Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.211
Peer-review started: July 15, 2020
First decision: August 8, 2020
Revised: August 17, 2020
Accepted: November 12, 2020
Article in press: November 12, 2020
Published online: January 6, 2021
Processing time: 170 Days and 4.1 Hours
Sclerosing angiomatoid nodular transformation (SANT) is a rare disease of the spleen. It has unique pathological features and mimics splenic tumor on radiological imaging.
A 47-year-old woman was incidentally found to have a splenic mass on abdominal ultrasound. She had a 10-cm postoperative scar in the lower abdomen due to previous cesarean sections. The patient had a past history of anemia of unknown etiology for 20 years. The patient underwent laparoscopic splenectomy. The postoperative course was uneventful, with a hospital stay of 7 d. The histopathological examination of the spleen revealed SANT. At the 6-mo follow-up, the patient remained disease-free.
SANT is a rare benign disease mimicking a malignant tumor. A definitive diagnosis can be made only on histopathology.
Core Tip: We report the case of a 47-year-old lady with long-standing anemia with an incidentally detected splenic mass of 5.9 cm × 5.1 cm that appeared malignant on computed tomography. The patient underwent laparoscopic splenectomy. Histopathological examination of the spleen revealed sclerosing angiomatoid nodular transformation (SANT). SANT is a rare benign disease of the spleen that mimics malignancy on radiological imaging. Preoperative diagnosis of SANT is difficult. SANT should be included in the differential diagnosis when treating patients with a splenic mass.
- Citation: Li SX, Fan YH, Wu H, Lv GY. Sclerosing angiomatoid nodular transformation of the spleen: A case report and literature review. World J Clin Cases 2021; 9(1): 211-217
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/211.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.211
Sclerosing angiomatoid nodular transformation (SANT) of the spleen is a rare benign lesion[1]. SANT was first reported and described as a benign splenic lesion by Martel et al[1]. SANT is more common in women than men and has no characteristic clinical or radiological features. Most patients undergo open or laparoscopic splenectomy due to a diagnostic dilemma. The final diagnosis is made based on histopathological examination of the spleen. We report a case of SANT and discuss the radiological and immunohistochemical characteristics of this disease.
A 47-year-old woman visited our hospital for a yearly health checkup.
She denied any recent fever, allergy, chills, or changes in bowel habits.
The patient had a past history of anemia of unknown etiology for 20 years. She also had a history of cesarean sections performed 23 years earlier.
She did not have any addictions or any significant family history.
On clinical examination, there was a 10-cm postoperative scar in the lower abdomen due to previous cesarean sections. The abdominal examination was unremarkable with no organomegaly.
Laboratory testing revealed a hemoglobin level of 85 g/L (normal, 115–150 g/L) suggestive of moderate anemia. The white blood cell and platelet counts were normal. The following tumor markers were within the normal range: serum carcinoembryonic antigen (0.58 ng/mL, normal < 5.0 ng/mL); cancer antigen (CA) 125 (9.46 U/mL, normal < 35 U/mL); alpha fetoprotein (5.38 ng/mL, normal < 20 ng/mL); and CA19-9 (5.97 U/mL, normal < 37 U/mL). The coagulation profile and liver function tests were within normal limits.
To determine the cause of the anemia, the patient underwent a bone marrow biopsy and smear examination, which revealed iron deficiency anemia.
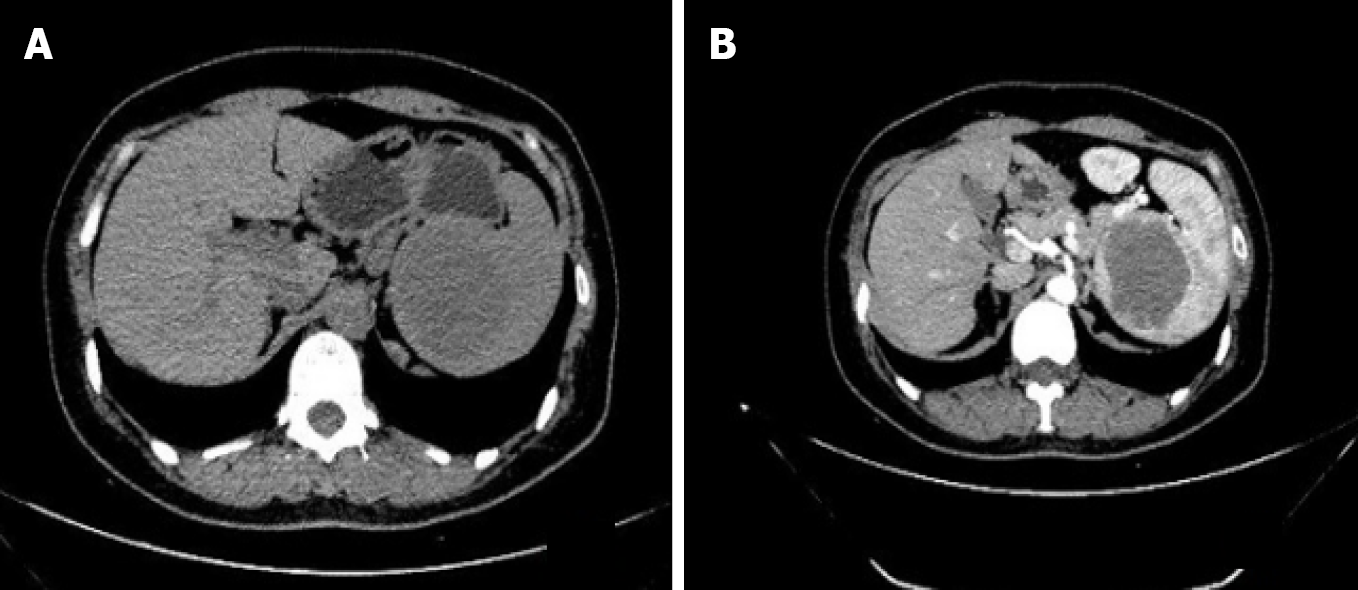
On abdominal ultrasound, a splenic lesion 5.9 cm × 5.1 cm in size and hypoechoic in nature was found at the upper pole of the spleen. A computed tomography (CT) scan showed a hypodense solid lesion, 5.9 cm × 5.1 cm, located at the upper pole of the spleen in the non-contrast phase (Figure 1A) and mild progressive enhancement in the contrast-enhanced phase (Figure 1B).
A final diagnosis of splenic tumor was made.
With a provisional diagnosis of splenic tumor, the patient was scheduled for laparoscopic splenectomy. The patient and her relatives were informed about the increased risk of overwhelming post-splenectomy infection (OPSI) after splenectomy. They were also informed about the need for an intraoperative histopathological examination of the spleen, and if the tumor was benign, a spleen autotransplantation would be considered[2]. However, due to the possibility of missing a malignancy on intraoperative histopathological examination and the low risk of OPSI in adults, the patient and her family members insisted on splenectomy and declined an intraoperative histopathological examination.
During the operation, a mass lesion measuring 7 cm × 5.5 cm × 5 cm was found at the upper pole of the spleen. Laparoscopic splenectomy was performed and the resected spleen was sent for histological examination. The operative time was 85 min, and estimated blood loss was 60 mL. The postoperative course was uneventful, with a hospital stay of 7 d.
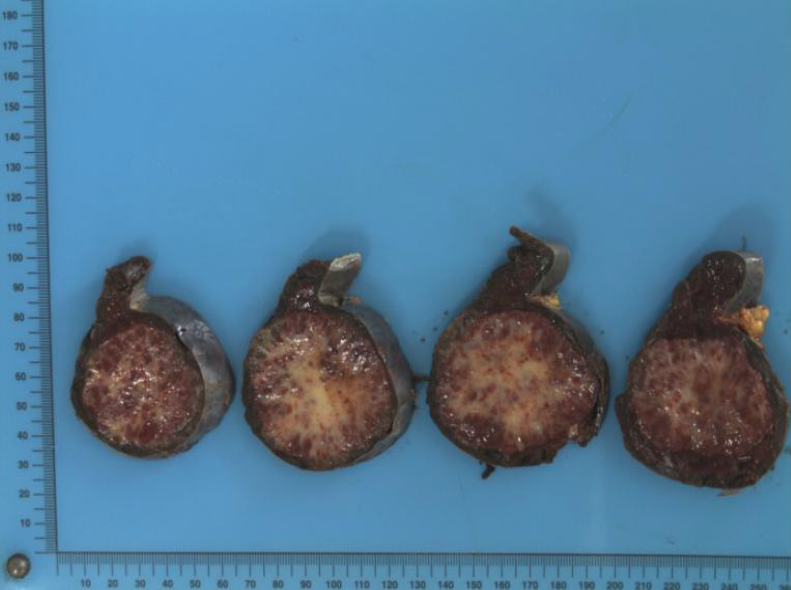
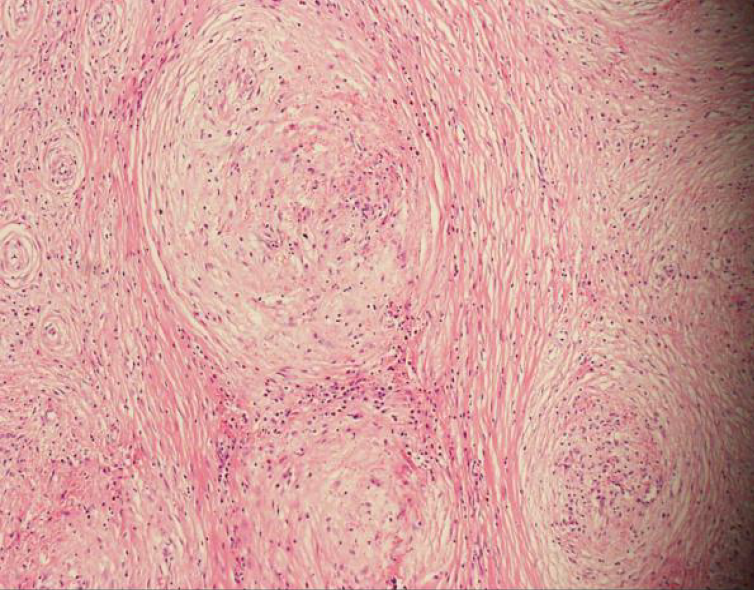
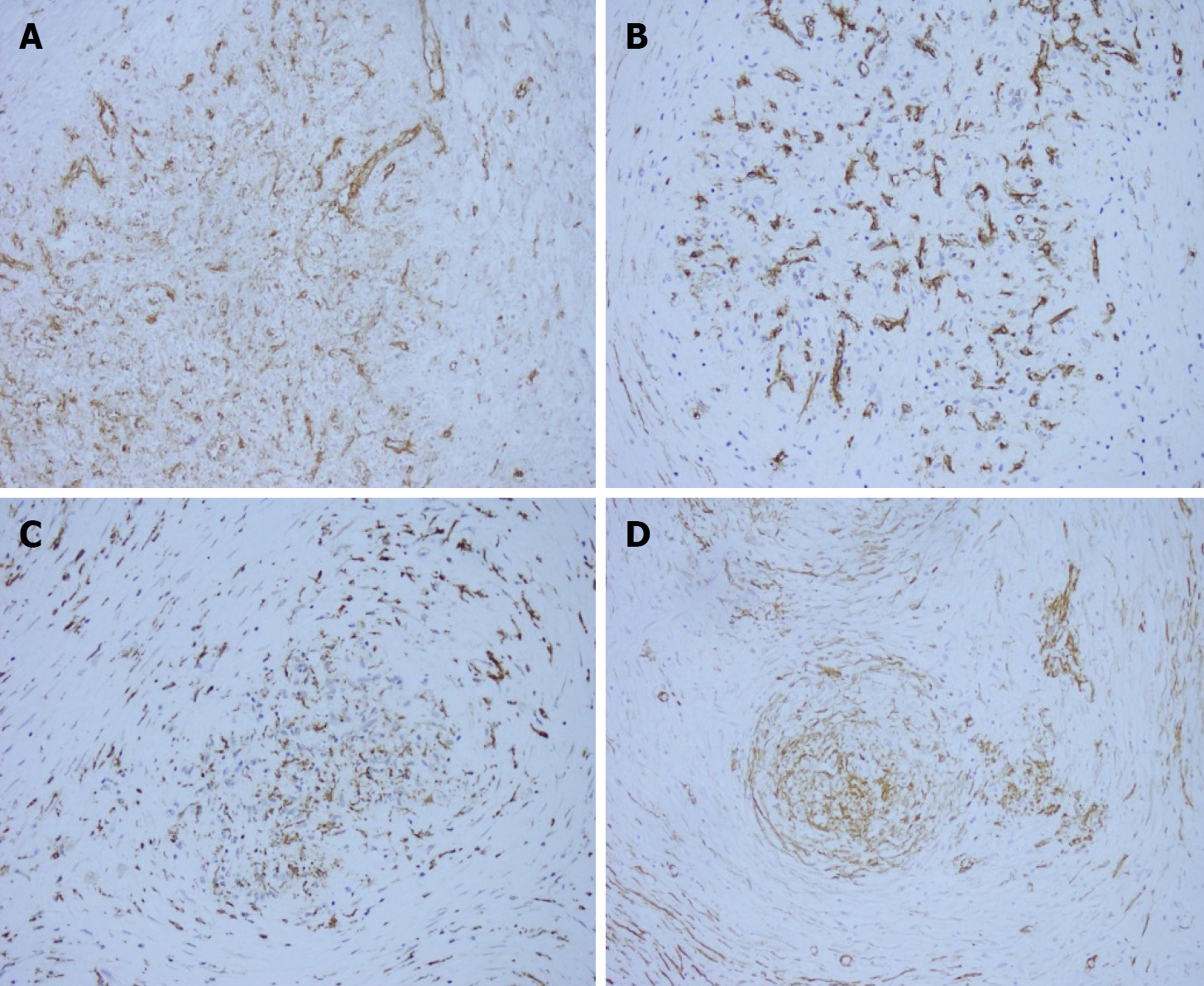
On macroscopic examination, the splenic lesion was well encapsulated, off-white and dull-red in color, and firm in consistency (Figure 2). On microscopic examination, there were multiple rounded hemangiomatous nodules surrounded by fibrosclerotic stroma (Figure 3). Immunohistochemistry revealed the following findings (Figure 4): cluster of differentiation (CD) 31 (+), CD34 (+), CD68 (+), Ki67 (1%), and smooth-muscle actin (SMA) (+). Based on these findings, a pathological diagnosis of SANT was made.
At the 6-mo follow-up, the patient remained disease-free and her anemia had resolved.
SANT is a rare benign lesion of the spleen. It was initially named multinodular hemangioma by Rosai et al[3]. In 2004, Martel et al[1] coined the term SANT for the first time. Based on their study of 25 cases, the authors concluded that SANT is a benign vascular lesion of the spleen probably caused by a reactive process.
The exact pathogenesis of SANT is unknown. Some researchers have detected Epstein-Barr virus RNA in resected specimens, suggesting that its pathogenesis could be similar to that of inflammatory pseudotumors[4]. Other studies have found a large number of IgG4-positive plasma cells in SANT samples, suggesting that SANT may be associated with IgG4-related autoimmune diseases[5].
SANT occurs more frequently in women than men. It is most often asymptomatic and has no characteristic clinical features (Table 1). The most common complaint is abdominal pain. In our case, the patient was asymptomatic. Laboratory tests may show an elevated erythrocyte sedimentation rate, leukocytosis, and throm-bocytopenia[1].
| Ref. | n | Mean age, yr | Gender, F/M | Clinical features (n cases) | Recurrence |
| Martel et al[1] | 25 | 48 | 17/8 | Incidental finding on physical examination (14); Abdominal discomfort (6); Splenomegaly (4); Fever (1); | No |
| Diebold et al[10] | 16 | 44 | 9/7 | Incidental finding on physical examination (7); Splenomegaly with anemia (5); Abdominal pain (2); Fever (2) | No |
| Hou et al[11] | 10 | 42 | 8/2 | Incidental finding on physical examination (8); Abdominal pain (1); Upper left abdominal pain (1) | No |
| Nomura et al[12] | 71 | 46 | 27/44 | Incidental finding on imaging studies (33); Abdominal or back discomfort, weight loss, fever (27); Not described (11) | NA |
| Pelizzo et al[13] | 1 | 9 wk | 1/0 | Severe abdominal distension and rectal bleeding (1) | No |
| Liao et al[14] | 1 | 50 | 1/0 | Persistent neutrophilia and unintentional weight loss (1) | No |
| Sánchez Belmar et al[15] | 1 | 42 | 0/1 | Vomiting and headaches and intravenous drug user (1) | No |
| Jin et al[16] | 37 | 45 | 16/19 | Incidental finding on imaging studies (35); Fever and leukocytosis (1); Abdominal pain (1) | NA |
| Chikhladze et al[17] | 2 | 45 | 1/1 | Lasting feeling of abdominal fullness (1); Weight loss and recurring vomiting (1) | No |
The most common imaging methods used for SANT are CT and magnetic resonance imaging of the abdomen (Table 2). In most of the reported cases, SANT presents as a solitary lesion but can be multifocal in rare cases. However, none of the radiological features are pathognomonic of SANT, and further studies are required to differentiate SANT from malignant lesions of the spleen. Raman et al[6] noted that it is difficult to differentiate SANT from other splenic diseases by radiological imaging alone. The final diagnosis is mainly based on histopathological and immunohistochemical examination of the resected specimen. The differential diagnosis of SANT includes hemangioma, splenic hamartoma, angiosarcoma, hemangioendothelioma, and inflammatory pseudotumor.
| Previous reports | Radiological characteristics |
| Li et al[8] | Low density with small calcification on plain CT scan and progressive enhancement of the lesion after enhancement |
| Zeeb et al[18] | Edge enhancement in arterial and portal phases. A stellate low-density region can be seen in the center of the delayed phase of the lesion |
| Thacker et al[19] | Persistent stellate low-density areas can be seen in the center of the lesion |
| Bamboat et al[20] | MRI T2-WI showed that the center of the lesion was stellate and low-density, and it appeared as a spoked-wheel configuration |
| Karaosmanoglu et al[21] | MRI and CT enhancement patterns of marginal enhancement and progressive spoked-wheel enhancement towards the center of the lesion |
| Nomura et al[12] | CT: Lesion is well circumscribed and of lower attenuation compared to the background spleen, and the mass becomes isodense in the delayed phase.MRI: On fat-saturated precontrast T1-weighted images SANT presented as a central hyperintense area consistent with hemorrhage, and on T2-weighted images, the lesion appeared as a spoke and wheel pattern. |
On histological examination with hematoxylin and eosin staining, multiple hemangioma-like nodules, fissure-like or sinus-like vascular cavities are visible in the center of the nodules, with a small number of tissue cells scattered around the lacunae, and a dense concentric distribution of smooth muscle or collagen fibers around the nodules. On immunohistochemical staining, three types of vascular structures are seen in SANT nodules: A capillary network, sinusoid vascular spaces, and small venous vessels. The cells of all three structures are CD31-positive. Moreover, the cells of the capillary network are CD34-positive while the cells of the sinusoid vascular spaces are CD8-positive[1,7]. In addition, there are spindle cells around the nodules that are positive for CD68, SMA, and vimentin. The cell proliferation (Ki-67) index is usually < 5%[7,8].
SANT of the spleen is a benign disease that does not require surgery unless the patient is symptomatic. However, due to diagnostic dilemma, most patients undergo splenectomy to rule out malignancy or another pathological disease of the spleen[9]. In our case, the patient underwent laparoscopic splenectomy and recovered without complications. At the 6-mo follow-up, the patient was free of disease.
SANT is a rare benign disease of the spleen that mimics malignant tumors on radiological imaging. Clinical differentiation between SANT and other splenic diseases is difficult. Splenectomy is both diagnostic and therapeutic in symptomatic cases. A definitive diagnosis can be made only with histopathological and immuno-histochemical examination of the resected spleen.
Manuscript source: Unsolicited manuscript
Specialty type: Biochemical research methods
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Carlo I S-Editor: Wang JL L-Editor: Webster JR P-Editor: Wu YXJ
| 1. | Martel M, Cheuk W, Lombardi L, Lifschitz-Mercer B, Chan JK, Rosai J. Sclerosing angiomatoid nodular transformation (SANT): report of 25 cases of a distinctive benign splenic lesion. Am J Surg Pathol. 2004;28:1268-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Toro A, Parrinello NL, Schembari E, Mannino M, Corsale G, Triolo A, Palermo F, Romano A, Di Raimondo F, Di Carlo I. Single segment of spleen autotransplantation, after splenectomy for trauma, can restore splenic functions. World J Emerg Surg. 2020;15:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Mahadevia PS, Tanaka K, Fineberg S. Rosai and Ackerman's surgical pathology, 9th edition author: Juan Rosai Mosby, Edinburgh, 2004. Diagn Cytopathol. 2006;34:382-383. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Weinreb I, Bailey D, Battaglia D, Kennedy M, Perez-Ordoñez B. CD30 and Epstein-Barr virus RNA expression in sclerosing angiomatoid nodular transformation of spleen. Virchows Arch. 2007;451:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Nagai Y, Hayama N, Kishimoto T, Furuya M, Takahashi Y, Otsuka M, Miyazaki M, Nakatani Y. Predominance of IgG4+ plasma cells and CD68 positivity in sclerosing angiomatoid nodular transformation (SANT). Histopathology. 2008;53:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Raman SP, Singhi A, Horton KM, Hruban RH, Fishman EK. Sclerosing angiomatoid nodular transformation of the spleen (SANT): multimodality imaging appearance of five cases with radiology-pathology correlation. Abdom Imaging. 2013;38:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Awamleh AA, Perez-Ordoñez B. Sclerosing angiomatoid nodular transformation of the spleen. Arch Pathol Lab Med 2007; 131: 974-978 [PMID: 17550330 DOI: 10. 1043/1543-2165(2007)131. |
| 8. | Li L, Fisher DA, Stanek AE. Sclerosing angiomatoid nodular transformation (SANT) of the spleen: addition of a case with focal CD68 staining and distinctive CT features. Am J Surg Pathol. 2005;29:839-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Bushati M, Sommariva A, Montesco MC, Rossi CR. Laparoscopic splenectomy for sclerosing angiomatoid nodular transformation of the spleen. J Minim Access Surg. 2017;13:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Diebold J, Le Tourneau A, Marmey B, Prevot S, Müller-Hermelink HK, Sevestre H, Molina T, Billotet C, Gaulard P, Knopf JF, Bendjaballah S, Mangnan-Marai A, Brière J, Fabiani B, Audouin J. Is sclerosing angiomatoid nodular transformation (SANT) of the splenic red pulp identical to inflammatory pseudotumour? Histopathology. 2008;53:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Hou J, Ji Y, Tan YS, Shi DR, Liu YL, Xu C, Zeng HY. [Sclerosing angiomatoid nodular transformation of spleen: a clinicopathologic study of 10 cases with review of literature]. Zhonghua Bing Li Xue Za Zhi. 2010;39:84-87. [PubMed] |
| 12. | Nomura R, Tokumura H, Katayose Y, Nakayama F, Iwama N, Furihata M. Sclerosing Angiomatoid Nodular Transformation of the Spleen: Lessons from a Rare Case and Review of the Literature. Intern Med. 2019;58:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Pelizzo G, Villanacci V, Lorenzi L, Doria O, Caruso AM, Girgenti V, Unti E, Putignano L, Bassotti G, Calcaterra V. Sclerosing angiomatoid nodular transformation presenting with abdominal hemorrhage: First report in infancy. Pediatr Rep. 2019;11:7848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Liao J, Musbahi A, Dasgupta K, Thibaut H, Gopinath B. Sclerosing angiomatoid nodular transformation of the spleen. BMJ Case Rep. 2019;12:e229757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Sánchez Belmar C, White A, Majeed M, Redmond HP. Sclerosing angiomatoid nodular transformation of the spleen: unusual case presentation in an intravenous drug user. BMJ Case Rep. 2020;13:e235648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Jin Y, Hu H, Regmi P, Li F, Cheng N. Treatment options for sclerosing angiomatoid nodular transformation of spleen. HPB (Oxford). 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Chikhladze S, Lederer AK, Fichtner-Feigl S, Wittel UA, Werner M, Aumann K. Sclerosing angiomatoid nodular transformation of the spleen, a rare cause for splenectomy: Two case reports. World J Clin Cases. 2020;8:103-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 18. | Zeeb LM, Johnson JM, Madsen MS, Keating DP. Sclerosing angiomatoid nodular transformation. AJR Am J Roentgenol. 2009;192:W236-W238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Thacker C, Korn R, Millstine J, Harvin H, Van Lier Ribbink JA, Gotway MB. Sclerosing angiomatoid nodular transformation of the spleen: CT, MR, PET, and 99(m)Tc-sulfur colloid SPECT CT findings with gross and histopathological correlation. Abdom Imaging. 2010;35:683-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Bamboat ZM, Masiakos PT. Sclerosing angiomatoid nodular transformation of the spleen in an adolescent with chronic abdominal pain. J Pediatr Surg. 2010;45:E13-E16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Karaosmanoglu DA, Karcaaltincaba M, Akata D. CT and MRI findings of sclerosing angiomatoid nodular transformation of the spleen: spoke wheel pattern. Korean J Radiol. 2008;9 Suppl:S52-S55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |