Published online Apr 26, 2020. doi: 10.12998/wjcc.v8.i8.1515
Peer-review started: January 10, 2020
First decision: February 29, 2020
Revised: March 27, 2020
Accepted: April 4, 2020
Article in press: April 4, 2020
Published online: April 26, 2020
Processing time: 103 Days and 5.8 Hours
Ulcerative colitis (UC), also known as chronic nonspecific UC, is an inflammatory bowel disease characterized by diffuse colonic mucosal inflammation. The incidence and prevalence of UC have risen markedly, and the disease seriously affects the quality of life of patients, and poses a great burden on the world health care infrastructure and economy.
We present a 60-year-old man who had ulcerative colitis for more than 10 years, with recurrent abdominal pain, bloody diarrhea with mucopurulent stool. The treatments with sulfasalazine, mesalazine, and traditional Chinese medicine were not effective, and herbs-partitioned moxibustion (HPM) was then applied at “Zhongwan” (RN12), “Tianshu”(ST25), and “Qihai” (RN6) once a day for about 30 min, 3 times per week, for 6 mo.His main clinical symptoms of abdominal pain, bloody diarrhea with mucopurulent stool gradually improved, and the mucosa had nearly healed, as observed under endoscopy by the 6th mo. The patient’s condition was alleviated without relapsing during the subsequent 3-mo follow-up period. HPM showed a significant effect in the treatment of ulcerative colitis in this case, and the effect would help the patient to maintain remission for at least 3 mo.
A series of symptoms of this UC patient significantly improved with the treatment of HPM.
Core tip: Ulcerative colitis (UC) is an inflammatory bowel disease characterized by diffuse colonic mucosal inflammation. Herbs-partitioned moxibustion (HPM) is a warm stimulation therapy based on traditional acupuncture theory. HPM was applied at “Zhongwan” (RN12), “Tianshu” (ST25), and “Qihai” (RN6) in an UC patient. The results showed that HPM can improve the clinical symptoms of this UC patient and the healing of colonic mucosa and help the patient maintain remission for at least 3 mo with no side effects. It can be a typical complementary and alternative therapy for UC.
- Citation: Lin YY, Zhao JM, Ji YJ, Ma Z, Zheng HD, Huang Y, Cui YH, Lu Y, Wu HG. Typical ulcerative colitis treated by herbs-partitioned moxibustion: A case report. World J Clin Cases 2020; 8(8): 1515-1524
- URL: https://www.wjgnet.com/2307-8960/full/v8/i8/1515.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i8.1515
Ulcerative colitis (UC) is highly recurrent and chronic, with the clinical manifestations of persistent or recurrent diarrhea, bloody mucopurulent stool accompanied by abdominal pain, tenesmus, and varying degrees of systemic symptoms as well as extra-intestinal manifestations in the skin, mucosa, and joints[1]. At present, the etiology and pathogenesis of UC are not clear. Most scholars suggest that it may be caused by the interaction of many factors, such as environmental, genetic, immunological, infectious, and psychological factors[2-4].In recent years, the incidence and prevalence of UC have risen markedly not only in Europe and North America[5] but also in Asian countries[6,7]. A long-term follow-up of UC in Hong Kong showed that the age-standardized incidence was 2.1 per 100000 in 2006 and 26.5 per 100000 in 2016, and this rate has increased 12-fold over the past 10 years[8]. Therefore, UC has become a major health problem that needs to be addressed urgently worldwide[9]. While seriously affecting the quality of life of patients, it also poses a great burden on the world health care infrastructure and economy.
One of the therapeutic goals of UC is to induce and maintain long-term clinical remission. Currently in clinical practice, the main treatments for UC include medication such as 5-Aminosalicylates, corticosteroids and immunosuppressants, surgery and other therapies, which have varying efficacy but have the disadvantages of high cost, high drug dependence, high recurrence rate, and significant side effects[10]. They fail to meet patient’s expectations for UC treatment and reduce compliance[9,11]. Therefore, increasingly more patients switch to complementary and alternative medicine for safe and effective treatments. Evidence from both traditional Chinese medicine (TCM) ancient books and modern research has demonstrated that acupuncture, as a typical complementary and alternative therapy[12-14], can improve the condition of UC patients or UC animal models with few side effects[15-17]. Moxibustion is a warm stimulation therapy based on traditional acupuncture theory and has been used to treat gastroenteropathy[18], cancer-related pain[19], insomnia[20], and other symptoms in clinical practice. Herbs-partitioned moxibustion (HPM) integrates moxibustion and Chinese herbal medicine and produces both pharmaceutical effects on the skin and physical thermal effects[21] to relieve abdominal pain, diarrhea, and other symptoms of UC patients, and it has a good efficacy. Joos etal[22] conducted a randomized controlled study on the therapeutic effect of moxibustion in UC patients. After treatment, the colitis activity index was significantly decreased compared with that before treatment, and the general condition of patients was also significantly improved, confirming the clinical efficacy of TCM moxibustion in the treatment of UC[22]. This case report describes a patient with longstanding, recurrent chronic UC whose condition significantly improved and maintained remission for at least 3 mo with the complementary treatment of HPM.
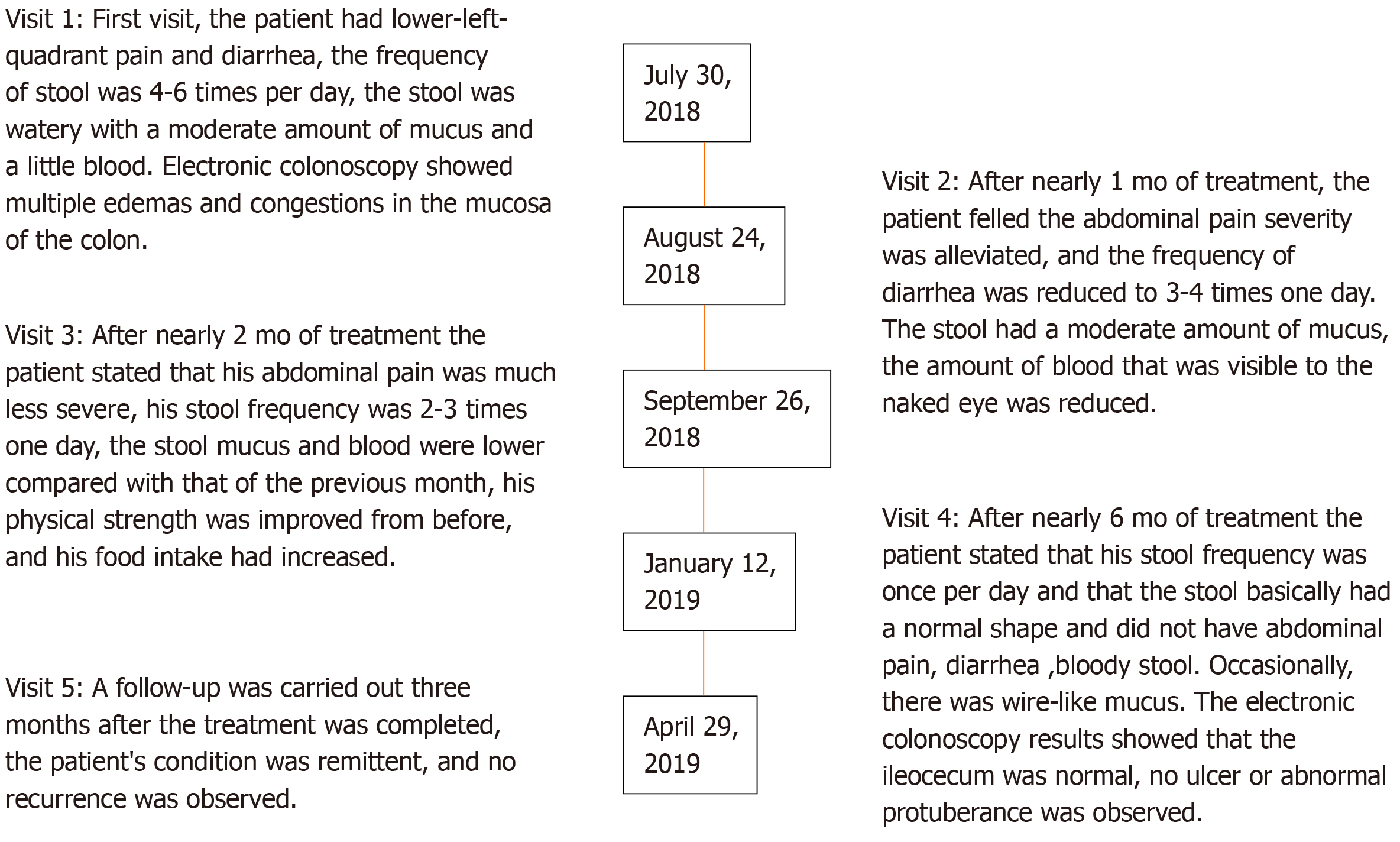
A 60-year-old male patient visited our hospital on July 30, 2018, with the manifestations of abdominal pain, bloody mucopurulent diarrhea for 10 years, and aggravated symptoms for 3 mo.
The patient presented to the clinic at our institution (visit 1). Patient’s symptoms started 10 years ago, and aggravated for 3 mo. Ten years earlier, the patient started to have lower-left abdominal pain without obvious inducement with increased frequency of defecation (4-5 times a day), and the stool consisted of a moderate amount of mucus and a small amount of blood. The patient then visited Shanghai East Hospital for treatment and was diagnosed with UC. The patient was given sulfasalazine [1.5 g ter in die (tid)], but the symptoms were not significantly relieved after 1 mo. Later (specific time unknown), the patient visited the Renji Hospital affiliated to Shanghai Jiaotong University for treatment. He was treated with mesalazine (Etiasa; 1 g tid), and the symptoms slightly improved. The medication was discontinued in March 2015 due to drug-induced liver injury, after which the medication was not taken regularly, and symptoms were intermittent. In 2017, the patient visited the Department of Traditional Chinese Medicine of the Renji Hospital and oral administration of Chinese herbal decoction (specific drug unknown) was prescribed. The medication was discontinued by the patient after the clinical symptoms slightly stabilized, and then the disease relapsed. The patient continued to use the same decoction, but the efficacy was poor.
The patient had no previous medical history.
None.
The patient’s temperature was 36.7 °C, heart rate was 86 beats/min, respiratory rate was 16 breaths/min, and blood pressure was 111/60 mmHg.
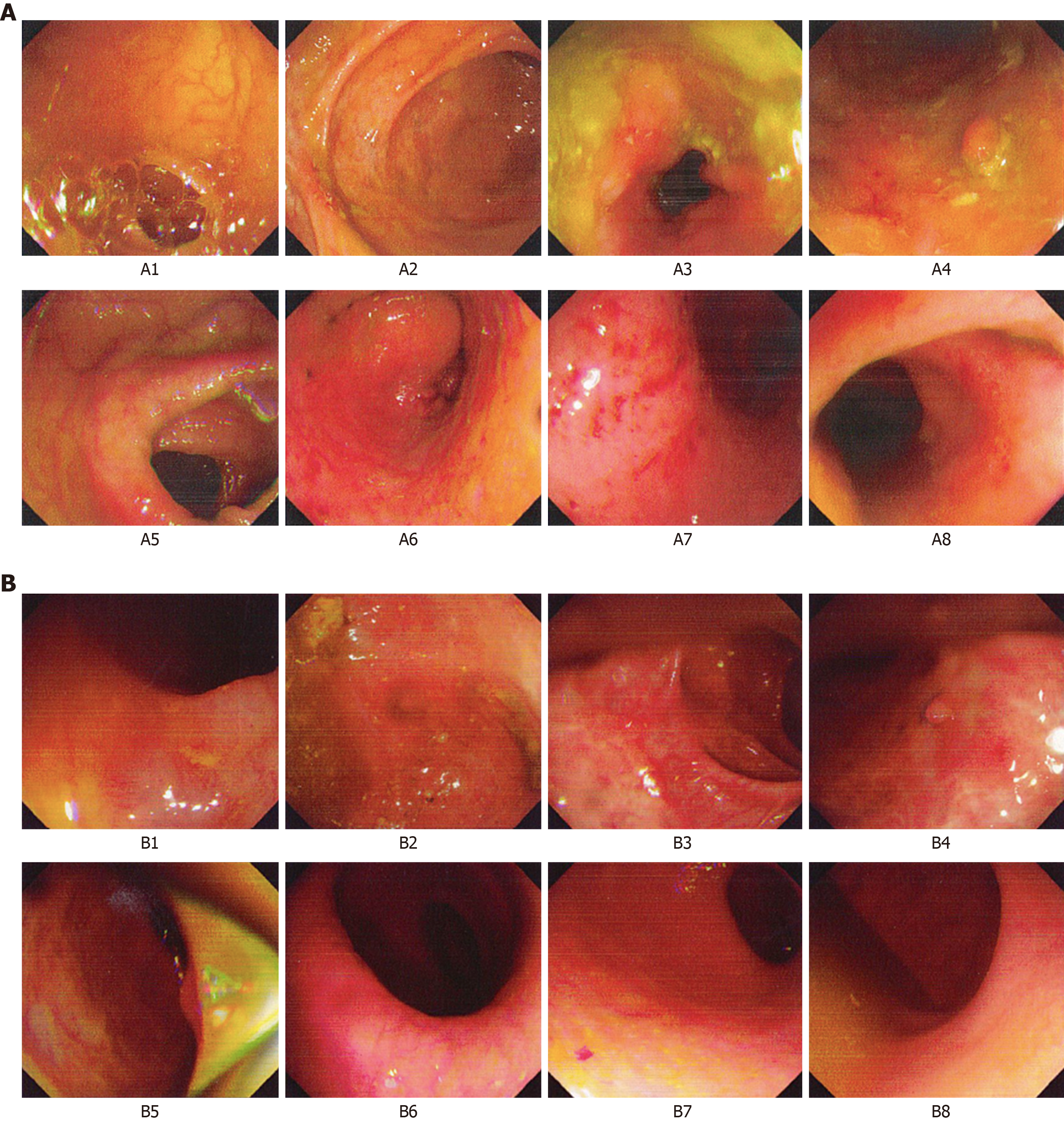
In March 2018, the patient had aggravated abdominal pain, the frequency of defecation was increased, and bloody mucopurulent stool occurred. The patient was back to the Renji Hospital and electronic colonoscopy showed mucosal edema and hyperemia in the hepatic flexure of the ascending colon, descending colon, sigmoid colon, and rectum; part of the transverse colon mucosa was normal. Biopsy results showed mild chronic colitis with activity in the hepatic flexure of the colon, occasional cryptitis, and local elongation and distortion (polypoid) of crypts. Moderate chronic colitis (full-thickness inflammation) with activity and architectural changes in the crypts were visible in the sigmoid colon(Figure 1). Intestinal computed tomography (CT) showed multiple diffuse thickenings of the intestinal wall in the sigmoid colon, splenic flexure of the colon, transverse colon, and hepatic flexure of the colon, as well as increased peripheral small vessels. The patient was diagnosed with active UC.
The patient was prescribed a TCM decoction (specific drug unknown) and mesalazine (Salofalk), but he refused to take mesalazine, and only took the TCM decoction due to previous drug-induced liver injury. After three months of treatment with this Chinese herbal decoction (July 30, 2018), the related laboratory test results [white blood cell count (WBC), red blood cell count (RBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP)] suggested that the disease condition was improved compared with before treatment (March 1, 2008), but the patient's clinical symptoms were not significantly relieved. On July 30, 2018, the patient visited our hospital presenting with lower left quadrant abdominal pain and diarrhea, the frequency of stool was 4-6 times a day, the stool was watery with a moderate amount of mucus and little blood, and the patient did not have fever or other discomfort. He also had no familial history of inflammatory bowel disease. Abdominal examination showed deep tenderness in the lower left quadrant, without palpable mass or draining fistula. The patient had a pale complexion, and there had been no significant weight loss since the onset. The patient had low spirit, poor appetite, fair sleep, and normal urination. The tongue was reddish, the coating of the tongue was white and greasy, and the pulse was weak.
Related laboratory tests (July 30, 2018) showed the following results: WBC: 12.42×109/L; RBC: 5.35× 1012/L; Hemoglobin (HGB): 164 g/L; Platelets (PLT): 300× 109/L; Hematocrit (HCT): 0.466 L/L; ESR: 10 mm/h; CRP (C-reactive proteins): 3.77 mg/L; and normal liver and kidney function (Table 1).
| Time | WBC (×109/L) | RBC (×1012/L) | HGB (g/L) | PLT (109/L) | HCT (L/L) | ESR (mm/h) | CRP (mg/L) |
| March 1, 2018 | 17.53 | 5.50 | 169 | 386 | 0.496 | 34 | 10.51 |
| April 16, 2018 | 10.17 | 5.10 | 153 | 245 | 0.450 | 16 | 18.10 |
| Visit1 | 12.42 | 5.35 | 164 | 300 | 0.466 | 10 | 3.77 |
| Visit2 | 9.40 | 5.37 | 160 | 279 | 0.469 | 5 | 1.46 |
| Visit3 | 10.77 | 5.32 | 160 | 262 | 0.460 | / | 2.84 |
| Visit4 | 10.31 | 5.22 | 162 | 285 | 0.464 | 7 | 0.54 |
| Visit5 | 9.91 | 5.38 | 165 | 294 | 0.484 | 10 | 2.80 |
Clinical assessment on July 30, 2018 showed the following: Score of the patient’s clinical symptoms (including the frequency, severity, and duration of abdominal pain; the frequency of diarrhea and stool characteristics; the amount of mucous stool and stool with pus and blood; the severity of abdominal distension and tenesmus; the percent reduction in food consumption; and symptoms of chills in the limbs and soreness in the waist and knees): 21 points; total score on the Inflammatory Bowel Disease Questionnaire(IBDQ), including systemic symptoms, intestinal symptoms, social competence, and emotional competence: 110 points; Mayo activity index (including diarrhea, hematochezia and mucosal manifestations): 6 points; Baron endoscopy score: 2 points; Visual Analog Scale(VAS) Score of abdominal pain: 21 points; and Hospital Anxiety and Depression Scale (HADS): HADS-A is 21 points, HADS-D is 15 points (Table 2).
| Time | Clinical symptoms(score) | IBDQ (score) | Mayo (score) | Baron (score) | VAS (score) | HADS-A (score) | HADS-D (score) |
| Visit1 | 21 | 110 | 6 | 2 | 21 | 21 | 15 |
| Visit2 | 19 | 121 | / | / | 17 | 17 | 13 |
| Visit3 | 15 | 150 | / | / | 14 | 10 | 8 |
| Visit4 | 4 | 201 | 1 | 0 | 0 | 5 | 0 |
| Visit5 | 3 | 203 | / | / | 0 | 5 | 0 |
The final diagnosis was ulcerative colitis.
Medical record is shown in Figure 2. The acupuncturist had at least a 5-year undergraduate education and was registered practitioners of traditional Chinese medicine.
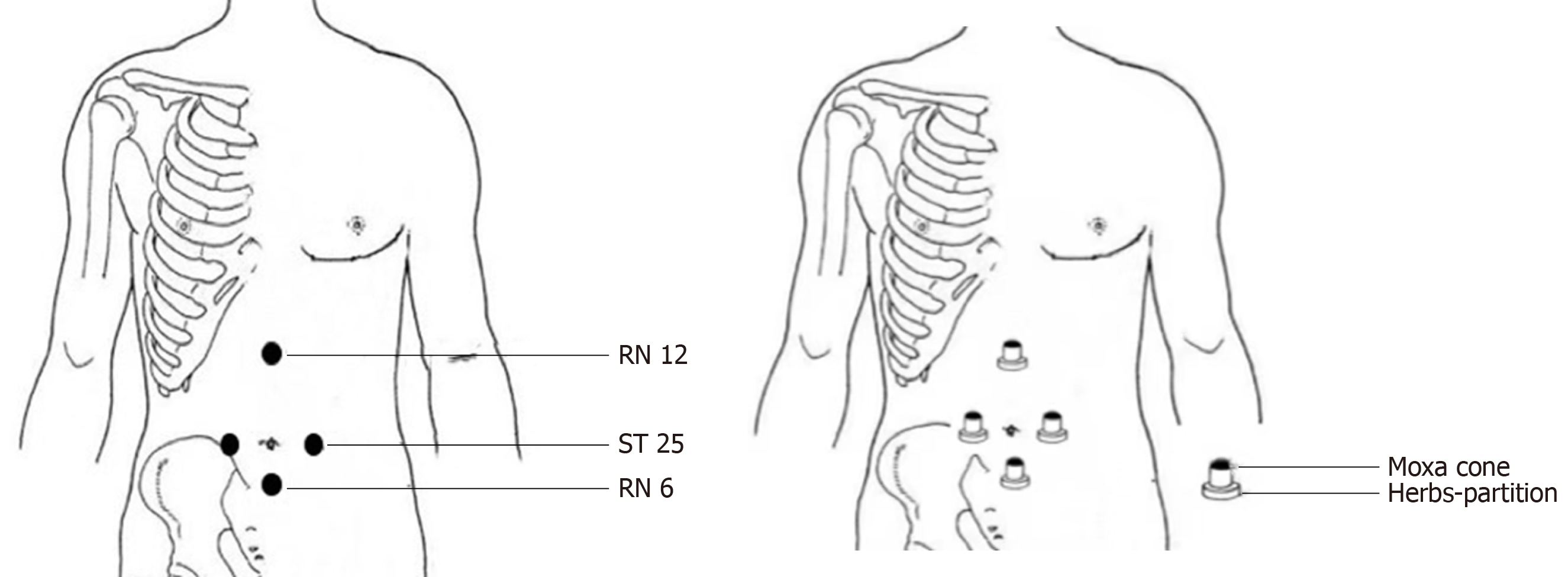
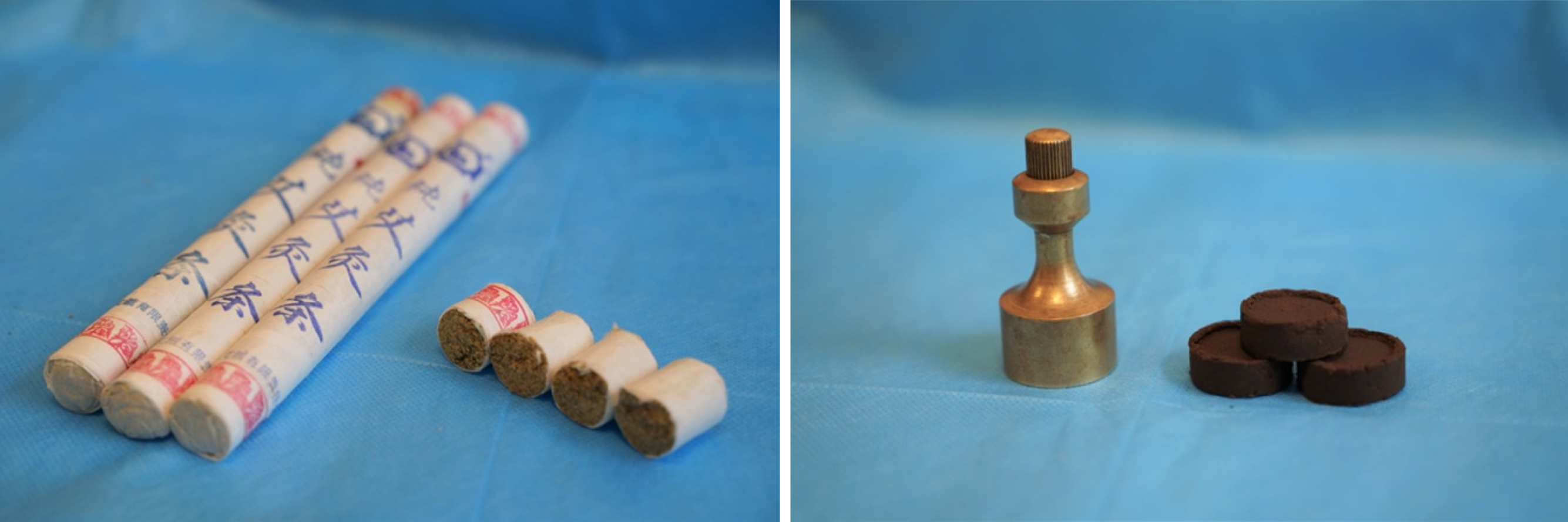
The following acupoints were selected: Zhongwan (RN12), Tianshu (bilateral, ST25), and Qihai (RN6) (Figure 3). Herbal cake formulation: Chinese aconite (Aconitum carmichaelii), Cinnamomum cassia, Salvia miltiorrhiza, Carthamustinctorius, Coptischinensis, common Vladimiria root, and other herbs were ground into fine powder for use. During treatment, the herbal powder was mixed with an appropriate amount of rice wine to make a thick paste, which was pressed with a mold to make an herbal cake with a diameter of 2.3 cm and thickness of 0.5 cm (containing 2.5 g herbal powder). Moxa cone: Three-year-old moxa sticks (Hanyi brand, Nanyang Hanyi Moxa Co., Ltd. China) were cut into a moxa cone with a diameter of approximately 1.7 cm, height of approximately 2 cm, and weight of approximately 2 grams (Figure 4). Dose of moxibustion: One moxibustion was performed per acupoint each time, for approximately 30 min. Treatment schedule: The treatment was applied once a day for about 30 min, 3 times per week (every Tuesday, Thursday, and Saturday). After 3 mo of treatment, the patient rested for 3 d, and the treatment was continued for another 3 mo; therefore, the treatment lasted approximately 6 mo for a total of 72 treatments.
During treatment, the diet should be light, and the patient was told to avoid pungent, spicy, raw, cold, greasy, “hot-energy” food and seafood,keep a regular work and rest schedule and avoid fatigue and staying up late.
After nearly 1 mo (August 24, 2018) of treatment, the abdominal pain was alleviated, and the frequency of diarrhea was reduced to 3-4 times a day. The stool had a moderate amount of mucus, and the amount of visible blood was reduced, and the rest of his symptoms were the same as before. Related laboratory tests showed WBC 9.40 × 109/L, RBC 5.37 × 1012/L, HGB 160 g/L, PLT 279 × 109/L, HC T0.469 L/L, ESR 5 mm/h, CRP 1.46 mg/L, and normal hepatic and renal functions (Table 1). Clinical assessment showed the following results: Score of patient's clinical symptoms: 19 points; IBDQ: 121 points; VAS Score of abdominal pain: 17 points; and HADS: HADS-A17 points and HADS-D 13 points (Table 2).
After nearly 2 mo (September 26, 2018) of treatment, the patient reported that his abdominal pain was alleviated compared with that of the previous month, his stool frequency was 2-3 times/d and the shape was pasty,mucus and blood in stool were reduced, his physical strength was improved, and his food intake increased. Laboratory tests showed WBC 10.77 × 109/L, RBC 5.32 × 1012/L, HGB 160 g/L, PLT 262 × 109/L, HCT 0.460 L/L, and CRP 2.84 mg/L (Table 1). Clinical assessment showed the following results: Score of patient’s clinical symptoms: 15 points; total IBDQ score: 150 points; VAS Score of abdominal pain: 14 points; and HADS: HADS-A 10 points and HADS-D 8 points (Table 2).
After nearly 6mo (January 12, 2019) of treatment, the stool frequency was once a day and that the stool basically had a normal shape. The patient did not have abdominal pain, diarrhea, or bloody stool. Occasionally, there was wire-like mucus. The patient had fair spirit, fair appetite, and fair sleep. The tongue was pale red, the tongue coating was thin and white, and the pulse was slippery. The patient received a reexamination in the Renji Hospital, and the electronic colonoscopy showed that the ileocecum was normal, a small amount of fecal water in the entire colon affected the visual field, ulcer scars and inflammatory polyps were visible in part of the intestines, no ulcer or abnormal protuberance was observed, and local mucosal edema and hyperemia were observed (Figure 1). Intestinal CT showed that the local intestinal wall of the sigmoid colon and splenic flexure was slightly thickened, which was improved compared with findings on March 13, 2018.
Related laboratory tests (January 12, 2019) showed WBC 10.31 × 109/L, RBC 5.22 × 1012/L, HGB 162 g/L, PLT 285 × 109/L, HCT 0.464 L/L, ESR 7 mm/h, CRP 0.54 mg/L, and normal hepatic and renal functions (Table 1). Assessment of the patient's condition showed the followings: Clinical symptom score: 4 points; total IBDQ score: 201 points; Mayo activity index: 1 point; Baron score: 0 point; VAS Score of abdominal pain: 0 points; and HADS: HADS-A 5 points and HADS-D 0 point (Table 2).
A follow-up was carried out three months after the treatment was completed (April 29, 2019). Laboratory tests showed WBC 9. 91 × 109/L, RBC 5.38 × 1012/L, HGB 165 g/L, PLT 294 × 109/L, HCT 0.484 L/L, ESR 10 mm/h, CRP 2.80 mg/L, and normal hepatic and renal functions (Table 1). Clinical assessment showed the following results: Clinical symptom score: 3 points; total IBDQ score: 203 points; VAS Score of abdominal pain: 0; and HADS: HADS-A 5 points and HADS-D 0 point. The patient's condition was alleviated, and no recurrence was observed (Table 2).
This patient had been suffering from UC for more than 10 years and took mesalazine orally, which led to drug-induced liver injury and hospitalization. The patient refused to take Western medicines. Although there was certain degree of improvement in the symptoms after oral administration of a Chinese herbal decoction, the symptoms of abdominal pain, diarrhea, and other symptoms recurred. Although this report only involves 1 case, the clinical symptom scores and the results of colonoscopy demonstrated that HPM is safe and effective for the treatment of UC. No adverse events such as serious burn in this case or in previous clinical studies were found[23,24]. The liver function of the patient was normal before and after the treatment with HPM, indicating that the HPM was safe and did not cause toxic side effects, and the clinical symptoms (including abdominal pain, diarrhea, bloody mucopurulent stool, and other symptoms) were improved. The comparison of the results of IBDQ before vs after treatment showed that the systemic symptoms, intestinal symptoms, social competence, and emotional competence of the patient all improved. After treatment, the mucosa was nearly healed on endoscopy, the mucosal edema and hyperemia were improved, and the Mayo score and Baron score were decreased compared with before treatment. HADS results showed that the anxiety and depression of the patient improved. Three months after the patient completed the treatment (visit 5), laboratory test results showed no abnormalities and were close to the values obtained at the end of the treatment (visit 4), and the clinical symptoms were well controlled, indicating that the therapeutic effect of HPM persisted after treatment, which helped to induce and maintain clinical remission.
Previous basic research reported that acupuncture stimulation facilitates gastrointestinal motility. The distal colon is stimulated via parasympathetic activation at acupointsST25, SP14, and ST37[25,26]. Previous clinical study results from our group showed that HPM significantly improved the clinical symptoms and signs of patients with UC, effectively improved the pathological changes in the colonic mucosa and the intestinal immune dysfunction, and controlled the inflammatory response and tissue damage[23]. According to the TCM theory, chronic non-specific ulcerative colitis belongs to the category of “Changpi” (bloody stool) and “Xiexie” (diarrhea), and results from deficiency and hypofunction of spleen and stomach, accumulation of damp-heat, stagnancy of the liver-qi and deficiency of spleen, or insufficiency of spleen-yang and kidney-yang. In a previous clinical study, good curative effect was achieved by the treatment of the moxibustion [performed at Zhongwan (RN12), Tianshu (ST25), and Qihai (RN6)], and moxibustion performed at Zhongwan (RN12), Tianshu (ST25), and Qihai (RN6) has the function of warming yang, promoting flow of qi and blood, improving the lesional blood circulation, and is helpful to hemostasis and the absorption of inflammatory products, and eventually attains the goal of the neogenesis of granulated tissue in the region of ulceration, and the repair of mucosa epithelium[24].
Our previous animal study showed that electro-acupuncture and HPM markedly down-regulated the Factor Associated Suicide (Fas) and Factor Associated Suicide Ligand (Fas L) expressions in colonic tissues of UC model rats and also decreased the number of apoptosis cells[27]. And acupuncture and moxibustion could inhibit the expression of inflammatory cytokines interleukin 1β(IL-1β) and Il-6mRNA in UC model rats, regulate the immunological abnormalities, reduce immunocyte response to inflammation, and then contribute to the elimination of inflammation and repair of tissue[28]. Furthermore, after moxibustion treatment, the levels of IL-12, IL-17, IL-23, interferon γ, Tumor Necrosis Factor α(TNF-α), TNFR1 and TNFR2, were significantly decreased, and anti-inflammatory cytokines IL-10 and TGF-β expression were increased in UC model rats, which demonstrated that moxibustion treatment may repair intestinal mucosal by down-regulating inflammatory cytokines and up-regulating anti-inflammatory cytokines[29]. All these findings preliminarily explain the anti-inflammatory immune mechanism of moxibustion in the treatment of UC.
In conclusion, UC has a long disease course and is prone to relapse, usually requiring long-term medication to control the disease. In some patients, due to the decline in their physical condition and the decrease in their working hours, their income is reduced. When the condition cannot be adequately managed, the recurrence rate increases. When relapse occurs, the patient needs to be hospitalized. Treatment methods include invasive diagnostic and treatment methods, biological agents, and even surgery. The treatment costs are expensive, which increases the social cost while increasing the financial burden on the patients. In this situation, challenged by the limited medical resources and growing demand for health care, HPM, as a complementary and alternative therapy, provides a safe, effective, and inexpensive treatment for patients with UC. However, its treatment process is complex and needs to be further simplified and improved in the future. Additionally, rigorous multicenter randomized controlled trials are required to verify its clinical efficacy.
A series of symptoms of this ulcerative colitis patient significantly improved under the treatment of HPM.
We thank the patient in this case for his informed consent for using some images for publication of this report.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiba T S-Editor: Zhang L L-Editor: MedE-Ma JY E-Editor: Qi LL
| 1. | Nostrant TT, Kumar NB, Appelman HD. Histopathology differentiates acute self-limited colitis from ulcerative colitis. Gastroenterology. 1987;92:318-328. [PubMed] |
| 2. | Regueiro M, Greer JB, Szigethy E. Etiology and Treatment of Pain and Psychosocial Issues in Patients with Inflammatory Bowel Diseases. Gastroenterology. 2017;152:430-439.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Selinger CP, Carbery I, Warren V, Rehman AF, Williams CJ, Mumtaz S, Bholah H, Sood R, Gracie DJ, Hamlin PJ, Ford AC. The relationship between different information sources and disease-related patient knowledge and anxiety in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J Psychosom Res. 2016;87:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 403] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 5. | Akhuemonkhan E, Parian A, Miller K, Hanauer S, Hutfless S. Prevalence and screening for anaemia in mild to moderate Crohn's disease and ulcerative colitis in the United States, 2010-2014. BMJ Open Gastroenterol. 2017;4:e000155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Li X, Song P, Li J, Tao Y, Li G, Li X, Yu Z. The Disease Burden and Clinical Characteristics of Inflammatory Bowel Disease in the Chinese Population: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2017;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Sandler RS, Glenn ME. Epidemiology of inflammatory bowel disease. Inflamm Bowel Dis. 2000;9:364–369. [DOI] [Full Text] |
| 8. | Chow DK, Leong RW, Tsoi KK, Ng SS, Leung WK, Wu JC, Wong VW, Chan FK, Sung JJ. Long-term follow-up of ulcerative colitis in the Chinese population. Am J Gastroenterol. 2009;104:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Bressler B, Marshall JK, Bernstein CN, Bitton A, Jones J, Leontiadis GI, Panaccione R, Steinhart AH, Tse F, Feagan B; Toronto Ulcerative Colitis Consensus Group. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148:1035-1058.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 10. | Kornbluth A, Sachar DB. Erratum: Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. AmJGastroenterol. 2010;105:500. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 418] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 12. | Sirois FM. Health-related self-perceptions over time and provider-based Complementary and Alternative Medicine (CAM) use in people with inflammatory bowel disease or arthritis. Complement Ther Med. 2014;22:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Opheim R, Hoivik ML, Solberg IC, Moum B; IBSEN Study Group. Complementary and alternative medicine in patients with inflammatory bowel disease: the results of a population-based inception cohort study (IBSEN). J Crohns Colitis. 2012;6:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Koning M, Ailabouni R, Gearry RB, Frampton CM, Barclay ML. Use and predictors of oral complementary and alternative medicine by patients with inflammatory bowel disease: a population-based, case-control study. Inflamm Bowel Dis. 2013;19:767-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Ji J, Huang Y, Wang XF, Ma Z, Wu HG, Im H, Liu HR, Wu LY, Li J. Review of Clinical Studies of the Treatment of Ulcerative Colitis Using Acupuncture and Moxibustion. Gastroenterol Res Pract. 2016;2016:9248589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Ji J, Lu Y, Liu H, Feng H, Zhang F, Wu L, Cui Y, Wu H. Acupuncture and moxibustion for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:158352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Lee DH, Kim JI, Lee MS, Choi TY, Choi SM, Ernst E. Moxibustion for ulcerative colitis: a systematic review and meta-analysis. BMC Gastroenterol. 2010;10:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Park JW, Lee BH, Lee H. Moxibustion in the management of irritable bowel syndrome: systematic review and meta-analysis. BMC Complement Altern Med. 2013;13:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Lee J, Yoon SW. Efficacy and Safety of Moxibustion for Relieving Pain in Patients With Metastatic Cancer: A Pilot, Randomized, Single-Blind, Sham-Controlled Trial. Integr Cancer Ther. 2014;13:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Gao X, Xu C, Wang P, Ren S, Zhou Y, Yang X, Gao L. Curative effect of acupuncture and moxibustion on insomnia: a randomized clinical trial. J Tradit Chin Med. 2013;33:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Zhang D, Ren YB, Wei K, Hong J, Yang YT, Wu LJ, Zhang J, Shi Z, Wu HG, Ma XP. Herb-partitioned moxibustion alleviates colon injuries in ulcerative colitis rats. World J Gastroenterol. 2018;24:3384-3397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Joos S, Wildau N, Kohnen R, Szecsenyi J, Schuppan D, Willich SN, Hahn EG, Brinkhaus B. Acupuncture and moxibustion in the treatment of ulcerative colitis: a randomized controlled study. Scand J Gastroenterol. 2006;41:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Wu HG, Gao ZW, Zhang L. A clinical and experimental study on the treatment of chronic nonspecific colitis by herbs-partitioned moxibustion. ZhongguoZhenjiuZazhi. 1992;012:28-31. |
| 24. | Wu HG, Zhou LB, Shi DR, Liu SM, Liu HR, Zhang BM, Chen HP, Zhang LS. Morphological study on colonic pathology in ulcerative colitis treated by moxibustion. World J Gastroenterol. 2000;6:861-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Gao X, Qin Q, Yu X, Liu K, Li L, Qiao H, Zhu B. Acupuncture at heterotopic acupoints facilitates distal colonic motility via activating M3 receptors and somatic afferent C-fibers in normal, constipated, or diarrhoeic rats. NeurogastroenterolMotil. 2015;27:1817-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Li YQ, Zhu B, Rong PJ, Ben H, Li YH. Neural mechanism of acupuncture-modulated gastric motility. World J Gastroenterol. 2007;13:709-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Wu HG, Gong X, Yao LQ, Zhang W, Shi Y, Liu HR, Gong YJ, Zhou LB, Zhu Y. Mechanisms of acupuncture and moxibustion in regulation of epithelial cell apoptosis in rat ulcerative colitis. World J Gastroenterol. 2004;10:682-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Wu HG, Zhou LB, Pan YY, Huang C, Chen HP, Shi Z, Hua XG. Study of the mechanisms of acupuncture and moxibustion treatment for ulcerative colitis rats in view of the gene expression of cytokines. World J Gastroenterol. 1999;5:515-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Qi Q, Liu YN, Jin XM, Zhang LS, Wang C, Bao CH, Liu HR, Wu HG, Wang XM. Moxibustion treatment modulates the gut microbiota and immune function in a dextran sulphate sodium-induced colitis rat model. World J Gastroenterol. 2018;24:3130-3144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |