Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.224
Peer-review started: August 17, 2020
First decision: October 18, 2020
Revised: October 21, 2020
Accepted: November 4, 2020
Article in press: November 4, 2020
Published online: January 6, 2021
Processing time: 137 Days and 4.8 Hours
Adult-onset Still's disease (AOSD) typically presents with a high spiking fever, polyarthritis, transient maculopapular rash, neutrophilic leukocytosis, and hepatosplenomegaly. It has a wide spectrum of clinical symptoms ranging from mild to severe, with extensive involvement of almost every organ. Although liver involvement in the form of increased hepatic enzymes and bilirubin is common, no AOSD case with liver involvement as the initial manifestation of AOSD has been reported.
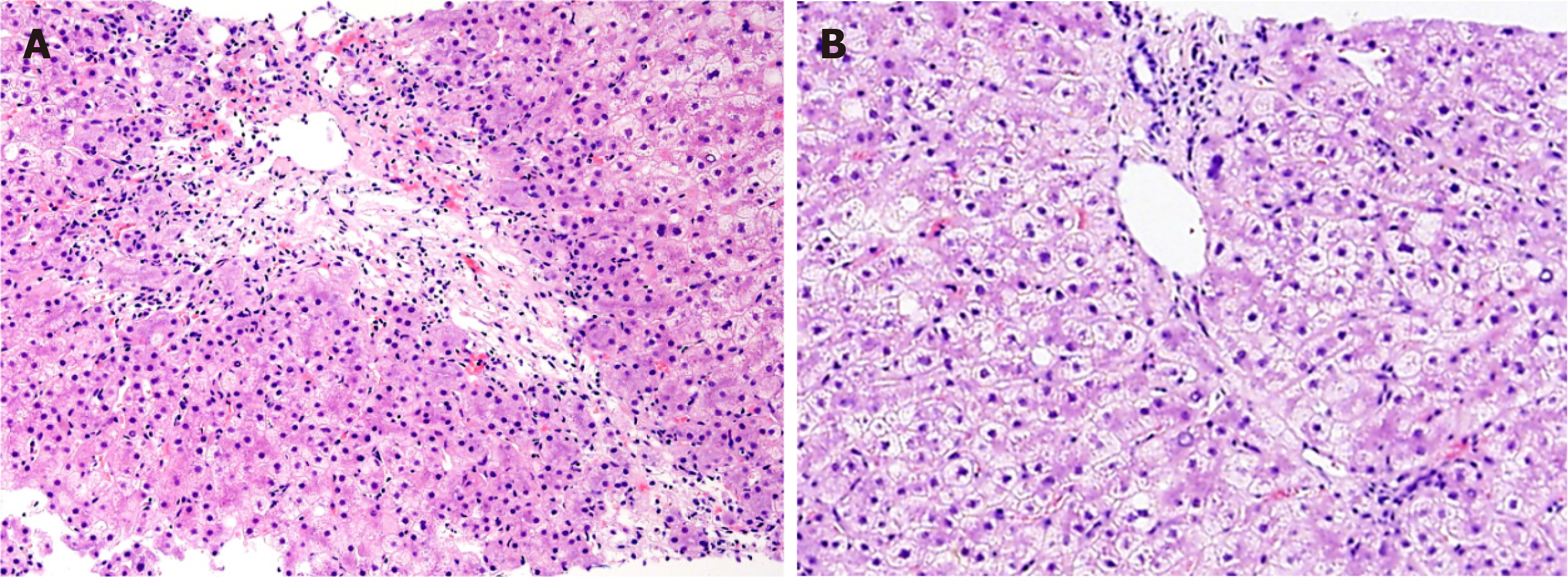
A 35-year-old woman presented to the hepatology department with progressively worsening jaundice for one week. Liver chemistry tests revealed a significantly increased liver enzymes and bilirubin level. Given that the clinical examination was unremarkable, liver biopsy was considered because the patient had a history of AOSD 6 years ago. Liver histopathology revealed that most hepatic lobules were still recognizable. Fusional necrosis was observed around most central veins. A few bridging necrotic zones were also found. Infiltration of multiple plasma cells were observed in the necrotic zone, and the reticular scaffold was still expanded. Additionally, no obvious fibrosis was observed in the portal area. Mild mixed inflammatory cell infiltration was noted in the interstitium of the portal area. Further examination was unremarkable except for a remarkably high level of ferritin. Collectively, a presumptive diagnosis of liver injury secondary to AOSD was made. The hepatic involvement responded well to glucocorticoid treatment.
This case highlights that hepatic involvement as an initial and sole manifestation could be a pattern of relapsed AOSD. The diagnosis of AOSD should be considered in the case of nonresolving liver injury after the exclusion of common etiologies for liver diseases. A liver biopsy can be useful for the differential diagnosis of liver injury associated with AOSD.
Core Tip: Liver involvement in the form of increased hepatic enzymes and bilirubin is common in adult-onset Still's disease (AOSD). Herein, we presented a patient with relapsed AOSD with hepatic involvement as an initial and sole manifestation responding well to glucocorticoid treatment. This case highlights that hepatic involvement as an initial and sole manifestation could be a pattern of relapsed AOSD. The diagnosis of AOSD should be considered in the case of nonresolving liver injury after the exclusion of common etiologies for liver diseases. A liver biopsy can be useful for the differential diagnosis of liver injury associated with AOSD.
- Citation: Yu F, Qin SY, Zhou CY, Zhao L, Xu Y, Jia EN, Wang JB. Atypical adult-onset Still’s disease with an initial and sole manifestation of liver injury: A case report and review of literature. World J Clin Cases 2021; 9(1): 224-231
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/224.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.224
Adult-onset Still’s disease (AOSD) is a rare condition characterized by a high spiking fever, polyarthritis, transient maculopapular rash, and neutrophilic leukocytosis[1,2]. It has a wide spectrum of clinical symptoms ranging from mild to severe, with extensive involvement of almost every organ. Moreover, life-threatening complications, such as myocarditis and disseminated intravascular coagulopathy (DIC), appear in approximately 15%-20% of patients with AOSD[3]. The early diagnosis and intervention of AOSD are vital to achieving sustained clinical remission and reducing the mortality rate[4,5]. However, early identification of AOSD in some cases remains a challenge because of highly varied symptoms, the involvement of multiple organs, and the lack of diagnostic tests and serologic markers[5-8]. Although hepatic involvement is commonly observed in AOSD with a prevalence of 50%-75%[9], liver injury is not a prerequisite to diagnose AOSD according to the Yamaguchi’s criteria[8], partly because many diseases can cause liver injury. Thus, it remains a challenge to decide whether the involvement of the liver as the initial organ is caused by AOSD or other etiologies. To date, no AOSD case with liver involvement as the initial manifestation of AOSD has been reported. We here present a patient with relapsed AOSD with hepatic involvement as an initial and sole manifestation.
A 35-year-old woman presented to the hepatology department with progressively worsening jaundice.
Patient’s symptoms started a week ago with progressively worsening jaundice. The patient denied fever, anorexia, fatigue, and abdominal distention.
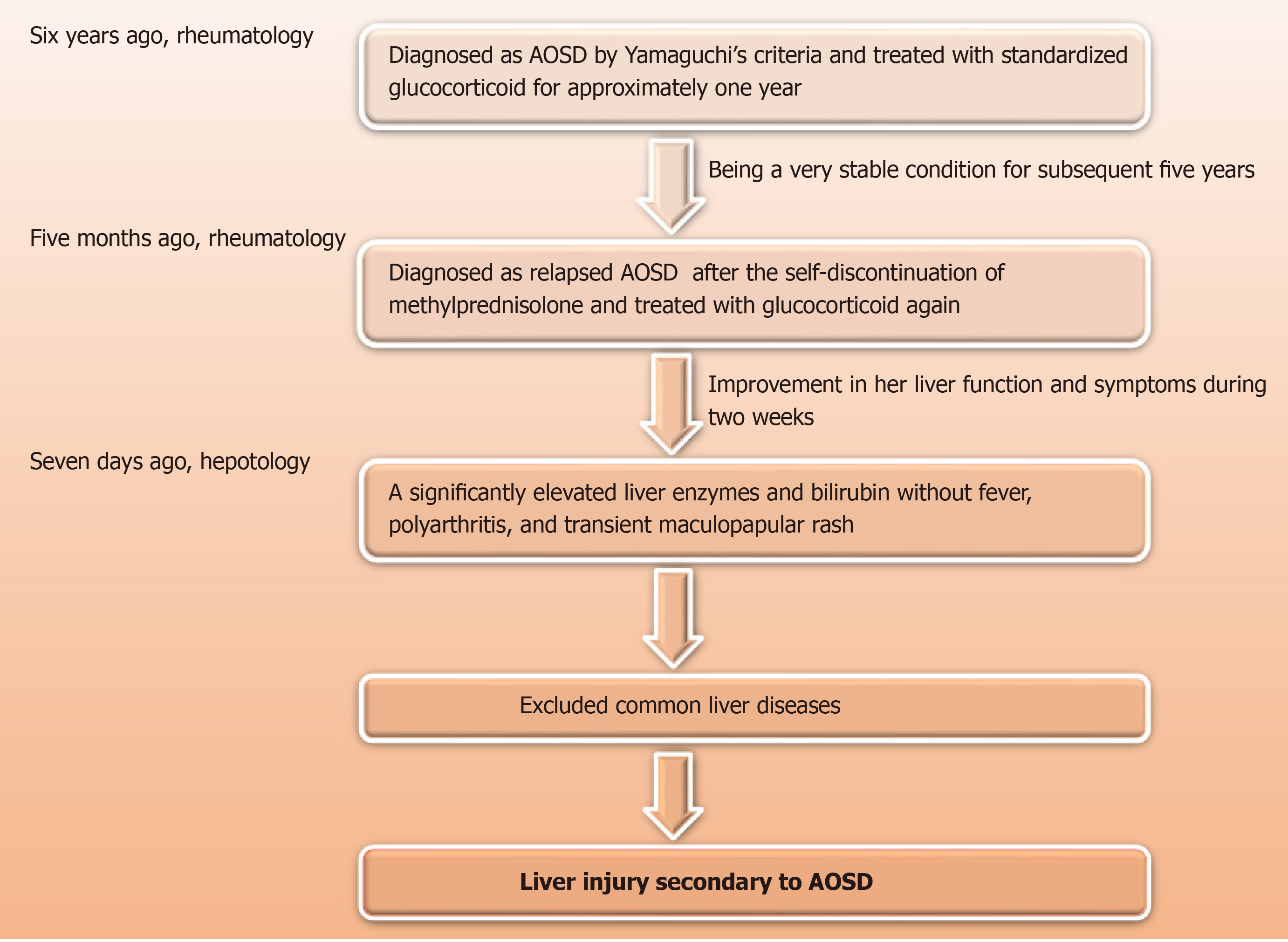
The patient had a history of AOSD 6 years previously and responded well to standardized glucocorticoid therapy in the first episode, but experienced a relapse 5 mo before the self-discontinuation of methylprednisolone. She had no risk factors for viral hepatitis or toxin. She does not smoke and drink. Her family history was not notable for autoimmune diseases or hepatic virus diseases. She had no risk factors for viral hepatitis.
No abnormalities.
The patient’s temperature was 36.7 °C, heart rate was 84 bpm, respiratory rate was 18 breaths per min, blood pressure was 116/75 mmHg and oxygen saturation in room air was 100%. The clinical examination revealed that the skin and sclera were jaundiced.
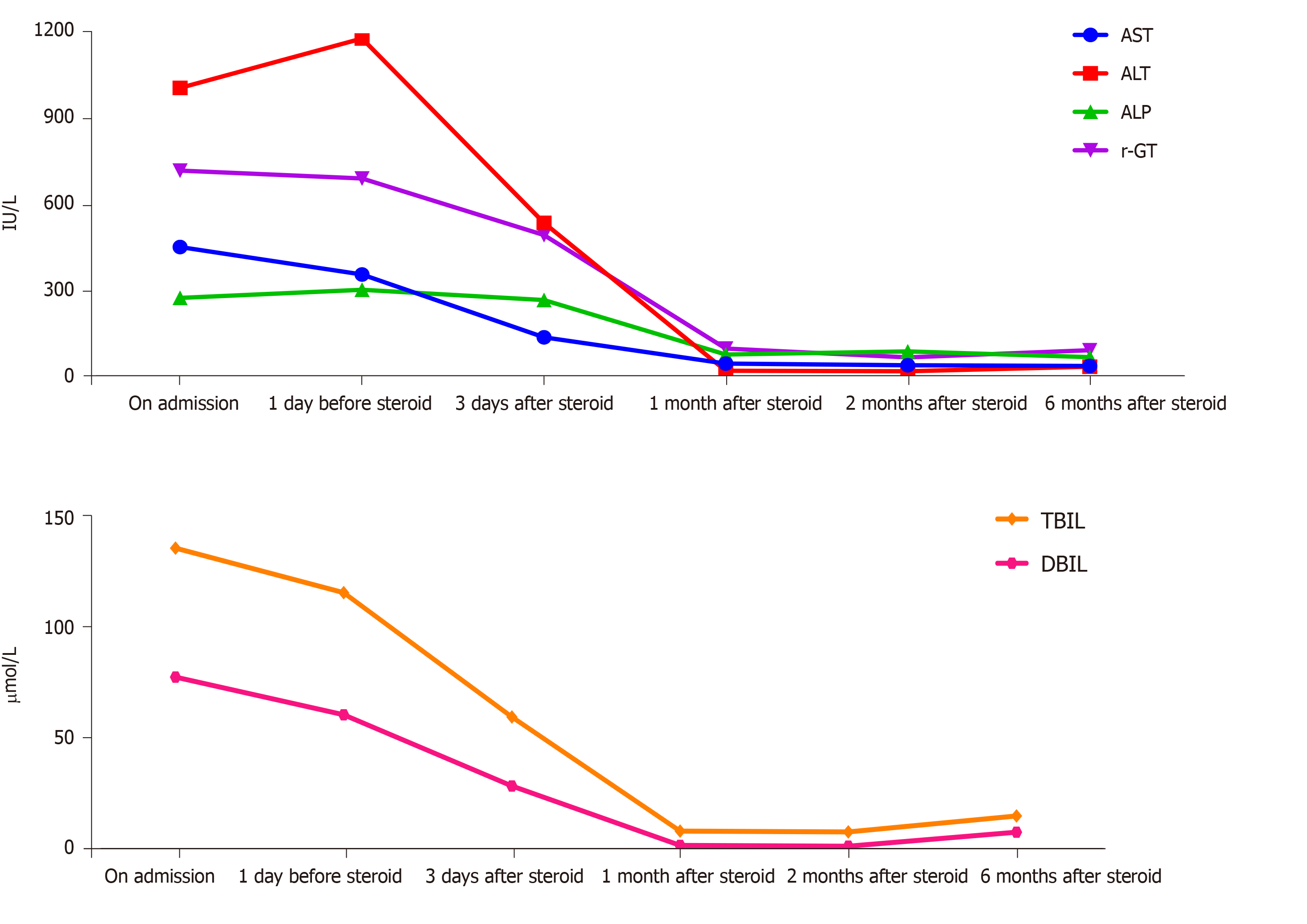
Liver chemistry tests revealed a significantly increased alanine transaminase (ALT) level (1009.71 IU/L; normal: 5-40 IU/L), aspartate transaminase level (455.41 IU/L; normal: 5-40 IU/L), gamma-glutamyl transferase level (721.85 IU/L; normal: 8-57 IU/L) and bilirubin level (137.59 µmol/L; normal: 0-21 µmol/L). Peripheral blood revealed a mildly elevated white blood cell (WBC) count (11.6 × 109/L; normal: 4-10 × 109/L) with 76.5% neutrophils (normal: 50.0%-70.0%) and a C-reactive protein (CRP) level of 31.22 mg/dL (normal: 0-8 mg/L). Results of other liver markers are demonstrated in Table 1. Full-blood screening analyses to determine the etiology of liver involvement were all negative or within normal limits.
| Serologic tests | Negative/positive |
| Anti-HAV | Negative |
| Anti-HAV | Negative |
| HBsAg | Negative |
| Anti-HBs | Positive |
| Anti-HBc IgM | Negative |
| Anti-HCV | Negative |
| Anti-HEV | Negative |
| Anti-EBV-VCA IgM | Negative |
| Anti-CMV IgM | Negative |
| Anti-HIV | Negative |
| HCV-RNA | Negative |
| Anti-nuclear antibody | Negative |
| Anti-HIV | Negative |
| Anti-nuclear antibody | Negative |
| Anti-smooth muscle antibody | Negative |
| Anti-soluble liver antibody | Negative |
| Antineutrophil cytoplasmic antibody | Negative |
| Antimitochondrial antibody | Negative |
| Anti-liver-kidney microsomal type-1 antibody | Negative |
Ultrasound showed a smoothly contoured liver and healthy thin-walled gallbladder. Thorax and abdominal computed tomography (CT) scans demonstrated generalized lymph nodes with the largest one measuring 1.1 cm × 1.2 cm; no solid masses were identified. Echocardiography and serum protein electrophoresis were normal.
Liver histopathology revealed that most hepatic lobules were still recognizable. Fusional necrosis was observed around most central veins. A few bridging necrotic zones were also present. Infiltration of multiple plasma cells was observed in the necrotic zone, and the reticular scaffold was still expanded, without obvious collagen deposition (Figure 1A). Additionally, no obvious fibrosis was observed in the portal area. Mild mixed inflammatory cell infiltration was noted in the interstitium of the portal area. Interfacial inflammation was not significant (Figure 1B).
Given that the clinical examination was unremarkable, liver biopsy was considered by the hepatology team at this point. However, it was decided to await rheumatology review because the patient had a history of AOSD 6 years ago, with symptoms including unexplained fever, arthritis, rash, and neutrophilic leukocytosis. Additionally, laboratory tests showed a remarkably high level of CRP and ferritin, and elevated liver enzymes. The patient responded well to standardized glucocorticoid therapy in the first episode but experienced a relapse 5 mo before the self-discontinuation of methylprednisolone. At the rheumatology review, the patient did not describe any systemic symptoms but felt constitutionally unwell with significant fatigue. There was no history of Raynaud’s phenomenon or any features suggestive of other autoimmune connective tissue diseases or vasculitis. Systemic examination was unremarkable except for a remarkably high level of ferritin (> 1650.0 ng/mL). After a discussion with the rheumatology team concerning diagnosis options, liver histological examination was still needed to determine whether the liver injury was caused by AOSD or liver disease per se (Figure 2).
There were two reasons why we considered liver injury as a result of relapsed AOSD rather than liver diseases. First, liver involvement is a well-characterized feature of AOSD, with highly varied manifestations ranging from minimally elevated hepatic enzymes to hyperbilirubinemia and even fulminant hepatic failure. However, the liver injury typically appears as one of the disease activity signs rather than as the only manifestation in patients with AOSD[2,10], further highlighting the importance of histological examination. Second, both fevers with unknown causes and elevated ferritin levels are features of active AOSD. Fever with unknown causes in Europe accounted for 3% - 20% of AOSD[11,12]. A retrospective study in China involving 517 individuals revealed that fever occurred in 472 (91.3%) patients with AOSD who satisfied the Yamaguchi criteria[13]. Additionally, hyperferritinemia still served as a marker of disease activity in AOSD[14], although its specificity remains to be confirmed[15]. Collectively, given the patient’s presenting features, especially the liver histological findings, in combination with the history of AOSD, a presumptive diagnosis of liver injury secondary to AOSD was made.
After early treatment with intravenous methylprednisolone (80mg/d ) for three days, the patient was switched to oral steroids (starting at prednisolone 40 mg daily), followed by a tapered dose. Additionally, she was referred for intensive physiotherapy, which is an essential part of overall management.
In response to treatment, the liver enzymes were normalized within four weeks and remained normal with symptom improvement (Figure 3). The patient also reported considerable improvements in anorexia and fatigue. Six months after discharge, the patient’s subsequent clinical course was stable, while the dose of oral methylpred-nisolone continued at 4 mg/d.
Although hepatic involvement is a well-characterized feature in patients with ASOD[16-19], it is uncommon for the prevalence of liver injury as the initial manifestation of AOSD. For the first time, we present an AOSD case with elevated hepatic enzymes and bilirubin as an initial and sole manifestation that was successfully treated with methylprednisolone.
It remains a challenge to make a differential diagnosis for hepatic enzyme elevations because many causes could induce liver injury. Seven AOSD cases with increased liver enzymes have been previously reported. The etiologies of liver injury in these cases included NSAID-induced liver injury (n = 2)[20,21], hepatic injury associated with AOSD itself (n = 2)[2,22], and AOSD with concurrent autoimmune hepatitis (n = 3)[23-25].
The largest challenge to identify the etiologies of liver injury in patients with AOSD is the lack of specific biomarkers and pathological findings. Highly variable hepatic pathological features in AOSD has been previously reported, ranging from mild portal inflammatory cell infiltration and Kupffer cell hyperplasia[20,21,26-28] to portal fibrosis and massive or submassive hepatic necrosis[29-33]. Taken together, no histological finding has been identified to be specific for liver injury secondary to AOSD. Although the value of liver biopsy in diagnosing AOSD remains debatable[34], the liver histological findings may provide valuable information to rule out liver disorders induced by viruses, autoimmune factors, and drugs, facilitating the early detection of AOSD and subsequent prompt intervention with methylprednisolone[29].
Several potential mechanisms responsible for liver injury in AOSD have been proposed[33]. Notably, the serum interleukin-18 (IL-18) concentration is markedly increased in patients with AOSD and active hepatitis and correlates with serum aminotransferase levels[35,36]. Moreover, previous studies have found a marked increase in IL-18 expression by activated macrophages and Kupffer cells within the liver parenchyma of a patient with AOSD[37]. However, it remains unknown whether serum IL-18 serves as an early predictor of liver injury in patients with AOSD[29].
Collectively, liver involvement in the form of elevated bilirubin and liver enzymes can be an initial and sole presentation of relapsed AOSD which responds well to glucocorticoid treatment. Therefore, the diagnosis of AOSD should be considered in the case of nonresolving liver injury after the exclusion of common etiologies for liver diseases.
Although liver involvement in the form of increased hepatic enzymes and bilirubin is common in AOSD patients, no AOSD case with liver involvement as the initial manifestation of AOSD has been reported. Liver involvement in the form of elevated bilirubin and liver enzymes can be an initial and sole presentation of relapsed AOSD which responds well to glucocorticoid treatment. Therefore, our study suggests that the diagnosis of AOSD should be considered in the case of nonresolving liver injury after the exclusion of common etiologies for liver diseases.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huan C, Yong D S-Editor: Gao CC L-Editor: MedE-Ma JY P-Editor: Xing YX
| 1. | Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still's disease. J Autoimmun. 2018;93:24-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 2. | Sahutoglu T, Kara E, Dogan IO, Gulluoglu M, Akyuz F, Besisik F. Acute Severe Hepatitis and Hemophagocytosis in Adult Onset Still's Disease. Arch Iran Med. 2015;18:724-728. [PubMed] |
| 3. | Kawaguchi H, Tsuboi H, Yagishita M, Terasaki T, Terasaki M, Shimizu M, Honda F, Ohyama A, Takahashi H, Miki H, Yokosawa M, Asashima H, Hagiwara S, Kondo Y, Matsumoto I, Sumida T. Severe Adult-onset Still Disease with Constrictive Pericarditis and Pleuritis That Was Successfully Treated with Tocilizumab in Addition to Corticosteroids and Cyclosporin A. Intern Med. 2018;57:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Cagatay Y, Gul A, Cagatay A, Kamali S, Karadeniz A, Inanc M, Ocal L, Aral O, Konice M. Adult-onset Still's disease. Int J Clin Pract. 2009;63:1050-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Sève P. Adult-onset Still's disease. Autoimmun Rev. 2014;13:708-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 389] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 6. | Fautrel B, Zing E, Golmard JL, Le Moel G, Bissery A, Rioux C, Rozenberg S, Piette JC, Bourgeois P. Proposal for a new set of classification criteria for adult-onset still disease. Medicine (Baltimore). 2002;81:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 330] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Jiang L, Wang Z, Dai X, Jin X. Evaluation of clinical measures and different criteria for diagnosis of adult-onset Still's disease in a Chinese population. J Rheumatol. 2011;38:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, Kashiwazaki S, Tanimoto K, Matsumoto Y, Ota T. Preliminary criteria for classification of adult Still's disease. J Rheumatol. 1992;19:424-430. [PubMed] |
| 9. | Linde B, Oelzner P, Katenkamp K, Hein G, Wolf G. [Fulminate liver failure in a 39-year-old female patient with leukocytosis, unclear fever, and arthralgic pain]. Med Klin (Munich). 2007;102:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Evensen KJ, Swaak TJ, Nossent JC. Increased ferritin response in adult Still's disease: specificity and relationship to outcome. Scand J Rheumatol. 2007;36:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Crispín JC, Martínez-Baños D, Alcocer-Varela J. Adult-onset Still disease as the cause of fever of unknown origin. Medicine (Baltimore). 2005;84:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Zenone T. Fever of unknown origin in adults: evaluation of 144 cases in a non-university hospital. Scand J Infect Dis. 2006;38:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Hu QY, Zeng T, Sun CY, Luo CN, Liu S, Ding TT, Ji ZF, Lu A, Yimaiti K, Teng JL, Cheng XB, Ye JN, Su YT, Shi H, Sun Y, Chi HH, Zhou ZC, Chen LJ, Xu J, Jiang LD, Wu LJ, Lin J, Yang CD, Liu HL. Clinical features and current treatments of adult-onset Still's disease: a multicentre survey of 517 patients in China. Clin Exp Rheumatol. 2019;37 Suppl 121:52-57. [PubMed] |
| 14. | Mehta B, Efthimiou P. Ferritin in adult-onset still's disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Efthimiou P, Kadavath S, Mehta B. Life-threatening complications of adult-onset Still's disease. Clin Rheumatol. 2014;33:305-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | Mehrpoor G, Owlia MB, Soleimani H, Ayatollahi J. Adult-onset Still's disease: a report of 28 cases and review of the literature. Mod Rheumatol. 2008;18:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Andrès E, Kurtz JE, Perrin AE, Pflumio F, Ruellan A, Goichot B, Dufour P, Blicklé JF, Brogard JM, Schlienger JL. Retrospective monocentric study of 17 patients with adult Still's disease, with special focus on liver abnormalities. Hepatogastroenterology. 2003;50:192-195. [PubMed] |
| 18. | Zhu G, Liu G, Liu Y, Xie Q, Shi G. Liver abnormalities in adult onset Still's disease: a retrospective study of 77 Chinese patients. J Clin Rheumatol. 2009;15:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Efthimiou P, Paik PK, Bielory L. Diagnosis and management of adult onset Still's disease. Ann Rheum Dis. 2006;65:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 20. | Ott SJ, Baron A, Berghaus T, Lamerz R, Beuers U. Liver failure in adult Still's disease during corticosteroid treatment. Eur J Gastroenterol Hepatol. 2003;15:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Omagari K, Matsunaga Y, Yamashita H, Nishiyama H, Hazama H, Oda H, Isomoto H, Mizuta Y, Murase K, Kohno S. Successful treatment with cyclosporin in adult-onset Still disease manifesting as acute hepatitis with marked hyperferritinemia. Am J Med Sci. 2003;326:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Bishara R, Braun-Moscovici Y, Dagan A, Toledano K, Saadi T, Sabo E, Balbir-Gurman A. Severe hyperferritinemia--a clue for severe hepatitis in a patient with adult-onset Still's disease. Clin Rheumatol. 2016;35:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Fujii K, Rokutanda R, Osugi Y, Koyama Y, Ota T. Adult-onset Still's disease complicated by autoimmune hepatitis: successful treatment with infliximab. Intern Med. 2012;51:1125-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Liu LL, Feng ML, Wang LN, Li XL, Yao L. A case report of successful treatment with plasma exchange for adult-onset Still's disease with autoimmune hepatitis. J Clin Apher. 2010;25:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Efe C, Purnak T, Ozaslan E. The diagnosis of autoimmune hepatitis in patients with adult-onset Still's disease. J Clin Apher. 2010;25:235; author reply 236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Kim HA, Kwon JE, Yim H, Suh CH, Jung JY, Han JH. The pathologic findings of skin, lymph node, liver, and bone marrow in patients with adult-onset still disease: a comprehensive analysis of 40 cases. Medicine (Baltimore). 2015;94:e787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Hot A, Toh ML, Coppéré B, Perard L, Madoux MH, Mausservey C, Desmurs-Clavel H, Ffrench M, Ninet J. Reactive hemophagocytic syndrome in adult-onset Still disease: clinical features and long-term outcome: a case-control study of 8 patients. Medicine (Baltimore). 2010;89:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Gallo M, Calvanese A, Oscuro F, Gallo A, Caso P, Annibale E, Farinato N. [Acute hepatitis in a patient with adult onset Still disease]. Clin Ter. 1997;148:183-187. [PubMed] |
| 29. | Lim KB, Schiano TD. Still disease and the liver-an underappreciated association. Gastroenterol Hepatol (N Y). 2011;7:844-846. [PubMed] |
| 30. | Thabah MM, Singh KK, Madhavan SM, Gupta R. Adult onset Still's disease as a cause of acute liver failure. Trop Gastroenterol. 2008;29:35-36. [PubMed] |
| 31. | Muta T, Yamano Y. Fulminant hemophagocytic syndrome with a high interferon gamma level diagnosed as macrophage activation syndrome. Int J Hematol. 2004;79:484-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Valluru N, Tammana VS, Windham M, Mekonen E, Begum R, Sanderson A. Rare Manifestation of a Rare Disease, Acute Liver Failure in Adult Onset Still's Disease: Dramatic Response to Methylprednisolone Pulse Therapy-A Case Report and Review. Case Rep Med. 2014;2014:375035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Hogan T, Kao KT, Tung J. A rare case of severe acute hepatitis associated with adult-onset still disease dramatically improved by high-dose steroid therapy. Gastroenterol Hepatol (N Y). 2011;7:841-844. [PubMed] |
| 34. | Andrès E, Locatelli F, Pflumio F, Marcellin L. Liver biopsy is not useful in the diagnosis of adult Still's disease. QJM. 2001;94:568-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Ogata A, Kitano M, Yamanaka J, Yamasaki T, Hashimoto N, Iwasaki T, Hamano T, Fujimoto J, Kakishita E. Interleukin 18 and hepatocyte growth factor in fulminant hepatic failure of adult onset Still's disease. J Rheumatol. 2003;30:1093-1096. [PubMed] |
| 36. | Yumoto E, Higashi T, Nouso K, Nakatsukasa H, Fujiwara K, Hanafusa T, Yumoto Y, Tanimoto T, Kurimoto M, Tanaka N, Tsuji T. Serum gamma-interferon-inducing factor (IL-18) and IL-10 levels in patients with acute hepatitis and fulminant hepatic failure. J Gastroenterol Hepatol. 2002;17:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Priori R, Barone F, Alessandri C, Colafrancesco S, McInnes IB, Pitzalis C, Valesini G, Bombardieri M. Markedly increased IL-18 liver expression in adult-onset Still's disease-related hepatitis. Rheumatology (Oxford). 2011;50:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |