory molecules and the aggravation of inflammatory reaction, which has made macrophages a potential target of RA drugs. Iguratimod is a kind of cyclo-oxygenase-2 inhibitor that affects macrophage polarity. It is speculated that its anti-inflammatory and anti-rheumatic effects may be related to the regulation of macrophage M1/M2 ratio.
To investigate the effects of Iguratimod on the polarity of mononuclear macrophages in elderly patients with RA.
Elderly patients with RA and joint effusion were selected, including 10 men and 25 women, with an average age of 66.37 ± 4.42 years. Patients were treated with oral administration of 25 mg Iguratimod (Iremod, State Food and Drug Administration Approval No. H20110084) twice daily for 12 wk. Disease Activity Score 28 and Health Assessment Questionnaire score were collected according to the disease severity before and after treatment. Venous blood and joint effusion fluid were collected, mononuclear macrophages were extracted and expression of cell surface markers CD86, CD64, CD163, and CD206 was analyzed by flow cytometry. The concentration of inflammatory factors interleukin (IL)-6, IL-1β, transforming growth factor-β, and IL-4 in the joint effusion fluid was analyzed by enzyme-linked immunosorbent assay. Expression of mononuclear cells inhibitor of nuclear factor-κB (IκB) and phosphorylated IκB in peripheral blood was analyzed by western blotting.
Disease Activity Score 28 score and Health Assessment Questionnaire score of patients treated with Iguratimod decreased significantly. The percentage of cell surface markers CD86 and CD64 decreased significantly, and the percentage of CD163 and CD206 increased significantly (P < 0.05). The inflammatory factors IL-6 and IL-1β decreased significantly, and transforming growth factor-β and IL-4 increased significantly. Western blot analysis showed that mononuclear cell inhibitor of nuclear factor-κB in peripheral blood was significantly increased after treatment, and its phosphorylation level was significantly decreased (P < 0.05).
Iguratimod can promote the transformation of mononuclear macrophages from M1 to M2 in elderly patients with RA by inhibiting the nuclear factor-κB pathway, thus improving symptoms of RA.
Core Tip: Elderly patients with rheumatoid arthritis (RA) with effusion in the joint cavity were treated by Iguratimod. Compared with before treatment, Disease Activity Score 28 score and Health Assessment Questionnaire score were decreased, the percentage of CD86 and CD64 decreased, the percentage of CD163 and CD 206 increased, interleukin (IL)-6 and IL-1β was reduced, while transforming growth factor-β and IL-4 was increased. Inhibitor of nuclear factor-κB (NF-κB) was expressed more, but phosphorylated inhibitor of NF-κB was expressed less, which suggested that Iguratimod can promote the transformation of monocyte macrophages from M1 type to M2 type in elderly patients with RA by inhibiting the NF-κB pathway, thus improving the symptoms of RA.
- Citation: Liu S, Song LP, Li RB, Feng LH, Zhu H. Iguratimod promotes transformation of mononuclear macrophages in elderly patients with rheumatoid arthritis by nuclear factor-κB pathway. World J Clin Cases 2021; 9(10): 2181-2191
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2181.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2181
Rheumatoid arthritis (RA) is an autoimmune disease involving innate and adaptive immunity. It is a multisystem inflammatory response that involves bones and joints[1]. The clinical manifestation is a chronic, symmetrical, progressive arthritis, often causing severe deformity of the joints and severely impacting the quality of life. The pathogenesis of RA is unclear. Existing studies have shown that inflammatory cells such as mononuclear macrophages, neutrophils, and lymphocytes play an important role in the pathogenesis of RA[2]. Studies have shown that there are a large number of macrophages in the synovial membrane and cartilage vasospasm of RA[3]. Macrophages not only kill and remove microbes from the body but also secrete a large number of inflammatory factors. Macrophages are classified into M1 type (proinflammatory) and M2 (anti-inflammatory). Macrophage type can switch from M1 type and M2 under the induction of different factors; this is the macrophage polarization phenomenon. In the occurrence and development of RA, the dynamic balance between M1 and M2 types of macrophages is broken, causing a proportional imbalance and resulting in more M1 proinflammatory macrophages that produce a large number of inflammatory factors and aggravating the inflammatory response[4,5]. Therefore, effective promotion of macrophage M1 to M2 transformation is beneficial to relieve inflammation and repair damaged tissues.
Iguratimod is a new type of antirheumatic drug, and a large number of in vitro experiments[6,7] and clinical trials[8,9] have confirmed the inhibitory effect of Iguratimod on cyclo-oxygenase (COX)-2. In order to avoid duplication, we did not study the changes in COX-2 in peripheral blood and synovial fluid before and after treatment. Iguratimod not only relieves bone and joint damage caused by chronic osteoarthritis and autoimmune diseases but also inhibits the production of inflammatory factors and immunoglobulins[10]. Studies of the effects of Iguratimod on cell polarity have not been reported. Combined with the above studies, our group concluded that Iguratimod affected the transformation of macrophage polarity, thereby alleviating the symptoms of RA in elderly patients.
We enrolled 35 patients aged 66.37 ± 4.42 years (25 female, aged 66.79 ± 4.42 years, 10 male, aged 65.88 ± 4.03 years) with RA with joint effusion at the Department of Rheumatology of the First Hospital of Qiqihar between October 2016 and September 2018. Inclusion criteria were: (1) Patients who did not receive any treatment and were newly diagnosed with RA; (2) Patients diagnosed in accordance with the 1987 American College of Rheumatology criteria[11]; (3) Patients aged ≥ 60 years; and (4) Patients with joint effusion. Exclusion criteria were: (1) Patients with RA who had received prior treatment; (2) Patients with diabetes, hyperlipidemia, or other endocrine diseases, cardiovascular or cerebrovascular diseases such as hypertension, liver or kidney diseases, or hematological diseases; and (3) Patients with Iguratimod contraindications. The patients took 25 mg Iguratimod (Iremod, State Food and Drug Administration Approval No.H20110084) orally twice daily for 12 wk. Venous blood from all patients was collected before and after treatment. The blood samples were treated with anticoagulant and preserved. Puncture aspiration was performed to collect joint cavity effusion fluid. The patients were informed of the experiment before receiving the treatment and gave signed informed consent. This study was approved by the Medical Ethics Committee of the First Hospital of Qiqihar.
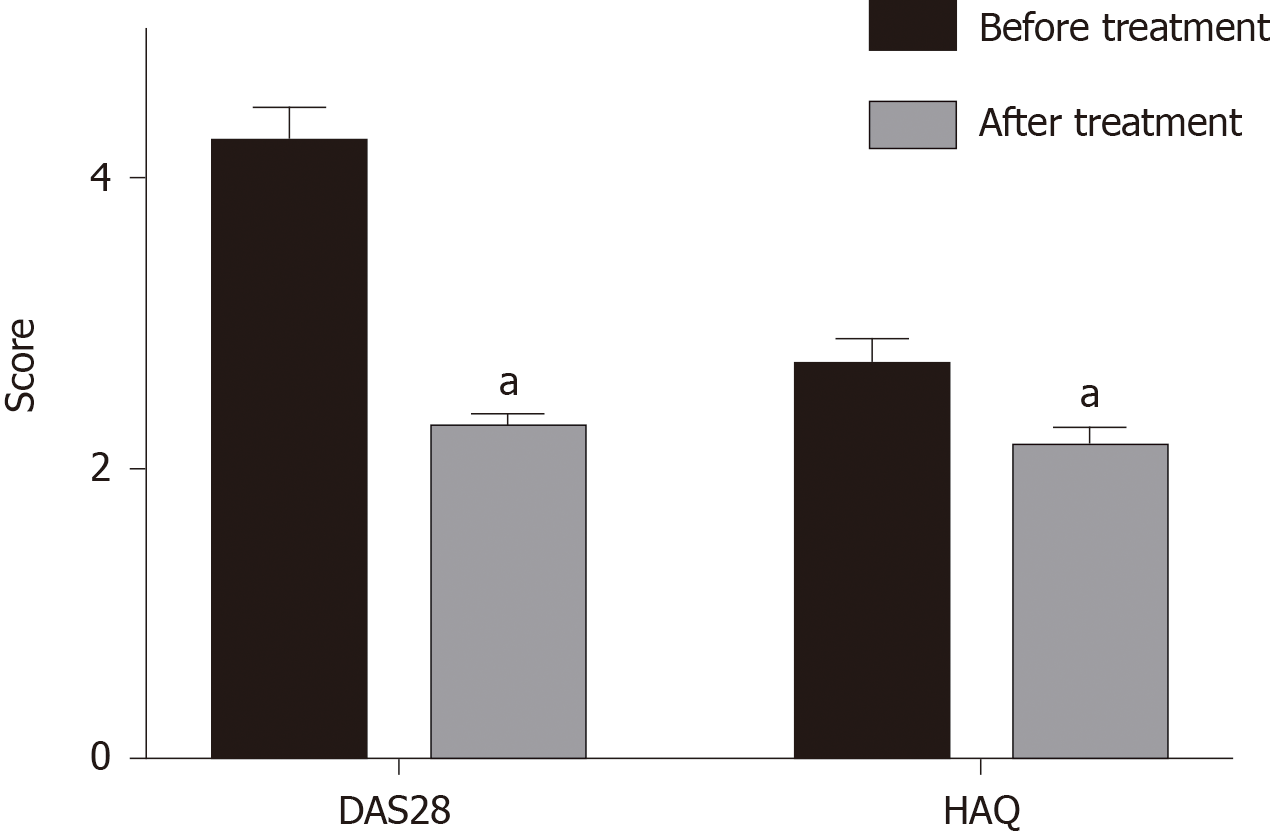
Disease Activity Score (DAS) 28 was recorded to judge the patients’ disease activity: DAS28 < 2.6 for disease remission, DAS28 > 3.2 for disease activity, and DSA28 > 5.1 for high disease activity. ΔDAS28 was the difference of DAS28 score before and after treatment. ΔDAS28 > 1.2 was a good treatment response, 0.6 < DAS28 ≤ 1.2 moderate treatment response, and 6 < DAS28 ≤ 0.6 nonresponse treatment. The Health Assessment Questionnaire (HAQ) score was used to assess various activities in daily life: 0: No difficulty; 1: Difficult; 2: Very difficult or needs assistance; and 3: Unfilled, and the total was the average scores of the eight questions.
Fluorescein isothiocyanate-labeled anti-CD86 antibody (ab77276), phycoerythrin-labeled anti-CD64 antibody (ab233449), phycoerythrin-labeled anti-CD163 antibody (ab233653), and Alexa Fluor® 488-labeled anti-CD206 antibody (ab195191) were purchased from Abcam (Cambridge, United Kingdom). Interleukin (IL)-6 (70-EK106), IL-1β (70-EK101B), transforming growth factor (TGF)-β (70-EK1811), and IL-4 (70-EK1041) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Lianke Biology Technology Co. Ltd. (Hangzhou, China). Bicinchoninic acid kit (P0009) was from Beyotime Biotechnology Co. Ltd. (Haimen, China). Phosphorylated inhibitor of nuclear factor-κB (IκB) (p-IκB)α antibody (2859) and IκBα antibody (4812) were from Cell Signaling Technology (Danvers, MA, United States). P-IκB kinase α antibody was from Baiao-Laibo (Beijing, China), p65 antibody was from Cell Signaling Technology, and internal reference glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (10494-1-AP) and horseradish peroxidase labeling secondary antibody (SA00001-2) were from Proteintech (Rosemont, IL, United States), and the Cytomics FC 500 flow cytometer from Beckman (Brea, CA, United States).
Venous blood was diluted with Hanks’ solution and slowly added to the tube containing 2 mL lymphocyte separation solution (Sigma, St. Louis, MO, United States) along the tube wall, with the interface kept clear. The sample was then centrifuged at 671 g for 20 min at room temperature. The upper layer of light yellow plasma was carefully discarded, the white layer (monocytes) was carefully aspirated, and 2 mL Hanks’ solution was added, and the sample was mixed and then centrifuged at 377 g for 10 min at room temperature. The supernatant was discarded, and 1 mL Hanks’ solution was added and resuspended, and the sample was temporarily stored at 4 °C. After the joint cavity effusion fluid was centrifuged, the supernatant was aspirated and stored at -80 °C for ELISA. The pellet was added to 1 mL Hanks’ solution and resuspended, and macrophages were analyzed as described above.
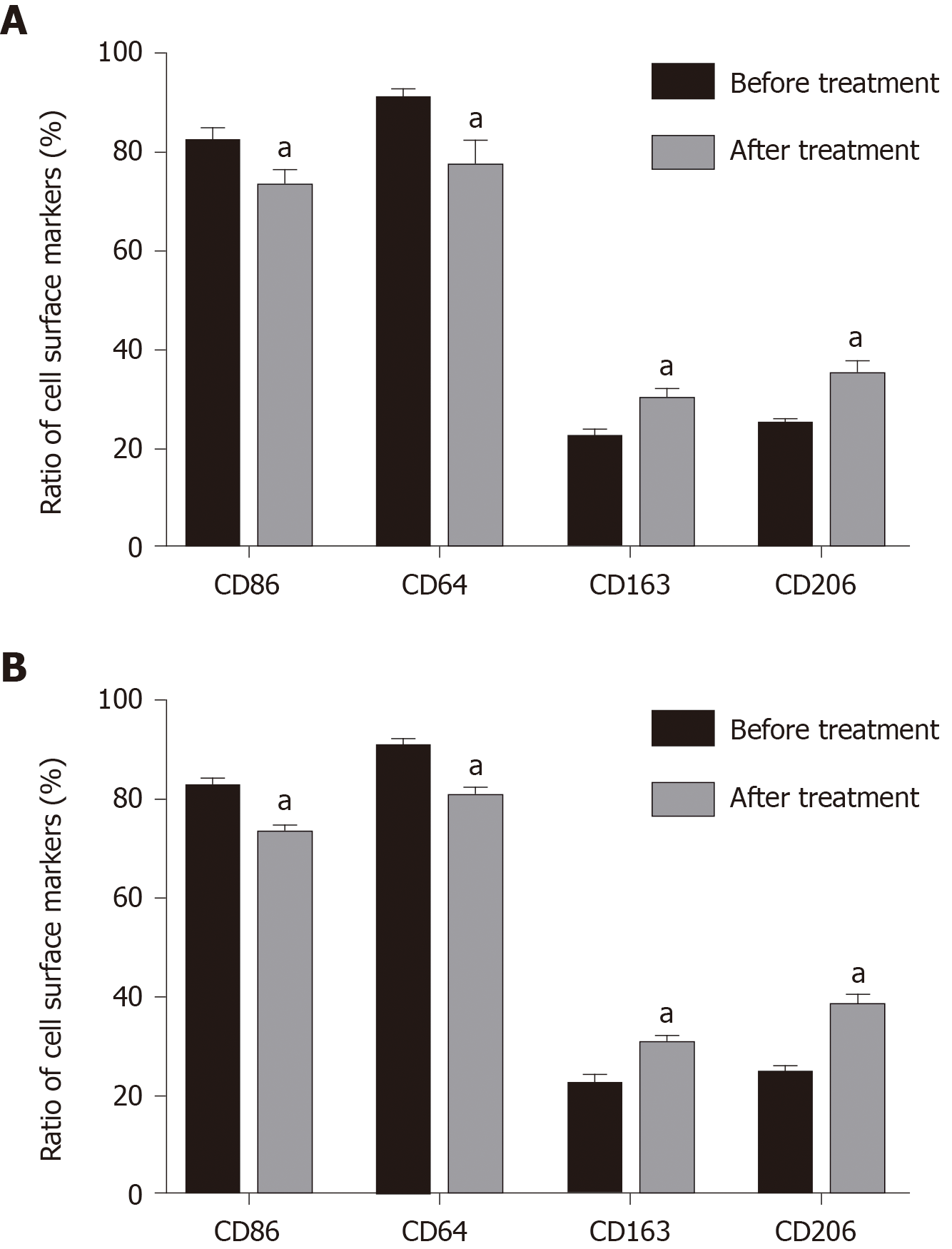
Two hundred microliters of resuspended mononuclear cells/macrophages were taken, and 10 mL of fluorescein-labeled CD86, CD64, CD163, and CD206 antibodies were added. The mixture was incubated for 30 min at 4 °C in the dark, centrifuged at 377 g for 5 min at room temperature and washed twice with Hanks’ solution; 500 mL Hanks’ solution was added for resuspension. Cell surface markers were detected, and CXP software was used to analyze the concentration of CD86, CD64, CD163, and CD206.
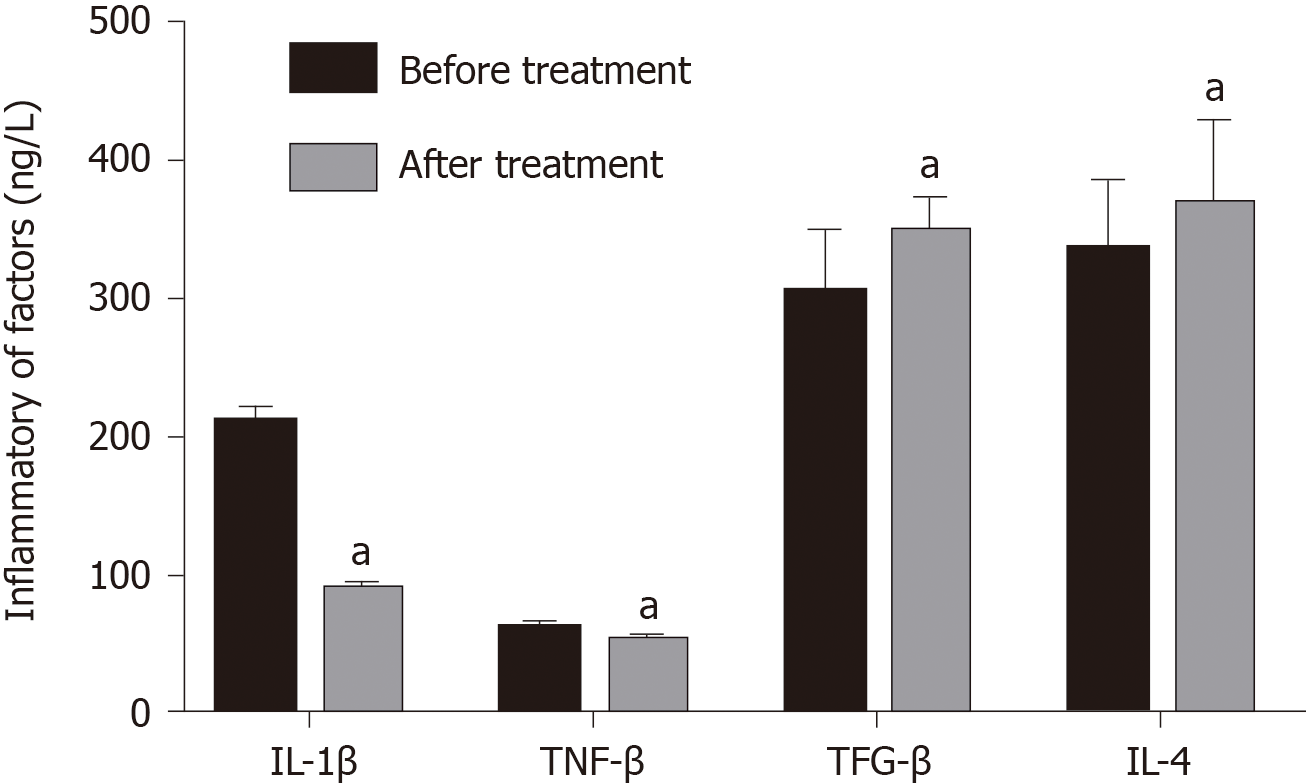
The ELISA kit was equilibrated to room temperature and the joint cavity effusion fluid was thawed at room temperature. The standard solution was diluted to establish six concentration gradients. Different concentrations of standard products were added at 50 μL per well, and two duplicates per well were set: Blank wells and sample wells. Forty microliters of sample was added to the well to be tested on the microplate, and then 10 μL biotin-labeled IL-6, IL-1β, TGF-β, and IL-4 antibodies were added. The sample was added to the bottom of the well without touching the wall. Fifty microliters of enzyme labeling reagent were added to each well, except the blank wells, and the samples incubated at 37 °C for 30 min after sealing. After that, the sealing membrane was removed, the liquid was discarded, and each well was filled with washing solution after it was dried. Washing solution was then discarded after standing for 30 s, and this was repeated five times. After draining, 50 μL of chromogenic reagents A and B was added to the wells to develop at 37 °C for 10 min, and 50 μL of stop solution was added to terminate the reaction. The absorbance of each well was measured at 450 nm with the blank well set to zero, and the data were inputted into the computer. The standard curve and the regression equation were used to calculate the concentrations of IL-6, IL-1β, TGF-β, and IL-4 in the sample wells.
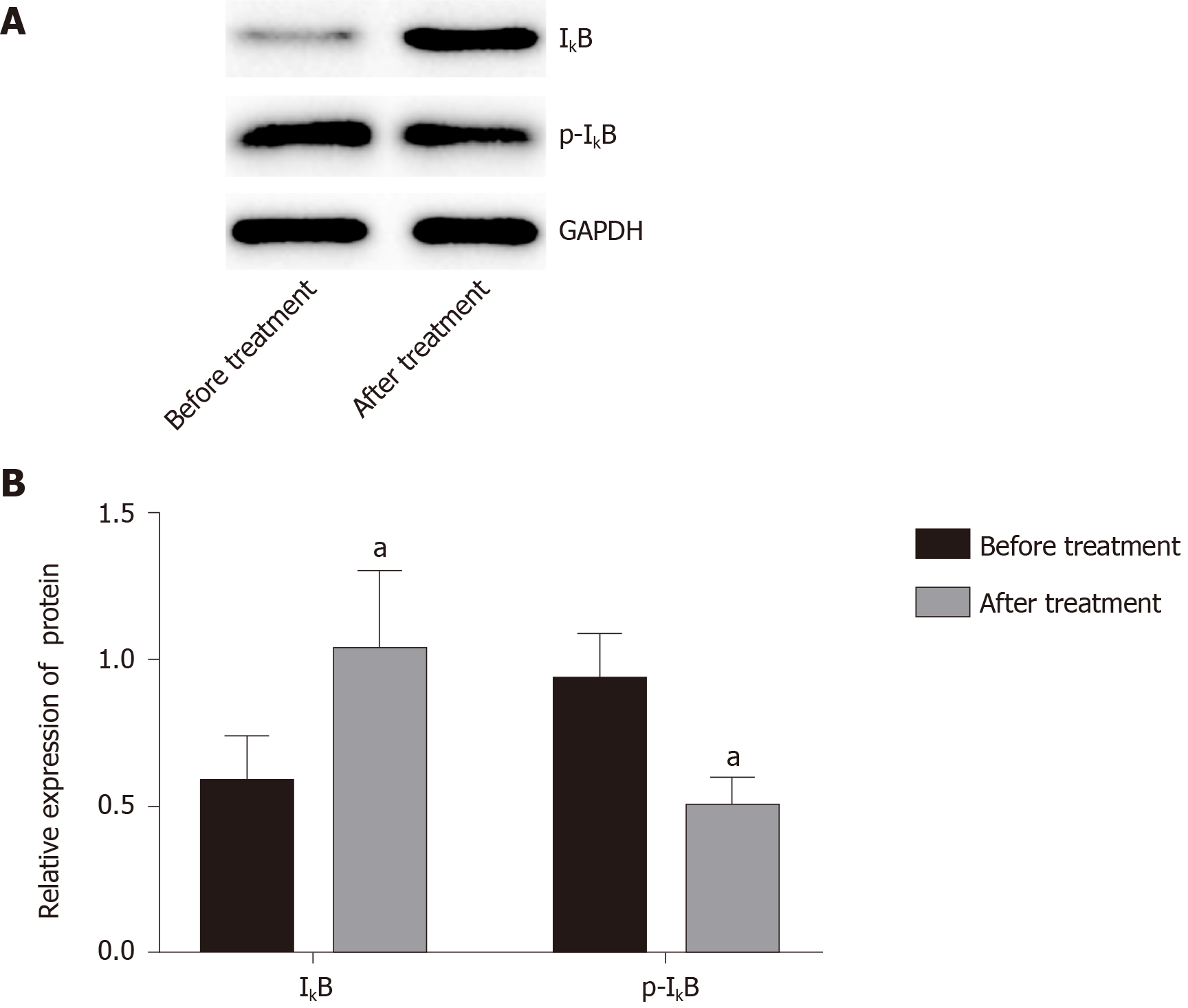
The monocytes extracted from the blood were centrifuged at 1509 g for 5 min, and the supernatant was obtained. The appropriate amount of ratchet-integrated pneumatic actuator lysate and protease inhibitor were added to the cell pellet, which was sonicated on ice for 5 min to fully lyse the cells, and then centrifuged at 12000 g for 15 min in a low-temperature centrifuge. Ten microliters of supernatant were collected for bicinchoninic acid protein quantification; the remaining supernatant was treated with 5 × loading buffer and boiled at 100 °C for 10 min. The sample amount was equal to that of total protein. Electrophoresis was performed with 5% upper gel and 10% lower gel, and 80 V constant electrophoresis was applied until bromophenol blue entered the lower gel; then 120 V was applied to separate the target strips. The protein was transferred to polyvinylidene difluoride membrane by wet transfer method at 275 mA for 80 min, and then blocked with 5% skim milk at room temperature for 2 h. Diluted IκB antibody and P65 antibody (diluted by 1:1000) were added and incubated at 4 °C overnight. After washing the membrane, 2% skim milk was added to the secondary antibody for 1 h at room temperature and then finally developed. Image J was used to analyze the gray value of the strip, GAPDH was used as an internal reference, and the protein expression was the ratio of the gray value of the target protein to the gray value of GAPDH.
SPSS version 23 (Armonk, NY, United States) was used for statistical analysis, and all experiments were repeated three times. The data were expressed as mean ± SD. The normality was tested first. If the data met a normal distribution, a t test was carried out between the two groups, and if it did not meet a normal distribution, a nonparametric test was performed; the statistical difference was set to P < 0.05.
DAS28 before treatment was 4.50 ± 0.62, indicating that RA was in the active stage. DAS28 and HAQ score were significantly decreased after treatment with Iguratimod (P < 0.05), and ΔDAS28 was 1.76 ± 0.73 (Figure 1).
Compared with before administration, the amount of joint effusion fluid decreased significantly after administration (16.74 ± 2.09 vs 0.57 ± 0.40, mL), the total number of cells dropped obviously (21.78 ± 2.33 vs 0.8 ± 0.05, × 109/L), and the ratio of macrophages raised from 35.73 ± 5.30 before administration to 66.83 ± 4.55 after administration. Compared with before treatment, the percentage of peripheral blood mononuclear cells and joint cavity macrophage surface markers CD86 and CD64 were significantly decreased after treatment (P < 0.05) (Figure 2). Expression of CD163 and CD206 in mononuclear macrophages in peripheral blood and joint effusion fluid were significantly increased after treatment with Iguratimod.
IL-6 decreased significantly from 190.69 ± 24.53 to 89.25 ± 6.41 ng/L after treatment, and IL-1β decreased significantly from 50.25 ± 6.41 to 40.94 ± 6.41 ng/L (P < 0.05), and the levels of TGF-β and IL-4 increased significantly after treatment (P < 0.05) (Figure 3).
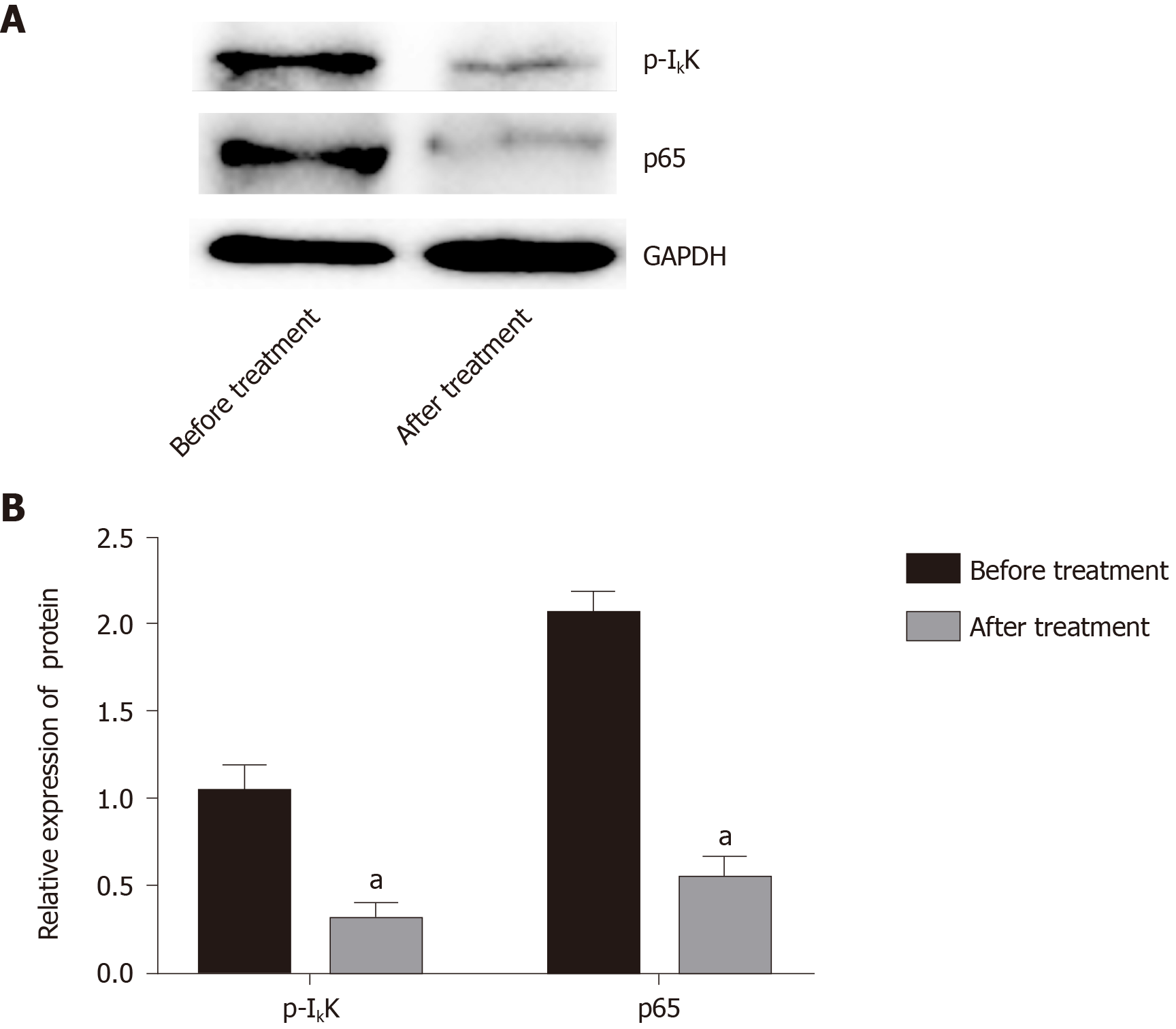
Compared with before treatment, IκB protein expression in blood mononuclear cells increased significantly after treatment from 0.63 ± 0.39 to 1.01 ± 0.27 (Figure 4), and the radio of p-IκB/IκB decreased significantly from 0.96 ± 0.44of before Iguratimod treatment to 0.54 ± 0.24 of after treatment (P < 0.05).
The prevalence of RA in different populations is 0.18%–1.07%, which shows some racial specificity. As the disease progresses, RA can involve multiple organs such as the heart and lungs. If it is not treated, the disability rate within 3 years is about 75%[12]. Elderly onset RA (EORA) is a form of RA with onset between age 60 and 65 years, which differs from young and middle-aged onset RA (YORA). Patients with EORA are more prone to sudden onset, and large joints such as the shoulder and hips joints are prone to rheumatoid polymyalgia-like characterization; while YORA patients are more likely to develop classic RA clinical characterization involving small joints[13]. EORA patients have a male to female ratio of 1.5-2:1, while YORA patients have a male to female ratio of 4-4.5:1. EORA patients are more prone to systemic symptoms such as fever, weight loss, and fatigue, with more acute onset, lower rheumatoid factor, and higher erythrocyte sedimentation rate[14]. Functional and anatomical outcomes of EORA are worse than those of YORA[15], and older age at onset is a predictor of death from cardiovascular disease[16].
Iguratimod is a new type of antirheumatic drug that has a good effect on RA. It can selectively inhibit the key enzyme cyclooxygenase-2 in prostaglandin synthesis and reduce prostaglandin production, thereby inhibiting the inflammatory response[17]. In this study, DAS28 in patients with RA was used to judge disease activity, and HAQ score was used to evaluate various activities in daily life. Changes in indicators, like blood tests, C-reactive protein, erythrocyte sedimentation rate, and body weight, were not included in our study. This is because the main purpose of the study was to investigate the polarity changes that occur after mononuclear macrophages are affected by Iguratimod. Several studies[18-20] have revealed the adverse effects of Iguratimod and changes in patients’ routine indicators and have yielded consistent and reliable conclusions for EORA. The phenomena observed in this study were also consistent with these conclusions, so there was no specific focus on indicator test results and drug adverse effects. The DAS28 of the 35 patients in our study was 4.50 ± 0.62, indicating that RA was in the active phase, and after 12 wk of treatment with Iguratimod, DAS28 score decreased to 2.74 ± 0.25, and ΔDAS28 was 1.76 ± 0.73. This indicates that Iguratimod can reduce the disease activity of RA, and although the treatment response is good, it cannot completely relieve the disease because Iguratimod is for symptom relief. It is impossible to change fundamentally the patients’ condition, and different types of RA and individual patient differences may affect drug efficacy. Therefore, in order to control better the development of RA, a combination of drugs has become the consensus of clinicians. Some studies have compared methotrexate and Iguratimod alone and in combination, and in combination they can control RA disease faster and more strongly and reduce disease activity[21,22].
Macrophages have strong plasticity and can be transformed into different types under the influence of different factors. According to their surface markers, they can be divided into M1 and M2 types, and M1 macrophages express CD64, CD80, CD86, and MHC II on their surface and secrete a variety of inflammatory factors, such as IL-6, IL-1β, and tumor necrosis factor-α. M2 type macrophages express CD206, CD163, CD209, and CD301 on their surface and secrete IL-4, IL-10, and TGF-β[23-25]. Zhu et al[26] showed that macrophages are mainly M1 type in joint effusion fluid, and Soler Palacios et al[27] also found that M1 type macrophage surface markers are dominant in joint effusion fluid, and cells highly express M1-related genes. These findings indicate that macrophages are mainly M1 type in the joint effusion fluid of RA. In addition to the above results, some studies have indicated that peripheral blood cytokine levels are low and difficult to detect and are not related to disease progression[28]; while increased cytokine levels in joint effusion fluid are activated by macrophages and related to the degree of joint damage[29]. Therefore, we chose joint cavity fluid instead of peripheral blood to study expression of cytokines. We found that after treatment with Iguratimod, M1 type cell surface markers and IL-6 and IL-1β inflammatory factors secreted by M1 type cell decreased significantly, and the M2 cell surface markers and their secretions IL-4 and TGF-β were significantly increased. The results showed that Iguratimod can promote the transformation of macrophage phenotype from the M1 into M2 to inhibit the inflammatory response.
Nuclear factor-κB (NF-κB) is an important nuclear transcription factor involved in the inflammatory response, immune response, apoptosis/antiapoptosis, cell cycle regulation, and other physiological activities[30]. NF-κB includes five subunits, RelA (p65), RelB, c-Rel, p50, and p52. When the cells are stimulated from the outside, IκB kinase is activated, which leads to its phosphorylation, and phosphorylated IκB is rapidly ubiquitinated. NF-κB dimer is released and activated and further transported to the nucleus to exert its biological effects. IκB is also considered to be the key to activation of the NF-κB pathway[31]. We did not extract adequate macrophages from the joint effusion fluid, and peripheral blood mononuclear cells were used for western blot analysis. The results showed that the level of IκB in the peripheral blood mononuclear cells after treatment were significantly increased compared with those before treatment, and the level of phosphorylated IκB was significantly decreased, indicating that Iguratimod can inhibit the NF-κB pathway. It has been shown that the transformation of macrophage polarity is regulated by the NF-κB pathway, and activation of the pathway can promote the transformation of macrophages to M1 type, promote expression of inflammatory factors, aggravate the inflammatory response, and induce osteoarthritis[32,33].
We believe that Iguratimod can regulate the transformation of RA macrophages from M1 to M2 type by inhibiting the NF-κB pathway, thereby reducing inflammation and alleviating RA symptoms. There were some limitations to this study. First, because of the small number of joint effusion fluid samples, only the activation of the NF-κB pathway in peripheral blood mononuclear cells was detected, and activation of the NF-κB pathway in joint effusion fluid macrophages needs further research. Second, the specific mechanism of Iguratimod regulation of the NF-κB pathway has not been elucidated (Figure 5).
Our study linked the anti-inflammatory effects of Iguratimod with macrophage polarity and transformation of macrophages, explored the therapeutic mechanism of Iguratimod in RA, and provided new ideas for clinical treatment of RA.
The role of macrophages in rheumatoid arthritis (RA) and its mechanism have attracted much attention in RA pathogenesis.
To investigate the effects of Iguratimod on the polarity of mononuclear macrophages in elderly patients with RA.
Macrophages accumulate in the synoviums of RA, and the proportion of M1 type pro-inflammatory macrophages is higher than that of M2 type anti-inflammatory macrophages, leading to the secretion of inflammatory molecules and the aggravation of inflammatory reaction, which makes macrophages potential targets of RA drugs.
Elderly patients with RA and joint effusion were selected. Patients were treated with oral administration of 25 mg Iguratimod (Iremod, Iremod, State Food and Drug Administration Approval No. H20110084). Venous blood and joint effusion fluid were collected, mononuclear macrophages were extracted, and expression of cell surface markers CD86, CD64, CD163, and CD206 was analyzed by flow cytometry. The concentration of inflammatory factors interleukin (IL)-6, IL-1β, transforming growth factor-β, and IL-4 in the joint effusion fluid was analyzed by enzyme-linked immunosorbent assay. Expression of mononuclear cells inhibitor of nuclear factor-κB (IκB) and phosphorylated IκB in peripheral blood was analyzed by western blotting.
Western blot analysis showed that mononuclear cell IκB in peripheral blood was significantly increased after treatment, and its phosphorylation level was significantly decreased (P < 0.05).
Iguratimod can promote the transformation of mononuclear macrophages from M1 to M2 in elderly patients with RA by inhibiting the nuclear factor-κB (NF-κB) pathway, thus improving symptoms of RA. Iguratimod can promote the transformation of mononuclear macrophages from M1 to M2 in elderly patients with RA. Iguratimod promotes the transformation of mononuclear macrophages from M1 to M2 by decreasing phosphorylation level of IκB
First, because of the small number of joint effusion fluid samples, only the activation of the NF-κB pathway in peripheral blood mononuclear cells was detected, and activation of the NF-κB pathway in joint effusion fluid macrophages needs further research. Second, the specific mechanism of Iguratimod regulation of the NF-κB pathway has not yet been elucidated.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sumi K S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Abeles AM, Pillinger MH. The role of the synovial fibroblast in rheumatoid arthritis: cartilage destruction and the regulation of matrix metalloproteinases. Bull NYU Hosp Jt Dis. 2006;64:20-24. [PubMed] |
| 2. | Cascão R, Rosário HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: More than simple final effectors. Autoimmun Rev. 2010;9:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Henneken M, Dörner T, Burmester GR, Berek C. Differential expression of chemokine receptors on peripheral blood B cells from patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2005;7:R1001-R1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Li J, Hsu HC, Mountz JD. Managing macrophages in rheumatoid arthritis by reform or removal. Curr Rheumatol Rep. 2012;14:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Andreakos ET, Foxwell BM, Brennan FM, Maini RN, Feldmann M. Cytokines and anti-cytokine biologicals in autoimmunity: present and future. Cytokine Growth Factor Rev. 2002;13:299-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Mucke HA. Iguratimod: a new disease-modifying antirheumatic drug. Drugs Today (Barc). 2012;48:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 7. | Li G, Yamasaki R, Fang M, Masaki K, Ochi H, Matsushita T, Kira JI. Novel disease-modifying anti-rheumatic drug iguratimod suppresses chronic experimental autoimmune encephalomyelitis by down-regulating activation of macrophages/microglia through an NF-κB pathway. Sci Rep. 2018;8:1933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Okamura K, Yonemoto Y, Okura C, Kobayashi T, Takagishi K. Efficacy of the clinical use of iguratimod therapy in patients with rheumatoid arthritis. Mod Rheumatol. 2015;25:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Suto T, Yonemoto Y, Okamura K, Sakane H, Takeuchi K, Tamura Y, Kaneko T, Ayabe K, Chikuda H. The three-year efficacy of iguratimod in clinical daily practice in patients with rheumatoid arthritis. Mod Rheumatol. 2019;29:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Tanaka K, Shimotori T, Makino S, Aikawa Y, Inaba T, Yoshida C, Takano S. Pharmacological studies of the new antiinflammatory agent 3-formylamino-7-methylsulfonylamino-6-phenoxy-4H-1-benzopyran-4-o ne. 1st communication: antiinflammatory, analgesic and other related properties. Arzneimittelforschung. 1992;42:935-944. [PubMed] |
| 11. | Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315-324 [PMID: 3358796 DOI: 10.1002/art. 1780310302;. |
| 12. | Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 589] [Article Influence: 29.5] [Reference Citation Analysis (2)] |
| 13. | Deal CL, Meenan RF, Goldenberg DL, Anderson JJ, Sack B, Pastan RS, Cohen AS. The clinical features of elderly-onset rheumatoid arthritis. A comparison with younger-onset disease of similar duration. Arthritis Rheum. 1985;28:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Soubrier M, Mathieu S, Payet S, Dubost JJ, Ristori JM. Elderly-onset rheumatoid arthritis. Joint Bone Spine. 2010;77:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Rexhepi S, Rexhepi M, Sahatçiu-Meka V, Rexhepi B, Bahtiri E, Mahmutaj V. Late onset rheumatoid arthritis an observational study. Reumatizam. 2016;63:1-5. [PubMed] |
| 16. | Naz SM, Farragher TM, Bunn DK, Symmons DP, Bruce IN. The influence of age at symptom onset and length of followup on mortality in patients with recent-onset inflammatory polyarthritis. Arthritis Rheum. 2008;58:985-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Yoshioka Y, Takahashi N, Kaneko A, Hirano Y, Kanayama Y, Kanda H, Takagi H, Ito T, Kato T, Saito K, Funahashi K, Asai S, Takemoto T, Terabe K, Asai N, Ishiguro N, Kojima T. Disease activity early in treatment as a predictor of future low disease activity in RA patients treated with iguratimod. Mod Rheumatol. 2016;26:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Hara M, Abe T, Sugawara S, Mizushima Y, Hoshi K, Irimajiri S, Hashimoto H, Yoshino S, Matsui N, Nobunaga M, Nakano S. Efficacy and safety of iguratimod compared with placebo and salazosulfapyridine in active rheumatoid arthritis: a controlled, multicenter, double-blind, parallel-group study. Mod Rheumatol. 2007;17:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Li J, Mao H, Liang Y, Lu Y, Chen S, Yang N, Shi G. Efficacy and safety of iguratimod for the treatment of rheumatoid arthritis. Clin Dev Immunol. 2013;2013:310628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Tanaka K, Yamaguchi T, Hara M. Iguratimod for the treatment of rheumatoid arthritis in Japan. Expert Rev Clin Immunol. 2015;11:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Xia Z, Lyu J, Hou N, Song L, Li X, Liu H. Iguratimod in combination with methotrexate in active rheumatoid arthritis : Therapeutic effects. Z Rheumatol. 2016;75:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Duan XW, Zhang XL, Mao SY, Shang JJ, Shi XD. Efficacy and safety evaluation of a combination of iguratimod and methotrexate therapy for active rheumatoid arthritis patients: a randomized controlled trial. Clin Rheumatol. 2015;34:1513-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Zhang YH, He M, Wang Y, Liao AH. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front Immunol. 2017;8:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 24. | Self-Fordham JB, Naqvi AR, Uttamani JR, Kulkarni V, Nares S. MicroRNA: Dynamic Regulators of Macrophage Polarization and Plasticity. Front Immunol. 2017;8:1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Han CC, Cui D, Li Y, Ma Y, Wei W. Is macrophage polarization important in rheumatoid arthritis? Int Immunopharmacol. 2017;50:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 26. | Zhu W, Li X, Fang S, Zhang X, Wang Y, Zhang T, Li Z, Xu Y, Qu S, Liu C, Gao F, Pan H, Wang G, Li H, Sun B. Anti-Citrullinated Protein Antibodies Induce Macrophage Subset Disequilibrium in RA Patients. Inflammation. 2015;38:2067-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Soler Palacios B, Estrada-Capetillo L, Izquierdo E, Criado G, Nieto C, Municio C, González-Alvaro I, Sánchez-Mateos P, Pablos JL, Corbí AL, Puig-Kröger A. Macrophages from the synovium of active rheumatoid arthritis exhibit an activin A-dependent pro-inflammatory profile. J Pathol. 2015;235:515-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Loubet-Lescoulié P, Constantin A, Mazières B, Tkaczuk J, de Préval C, Cantagrel A. Decreased peripheral blood T cell cytokine gene expression in rheumatoid arthritis. Scand J Rheumatol. 1999;28:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Weyand CM, Zeisbrich M, Goronzy JJ. Metabolic signatures of T-cells and macrophages in rheumatoid arthritis. Curr Opin Immunol. 2017;46:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 30. | Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2488] [Cited by in RCA: 2492] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 31. | Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 32. | Kono Y, Kawakami S, Higuchi Y, Yamashita F, Hashida M. In vitro evaluation of inhibitory effect of nuclear factor-kappaB activity by small interfering RNA on pro-tumor characteristics of M2-like macrophages. Biol Pharm Bull. 2014;37:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Hah YS, Cheon YH, Lim HS, Cho HY, Park BH, Ka SO, Lee YR, Jeong DW, Kim HO, Han MK, Lee SI. Myeloid deletion of SIRT1 aggravates serum transfer arthritis in mice via nuclear factor-κB activation. PLoS One. 2014;9:e87733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |