Published online Apr 26, 2020. doi: 10.12998/wjcc.v8.i8.1547
Peer-review started: January 24, 2020
First decision: February 26, 2020
Revised: April 3, 2020
Accepted: April 8, 2020
Article in press: April 8, 2020
Published online: April 26, 2020
Processing time: 91 Days and 3.8 Hours
Cesarean scar molar pregnancy is extremely rare, but the incidence has been rising due to the continuous increase in the rate of cesarean section. The presence of a hydatidiform mole in the scar left on the uterus by the procedure may lead to severe complications. We performed a literature review and found only seven reported cases of cesarean scar molar pregnancy. Accurate diagnosis and appropriate treatment are extremely important for the patients’ prognosis.
A 35-year-old woman, gravida 4, para 1, complained of vaginal bleeding lasting more than 1 mo and amenorrhea lasting more than 2 mo. The patient’s serum human chorionic gonadotropin was 4287800 IU/L. Ultrasound showed a 11.5 cm × 7.5 cm mass at the anterior lower wall of the uterus. The patient underwent suction evacuation, and partial grape-like tissue mixed with blood clots was removed. Uterine arterial embolization was performed to control intraoperative and postoperative bleeding. Histological examination confirmed the presence of a hydatidiform mole in uterine scar. After surgery, there was still a mass with heterogeneous intensity near the isthmus of the uterus on magnetic resonance imaging. The patient then underwent chemotherapy. During the 6-mo follow-up period, the mass disappeared and the serum human chorionic gonadotropin level gradually decreased to normal level.
We report a case of cesarean scar molar pregnancy successfully cured by comprehensive treatment. We found that cesarean scar molar pregnancy was subject to intraoperative bleeding, and uterine arterial embolization before surgery may be helpful.
Core tip: We here report a case of cesarean scar molar pregnancy cured by comprehensive treatment including suction evacuation, uterine arterial embolization, and chemotherapy. We also performed a literature review and found that most cases of cesarean scar molar pregnancy were associated with intraoperative bleeding. Uterine arterial embolization might help prevent massive bleeding if used before surgery.
- Citation: Jiang HR, Shi WW, Liang X, Zhang H, Tan Y. Hydatidiform mole in a scar on the uterus: A case report. World J Clin Cases 2020; 8(8): 1547-1553
- URL: https://www.wjgnet.com/2307-8960/full/v8/i8/1547.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i8.1547
Cesarean scar pregnancy is a rare type of ectopic pregnancy, which means that the gestational sac is implanted in the myometrium at the site of a previous cesarean section[1]. Hydatidiform mole pregnancy embedded in cesarean scars is also extremely rare[2-5]. It can lead to severe complications, such as uterine rupture, hemorrhage, and maternal death[6]. Here, we report a case of cesarean scar molar pregnancy cured by comprehensive treatment and summarize its clinicopathologic and imaging characteristics based on the previous literature.
A 35-year-old woman complained of vaginal bleeding lasting more than 1 mo and amenorrhea lasting more than 2 mo.
The patient usually had a regular menstrual period lasting 7 d on a 26-d cycle with no dysmenorrhea. There was no obvious cause of continuous vaginal bleeding at any point in the 2 mo leading up to treatment. There were no early pregnancy reactions such as nausea or vomiting and no discomfort such as lower abdominal pain, dizziness, or fatigue. The patient had normal appetite, sleep, and urination despite the bleeding, and she had no weight loss.
The patient had undergone three previous surgical terminations of pregnancy, 10, 6, and 2 years prior, respectively, and one cesarean delivery 10 years prior (gravida 4, para 1).
Physical examination showed a markedly enlarged uterus.
Upon admission, the serum human chorionic gonadotropin (HCG) and β-HCG levels were 4287800 IU/L and 1512540 IU/L, respectively. After suction evacuation under ultrasound guidance and uterine arterial embolization, the HCG and β-HCG levels were 187780 and 66312 IU/L, respectively. Two weeks later, the patient underwent seven cycles of chemotherapy with fluorouracil (5-Fu) and kengshemycin (KSM) once every 3 wk. After the third cycle, the HCG and β-HCG levels were 29540 and 8869 IU/L, respectively. After the sixth cycle, the HCG and β-HCG levels were 381.8 and 115 IU/L, respectively. After the seventh cycle, the β-HCG level was 38.7 IU/L. The HCG/β-HCG level had dropped to normal after 6 wk.
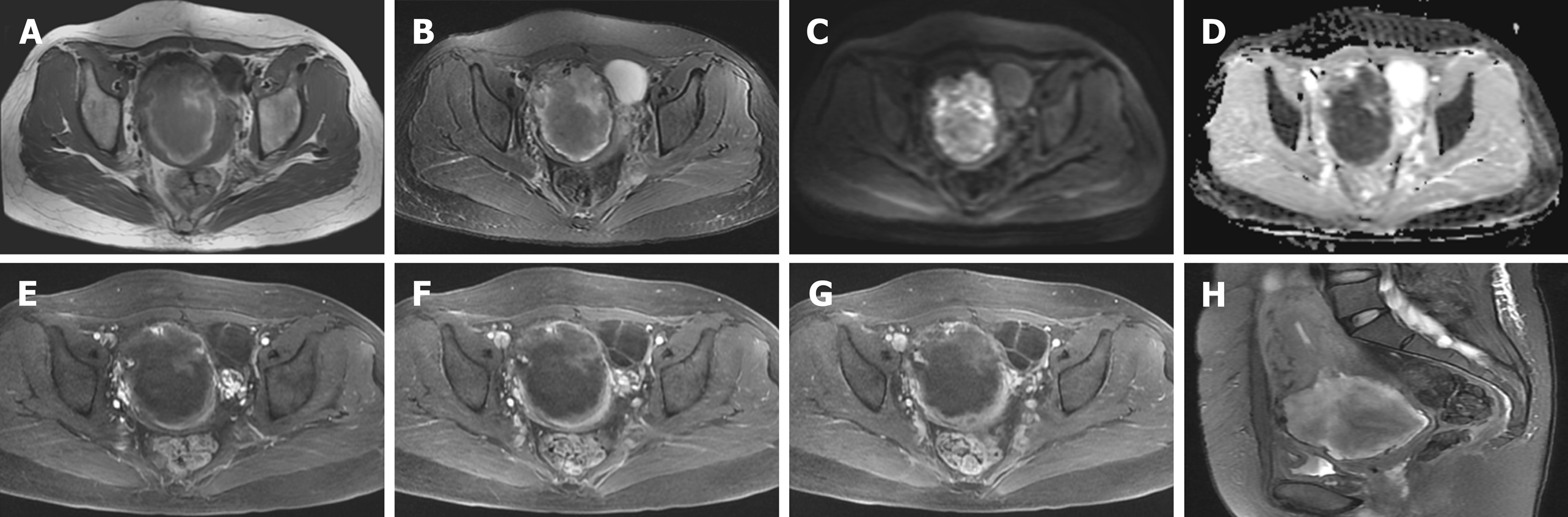
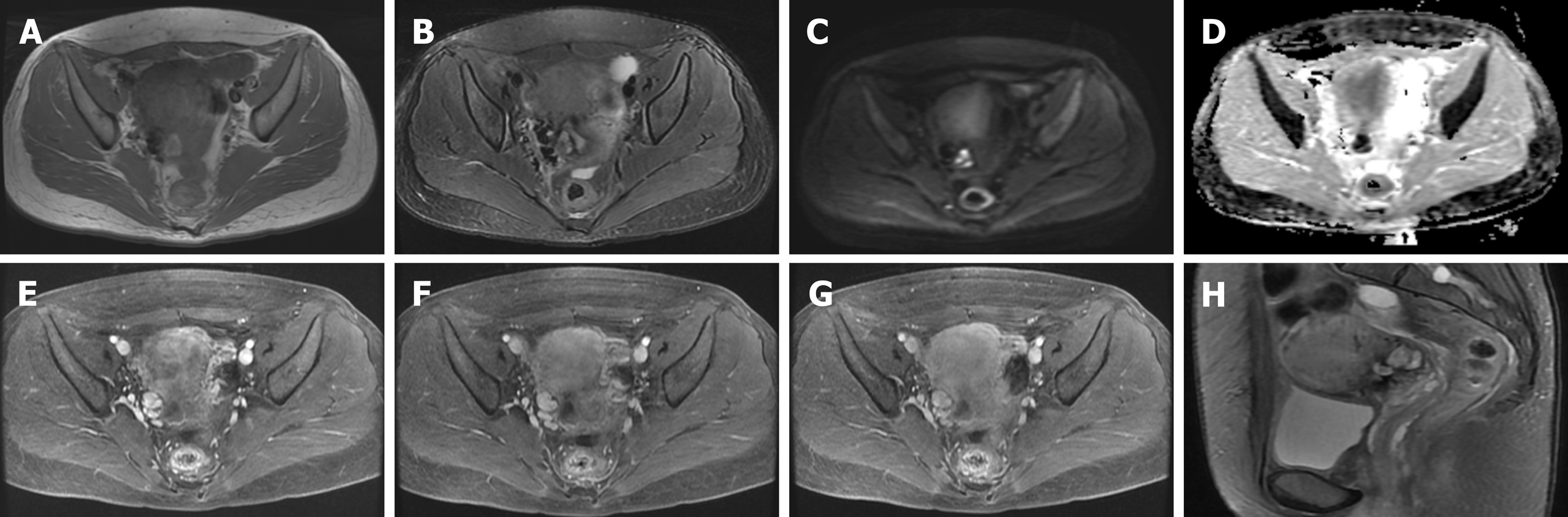
At admission, ultrasound indicated a 11.5 cm × 7.5 cm mass at the anterior lower wall of the uterus, in which an anechoic 5.1 cm × 2.8 cm area was noted inside; the echo of the myometrium was heterogeneous. Ten days after embolization, magnetic resonance imaging (MRI) (Figure 1) demonstrated that there was still a mass measuring 6.7 cm × 6.5 cm × 6.0 cm in the proximal isthmus. The lesion showed heterogeneous intensity on both T1-weighted image (WI) and T2WI, heterogeneous hyperintensity on diffusion-weighted image, and hypointensity on apparent diffusion coefficient maps. The mass showed mild rim enhancement in the arterial phase and continued enhancement in the venous and delayed phases; multiple distorted vascular masses were seen in the anterior wall of the uterus. After the sixth cycle of chemotherapy, MRI (Figure 2) showed that the lesion was visibly diminished in size.
Histological examination confirmed the diagnosis of hydatidiform mole in a uterine scar (Figure 3).
The patient underwent a suction evacuation using the No. 8 suction tube (MKL, Suzhou, China) under ultrasound guidance. Abundant grape-like tissue mixed with blood clots was shaved out, but there was a massive hemorrhage during and after the operation. Therefore, the patient was infused with concentrated erythrocyte 4 U and fresh frozen plasma 400 mL, and a Foley catheter balloon was used to restrain blood loss for more than 10 min. Also, emergency uterine arterial embolization was performed to control active bleeding. The Seldinger technique was applied to puncture and catheterize the bilateral internal iliac arteries through the right femoral artery, and the procedure was performed under local anaesthesia. Angiography confirmed that the vessels were uncomplete, tortuous, and disordered. Thus, superselective embolization of both uterine arteries was performed using gelatin sponge granules. Postembolization angiography showed no obvious bleeding. The hemoglobin level was 22 g/L and 48 g/L before and after the embolization, respectively. Then, the patient underwent seven cycles of chemotherapy with 5-Fu and KSM.
During the 6-mo follow-up period, the mass disappeared and the serum HCG gradually decreased to normal level, and the patient had no complaint.
Cesarean scar molar pregnancy is extremely rare, but the incidence has been rising due to the continuous increase in the rate of cesarean section[2]. The myometrium of the uterus usually thins and merges with the thin and fibrous scar after caesarean section, so hydatidiform moles in the uterine scar may have severe complications, such as uterine rupture, hemorrhage, hysterectomy, and serious maternal morbidity[6].
To our knowledge, only seven cases have been reported, including one case of cesarean scar invasive molar pregnancy[3], four cases of partial molar pregnancy[2,4,5,7], one case of complete molar pregnancy[8], and one case in which the author did not specify the type[9]. Clinicopathological features of the eight cases including our case are listed in Table 1. Among these cases, the median maternal age was 34 years (range from 28 to 44 years) with a median gravidity of 4 (range from 2 to 8) and at least one parity. All patients had at least two prior uterine curettages. The concentration of HCG/β-HCG also increased abnormally. The clinical manifestations were not fully identical, but the most common symptom was vaginal bleeding for more than 1 mo (6/8 cases, 75%). Other presentations include symptoms of pregnancy[2], abdominal pain[3], and amenorrhea[5], and only one patient was asymptomatic on admission[8].
| Ref. | Age (yr) | Presentation | Gravida/para | HCG (IU/L) | Diagnostic method | Treatment |
| Wu et al[4], 2006 | 31 | Persistent symptoms | 8/1 | 61798 (β-HCG) | Ultrasound, pathology | Dilation and curettage |
| Michener et al[9], 2009 | 33 | Vaginal hemorrhage | 5/3 | 161 (β-HCG) | Emergency hysterectomy | Intragestational sac MTX injection |
| Jin et al[7], 2011 | 44 | Vaginal bleeding, lower abdominal pain | 4/2 | 94724 (β-HCG) | Transvaginal sonography, pathology | Suction curettage |
| Ko et al[2], 2012 | 34 | Persistent symptoms of pregnancy | 4/2 | 21925 (HCG) | Ultrasound, pathology | Suction evacuation, uterine arterial embolization |
| Kaluarachchi et al[8], 2013 | 40 | Asymptomatic | 4/2 | 6743 (β-HCG) | Laparotomy | Hysterectomy |
| Vimercati et al[3], 2016 | 34 | Abdominal pain and vaginal bleeding | 4/1 | 51547 (β-HCG) | Ultrasound, pathology | Local and systemic MTX, resection of the uterine |
| Ling et al[5], 2018 | 28 | Abdominal pain, vaginal bleeding, amenorrhea | 3/1 | 7984 (HCG) | Ultrasound, MRI, pathology | Uterine arterial embolization, suction evacuation |
| Present case | 35 | Vaginal bleeding, menopause | 4/1 | 1515540 (β-HCG) | Ultrasound, MRI, pathology | Suction evacuation, uterine arterial embolization, chemotherapy |
To date, MRI has not been used very often in the diagnosis of hydatidiform mole embedded in the scar on the uterus. Only two cases have been reported: One was an ectopic molar pregnancy, which showed isointensity on T1WI, hyperintensity on T2WI, and distinct contrast enhancement[10], and the other was a cesarean scar molar pregnancy, which showed a low signal on T1WI and heterogeneous high signal on T2WI, and the myometrium of the anterior wall of the uterine isthmus incision was not continuous[5]. All three cases showed different signals on T1WI and T2WI, which could be caused by hemorrhage or cystic components. Ash et al[6] stated that MRI could be used to show the gestational sac embedded in the anterior lower uterine segment, evaluate pelvic anatomy, improve intra-operative orientation, and assess the possibility of myometrial invasion. Whether MRI is available to the application of hydatidiform mole in the scar of uterus remains to be further studied.
In histopathology, invasive mole often presents molar villi in the myometrium and vascular permeation, while hydatidiform mole usually presents mildly or markedly hydropic villous swelling with central cistern formation[11]. Missaoui et al[12] found that p57 KIP2 expression was present in partial hydatidiform mole, and the expression of p53 and Ki-67 could reflect the pathological development of hydatidiform mole. This may be helpful to the diagnosis of hydatidiform moles. Among all cases, only one patient underwent immunohistochemical examination with positive p57 expression and proliferation index (Ki-67) of trophocytes of 10%[5].
Gestational trophoblastic disease (GTD) includes the tumour spectrum of hydatidiform mole (complete and partial), invasive mole, choriocarcinoma, and placental-site trophoblastic tumour. Recently, the majority of all the reported cases of cesarean scar GTD are molar pregnancy, and only two cases of cesarean scar choriocarcinoma have been described. Sorbi et al[13] reported a case of cervical choriocarcinoma that had been misdiagnosed as a cesarean scar ectopic pregnancy in 2013. The patient was admitted to hospital for irregular vaginal bleeding of 10 d duration and sovrapubic pain. Qian et al[14] revealed a second case of cesarean scar choriocarcinoma, which was also misdiagnosed as a normal cesarean scar pregnancy in 2014. The patient complained amenorrhea for 47 d and irregular vaginal bleeding for half a month. The rarity and non-specific clinical signs and symptoms of this disease highlight the importance of early consideration of choriocarcinoma when suspecting a GTD in the cesarean scar.
So far, there has been no definitive consensus regarding the treatment of cesarean scar molar pregnancy[2]. Based on our literature review, two cases were treated by suction evacuation, one case was managed with methotrexate, one case was treated with methotrexate and hysterectomy, two cases were cured by suction evacuation and uterine arterial embolization, one case was treated by hysterectomy, and our patient was treated by suction evacuation, uterine arterial embolization, and chemotherapy. In all cases, except for one patient who was lost to follow-up[9], the levels of HCG/β-HCG returned to normal and no corresponding complications occurred. In our case, the HCG decreased visibly after surgery and declined to normal after subsequent chemotherapy. However, intraoperative hemorrhage took place. This involves a high risk of hysterectomy and maternal death, and subsequent uterine arterial embolization was performed.
In conclusion, we have documented a case of cesarean scar molar pregnancy successfully cured by comprehensive treatment including suction evacuation, uterine arterial embolization, and chemotherapy. We also performed a literature review and found that MRI was only rarely used in the diagnosis of hydatidiform moles. We also found that most cases of cesarean scar molar pregnancy are associated with intraoperative bleeding, and uterine arterial embolization before surgery might help to prevent massive bleeding.
We thank the Gynaecology and Obstetrics Department of the First Hospital of Shanxi Medical University for providing the case information and the assistance of the patient.
Manuscript source: Invited Conference Manuscripts
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Isik A, Sel G, Vagholkar K S-Editor: Wang YQ L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | Sel G, Sucu S, Harma M, Harma Mİ. Successful management of cesarean scar pregnancy with vacuum extraction under ultrasound guidance. Acute Med Surg. 2018;5:358-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Ko JK, Wan HL, Ngu SF, Cheung VY, Ng EH. Cesarean scar molar pregnancy. Obstet Gynecol. 2012;119:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Vimercati A, de Gennaro AC, Resta L, Cormio G, Cicinelli E. Sonographic and Power Doppler Evaluation of an Invasive Mole Located in a Cesarean Scar Pregnancy. J Ultrasound Med. 2016;35:1608-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Wu CF, Hsu CY, Chen CP. Ectopic molar pregnancy in a cesarean scar. Taiwan J Obstet Gynecol. 2006;45:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Ling C, Zhao J, Qi X. Partial molar pregnancy in the cesarean scar: A case report and literature review. Medicine (Baltimore). 2018;97:e11312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG. 2007;114:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 331] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 7. | Jin FS, Ding DC, Wu GJ, Hwang KS. Molar Pregnancy in a Cesarean Section Scar of Uterus. J Med Sci. 2011;31:173-176. Available from: https://www.researchgate.net/publication/266421166. |
| 8. | Kaluarachchi C, Tissera A, Gananatha Karunarathna S. Caesarean scar site complete molar pregnancy. Sri Lanka J Obstet Gynaecol. 2013;35:62-64. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 9. | Michener C, Dickinson JE. Caesarean scar ectopic pregnancy: a single centre case series. Aust N Z J Obstet Gynaecol. 2009;49:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Yamada Y, Ohira S, Yamazaki T, Shiozawa T. Ectopic Molar Pregnancy: Diagnostic Efficacy of Magnetic Resonance Imaging and Review of the Literature. Case Rep Obstet Gynecol. 2016;2016:7618631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Cheung AN. Pathology of gestational trophoblastic diseases. Best Pract Res Clin Obstet Gynaecol. 2003;17:849-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Missaoui N, Landolsi H, Mestiri S, Essakly A, Abdessayed N, Hmissa S, Mokni M, Yacoubi MT. Immunohistochemical analysis of c-erbB-2, Bcl-2, p53, p21WAF1/Cip1, p63 and Ki-67 expression in hydatidiform moles. Pathol Res Pract. 2019;215:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Sorbi F, Sisti G, Pieralli A, Di Tommaso M, Livi L, Buccoliero AM, Fambrini M. Cervicoisthmic choriocarcinoma mimicking cesarean section scar ectopic pregnancy. J Res Med Sci. 2013;18:914-917. [PubMed] |
| 14. | Qian ZD, Zhu XM. Caesarean scar choriocarcinoma: a case report and review of the literature. Eur J Med Res. 2014;19:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |