Published online Apr 26, 2020. doi: 10.12998/wjcc.v8.i8.1444
Peer-review started: December 13, 2019
First decision: February 20, 2020
Revised: March 5, 2020
Accepted: April 1, 2020
Article in press: April 1, 2020
Published online: April 26, 2020
Processing time: 133 Days and 8.4 Hours
Very few studies have been published on the hemodynamic changes associated with spinal anesthesia induced with ropivacaine during cesarean deliveries in preeclamptic women.
To record and analyze hemodynamic data in women with preeclampsia undergoing cesarean delivery after spinal anesthesia induced with ropivacaine.
Ten eligible women with preeclampsia were enrolled in this prospective observational study. Spinal anesthesia was performed with 2.4 mL of 0.5% ropivacaine. Hemodynamic changes were then analyzed at multiple time points. The hemodynamic responses to vasopressor interventions and uterotonic agents, as well as maternal and neonatal outcomes were also recorded.
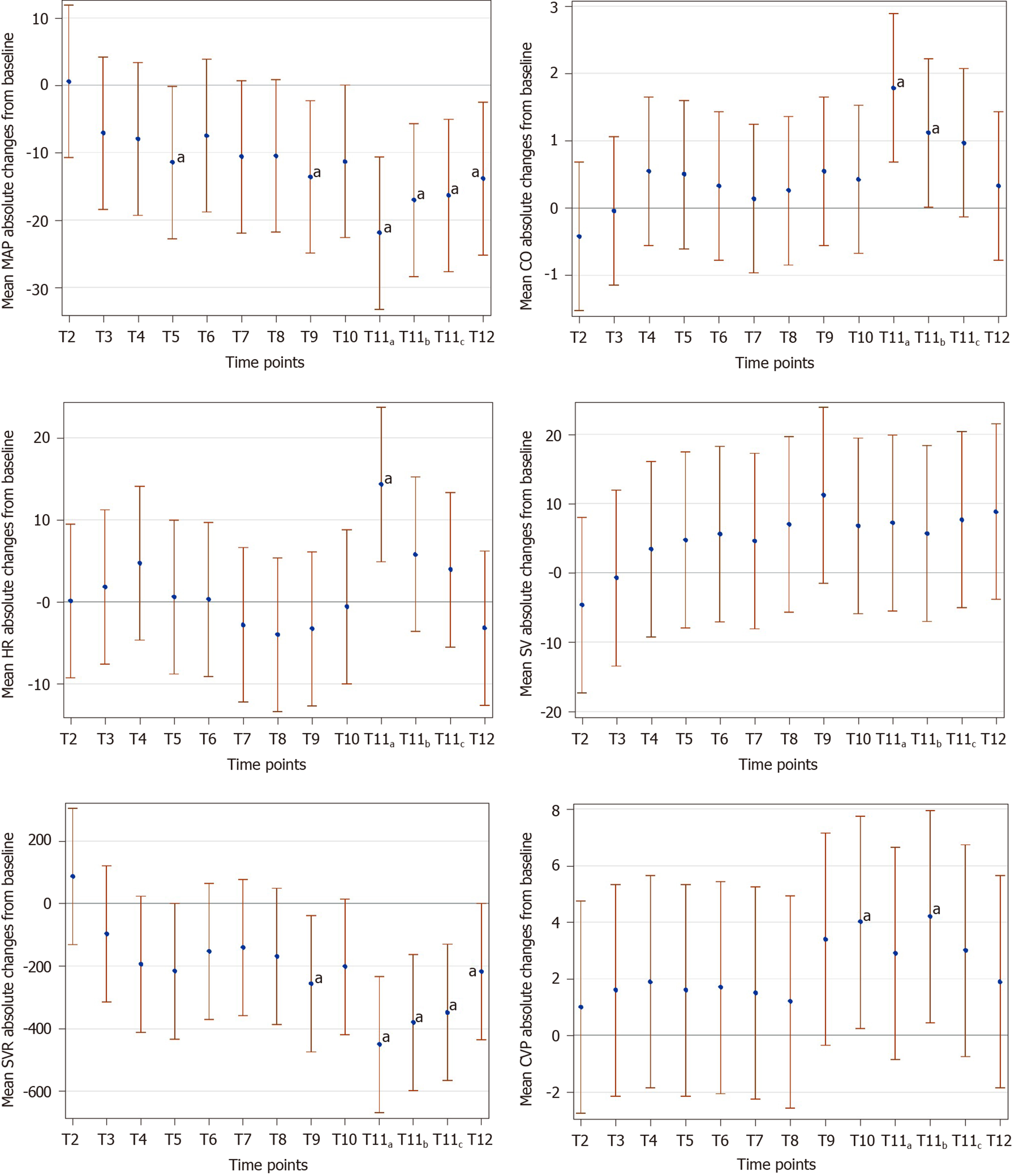
Stable hemodynamic trends were observed in this study. Cardiac output (CO) and stroke volume increased mildly during surgery. In contrast, mean arterial pressure and systemic vascular resistance showed a moderate decrease from induction until the end of surgery. Central venous pressure dramatically increased after delivery. Oxytocin administration was associated with the most significant hemodynamic fluctuations during surgery, namely, an increase in CO and heart rate. Phenylephrine intervention was only required in three patients, and caused an increase in mean arterial pressure and systemic vascular resistance along with a decrease in heart rate, stroke volume, and CO. No maternal and neonatal complications were observed during this study, except transient episodes of hypotension.
Spinal anesthesia for caesarian delivery with ropivacaine in women with preeclampsia is linked to modest hemodynamic changes of no clinical significance in this study. Careful cardiovascular monitoring is still recommended, particularly after the delivery of the fetus or the use of oxytocin.
Core tip: This study is the first to investigate in detail the hemodynamic changes induced by spinal anesthesia with ropivacaine during cesarean delivery in women with preeclampsia. Intrathecal low-dose ropivacaine produces satisfactory anesthesia in cesarean deliveries and stable hemodynamics in preeclampsia patients. Based on the hemodynamic trends seen in this study, large amounts of pre-operative fluids or prophylactic vasopressors before anesthesia aimed at preventing intra-operative hypotension may not be necessary in patients with preeclampsia undergoing spinal anesthesia with low-dose ropivacaine.
- Citation: Zhao N, Xu J, Li XG, Walline JH, Li YC, Wang L, Zhao GS, Xu MJ. Hemodynamic characteristics in preeclampsia women during cesarean delivery after spinal anesthesia with ropivacaine. World J Clin Cases 2020; 8(8): 1444-1453
- URL: https://www.wjgnet.com/2307-8960/full/v8/i8/1444.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i8.1444
Preeclampsia is a significant complication of pregnancy[1-3] and is associated with an increased risk of cardiovascular disease later in life[4,5]. Preeclampsia is the third most common direct cause of maternal mortality worldwide (9%-26% of deaths)[6] and is also associated with 15% of preterm deliveries[2]. Due to a lack of early diagnosis or treatment options, among other factors, maternal mortality caused by preeclampsia in developing countries remains stubbornly high at up to 15% (whereas in developed countries it is only 0%-1.8%)[7].
Elective cesarean delivery (CD) is the most frequently adopted delivery mode to terminate pregnancy in women suffering from preeclampsia[8,9]. The currently preferred anesthesia modality for women with preeclampsia undergoing CD is spinal anesthesia (SA)[10-12], but hypotension related to SA is still a common complication. Some studies have reported less hypotension and lower vasopressor requirements during SA in parturients with preeclampsia than in otherwise healthy patients[13-15], suggesting that preeclampsia has its own hemodynamic characteristics.
The goal of hemodynamic management during CD is the maintenance of maternal cardiac output (CO) and utero-placental perfusion. Early research has shown that, compared with upper arm blood pressure, changes in CO were more closely related to utero-placental blood flow[16]. In the past several decades, thermodilution using a pulmonary artery catheter (PAC) was established as a gold standard technique to monitor CO[17,18]. However, the use of PAC in preeclamptic parturients is controversial because of its invasive nature and associated risks[19]. Therefore, a less-invasive CO monitoring technique was adopted in this study to monitor hemodynamic parameters.
Compared with bupivacaine, ropivacaine is less potent in inducing paralysis, but our clinical experience has shown that it can provide a satisfactory anesthetic effect in CD. However, the hemodynamic data in patients with preeclampsia undergoing SA induced with ropivavaine in current practice has so far been limited. This study was designed to observe the hemodynamic parameters of women with preeclampsia undergoing CD after SA induced with ropivacaine via a moderately invasive monitoring system. By doing so, we hope to help improve our understanding of the anticipated intraoperative hemodynamic changes in such patients.
This was a prospective, observational study carried out at an urban academic teaching hospital offering tertiary-level care in obstetrics and gynecology in Beijing, China. Ethical approval was granted by the hospital’s ethics review board and all subjects provided written informed consent.
Parturients, aged 18 to 45 years old, diagnosed with preeclampsia between December 2017 and May 2018 and requiring urgent CD were screened for enrollment. Preeclampsia was defined as blood pressure ≥ 140/90 mmHg with proteinuria ≥ 300 mg in 24 h or, in the absence of proteinuria, new-onset hypertension combined with new-onset end-organ dysfunction[20]. Severe preeclampsia was defined as blood pressure ≥ 160/110 mmHg, platelet count < 100 × 109/L, or new-onset cerebral, cardiac, hepatic, or renal disturbances. The exclusion criteria incorporated included parturients with any contraindication to SA, chronic hypertension, cardiovascular diseases, multiple fetuses, active labor, pathological pregnancy (e.g., placental abruption, placenta previa, cord prolapse, or a non-reassuring fetal heart tracing), or patient refusal.
All parturients with preeclampsia received antepartum management with anti-hypertensive and seizure prophylaxis medications upon admission according to our institution’s standardized protocol. If CD was anticipated, all subjects underwent echocardiography to exclude abnormal cardiac function and arrhythmia before surgery, had a central venous line placed via the right internal jugular vein, and had an arterial line placed in the left radial artery on the day of surgery.
In the operating room, combined spinal and epidural anesthesia was administered to the patient in a lateral recumbent position, with a 25-gauge spinal needle inserted in the L2–3 interspace followed by a 19-gauge catheter placed in the epidural space at the same location. All participants received 2.4 mL of 0.5% ropivacaine (1% ropivacaine diluted with an equal amount of cerebrospinal fluid) by intrathecal injection. Intravenous crystalloid fluid (250 mL) was rapidly initiated immediately after the beginning of intrathecal injection. After the procedure, the patient was returned to the supine position. Block level was assessed using cold sensitivity. If SA failed, the participant was withdrawn from this study. Following the delivery of the fetus, all women received 20 units of oxytocin injected into the muscular wall of the uterus immediately. If the mean arterial pressure (MAP) was more than 20% below baseline, 50 μg of phenylephrine was administered intravenously; this dose could be repeated until the MAP recovered. After surgery, all women received 2 mg of epidural morphine and a patient controlled epidural pump with 0.15% ropivacaine added with 0.5 μg/mL sufentanil for 24 h of postoperative analgesia.
All participants received routine noninvasive monitoring (noninvasive blood pressure, electrocardiograph, and pulse oximetry), as well as invasive monitoring via a central line for central venous pressure (CVP) and a FloTrac/VigileoTM monitor (Edwards Lifesciences Corporation, Irvine, CA; software version 4.0) for CO and other hemodynamic parameters. The FloTrac/VigileoTM system collected hemodynamic data from peripheral arterial pressure waveforms with no external calibration needed. It derived a MAP value every 20 s and updated all parameters every minute. After patient data (age, gender, body height, and weight) were entered into the system, the arterial line waveform was checked, the system was calibrated, and continuous measurements were initiated. The hemodynamic changes at key time points were recorded. With the input of a CVP value, the systemic vascular resistance (SVR) would be computed for each patient.
We recorded perioperative hemodynamic parameters as follows: CO, MAP, CVP, heart rate (HR), cardiac index (CI), stroke volume (SV), stroke volume index, SVR, and stroke volume variation (SVV). An independent observer carried out data recording. We assessed and focused on hemodynamic changes at 14 key time points, including: T1 (baseline value, on arrival to the operating room); T2 (immediately before intrathecal injection); T3-T7 (2, 4, 6, 8, and 10 min after intrathecal injection); T8 (beginning of skin incision); T9 (beginning of uterine incision); T10 (immediately after newborn delivery); T11a, T11b, and T11c (1, 3, and 5 min after oxytocin administration); T12 (end of surgery).
The measurement data are expressed as the mean ± SD. Statistics were computed using Statistical Analysis System version 9.4 (SAS Institute, Cary, NC, United States). Mean changes from baseline were estimated with mixed models that allowed a general variance-covariance structure for repeated measures. Multiple comparisons and 95% confidence intervals of differences between measures and baselines were adjusted using the Dunnett correction. A P value less than 0.05 was considered statistically significant.
Ten eligible parturients were enrolled in our study, eight of whom were diagnosed with severe preeclampsia. Table 1 shows their characteristics (e.g., age, height, weight, and gestational age), as well as preoperative medications and baseline MAP.
| Case | Age (yr) | Weight (kg) | Height (cm) | BMI | G/P | GA (wk) | Preoperative medication(s) | Diagnosis | B-MAP (mmHg) |
| 1 | 37 | 98 | 174 | 32.4 | 1/0 | 39 | Magnesium sulfate | Severe PE | 107 |
| 2 | 39 | 84 | 156 | 34.5 | 6/2 | 36 | Magnesium sulfate | Severe PE | 117 |
| 3 | 28 | 92 | 161 | 35.5 | 2/0 | 33 | Labetalol | Severe PE | 128 |
| 4 | 30 | 93 | 167 | 33.3 | 2/0 | 38 | Labetalol, magnesium sulfate | Mild PE | 99 |
| 5 | 40 | 89 | 160 | 34.8 | 5/1 | 37 | Labetalol, magnesium sulfate | Mild PE | 76 |
| 6 | 36 | 90 | 168 | 31.9 | 3/1 | 32 | Labetalol, magnesium sulfate | Severe PE | 129 |
| 7 | 31 | 90 | 162 | 34.3 | 1/0 | 34 | Labetalol | Severe PE | 125 |
| 8 | 43 | 84.5 | 164 | 31.4 | 3/1 | 35 | Magnesium sulfate | Severe PE | 109 |
| 9 | 39 | 81 | 168 | 28.7 | 4/2 | 32 | Nicardipine, magnesium sulfate | Severe PE | 126 |
| 10 | 35 | 80 | 160 | 31.2 | 3/0 | 33 | Magnesium sulfate | Severe PE | 113 |
All included patients completed this trial without complications. All newborns were healthy, and no Apgar scores less than 7 were noted (at 1 and 5 min). The average neonate weight was 2449 ± 839.7 g. Satisfactory anesthesia was provided to all patients, achieving a maximum block height at the T4 dermatomal level before the start of surgery. The average time from induction of SA to skin incision was 11.9 ± 3.1 min, and to the end of surgery was 57.9 ± 10.7 min. The average blood loss was 430 ± 141.8 mL, and the average infusion volume was 510 ± 137 mL. Only crystalloids were infused.
Table 2 shows main hemodynamic values at the observed time points. Overall, hemodynamics remained stable throughout each operation, except after the use of oxytocin, which resulted in significant hemodynamic fluctuations. Mean absolute changes from baseline values for all hemodynamic variables at defined time points are displayed in Figure 1. The absolute values of MAP at defined time points decreased by less than 20 mmHg, except after the use of oxytocin, and CO was above baseline throughout surgery.
| Time point | CO | CVP | HR | MAP | SV | SVR | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| T1 | 7.2 | 0.8 | 6.7 | 4.7 | 84.6 | 18.8 | 115.9 | 17.9 | 86.5 | 18.1 | 1233.7 | 246.1 |
| T2 | 6.7 | 1 | 7.8 | 5.7 | 84.7 | 16.6 | 117.6 | 16.7 | 81.4 | 23.1 | 1336.3 | 321.5 |
| T3 | 7 | 0.9 | 8.4 | 4.5 | 86 | 14.6 | 110.6 | 14.5 | 84.6 | 23.9 | 1183.3 | 242.3 |
| T4 | 7.5 | 0.9 | 8.7 | 6.1 | 84.6 | 14.8 | 113.2 | 21.6 | 91.1 | 22.5 | 1133.9 | 277.6 |
| T5 | 7.6 | 1.2 | 8.4 | 5.2 | 81.9 | 15.6 | 108.5 | 19.8 | 93 | 23.7 | 1081.2 | 285.8 |
| T6 | 7.5 | 1 | 8.5 | 5.1 | 84.3 | 18.1 | 108 | 18.1 | 92.7 | 25.4 | 1077.5 | 250.4 |
| T7 | 7.5 | 1 | 8.3 | 5 | 83.3 | 13.9 | 107.5 | 20.2 | 91.1 | 24.7 | 1078.3 | 256.1 |
| T8 | 7.3 | 1.2 | 8 | 3.7 | 82.1 | 17.1 | 107.6 | 20.5 | 91.8 | 24.2 | 1129.9 | 397.1 |
| T9 | 7.3 | 1.5 | 9.8 | 4.3 | 80.9 | 16.2 | 100.2 | 21.5 | 93.5 | 27.2 | 1031.1 | 268.3 |
| T10 | 7.2 | 1.8 | 10.2 | 4.4 | 81.7 | 12.1 | 101.5 | 21.5 | 90.7 | 28.2 | 1072.8 | 319.9 |
| T11a | 8.6 | 1.7 | 9.2 | 4.5 | 96.3 | 10.8 | 91.3a | 20.8 | 91.4 | 19 | 807.5a | 280.9 |
| T11b | 7.9 | 1.2 | 10.7 | 3.9 | 88.2 | 11.6 | 97.5 | 19.1 | 89.6 | 19 | 893.6 | 189.8 |
| T11c | 8 | 1 | 9.5 | 3.4 | 87 | 12.3 | 94.8 | 22.3 | 93.6 | 18.1 | 856.0a | 202.1 |
| T12 | 7.4 | 1.3 | 8.2 | 3.5 | 80.9 | 16 | 98.8 | 12.5 | 94.2 | 26.2 | 1008.1 | 211.9 |
CO measurements slightly increased after intrathecal anesthetic administration, peaked after delivery and oxytocin use, and gradually fell back to baseline levels by the end of surgery. To show the fluctuations in individual CO at defined time points, we depicted the CO percentage changes from baseline in Figure 2. The fluctuation range of CO was ± 25%, and the overall trend was upward. Two CO values from two different patients decreased outside the range of 25% below the baseline value, which was seen in the setting of phenylephrine use.
Phenylephrine was required in three out of the ten patients; and its intervention resulted in an increase in MAP and SVR with a decrease in HR, SV, and CO. Only two of ten patients received the second line uterotonic agent, carboprost (single dose, 250 μg). In these two patients, carboprost did not lead to significant hemodynamic fluctuations.
Hypotension related to SA is a common complication. Careful hemodynamic monitoring and management are a priority in clinical practice, to help reduce maternal and neonatal complications during the perinatal period. Compared with healthy women, patients with preeclampsia have unique pathophysiological characteristics. Understanding and anticipating perioperative hemodynamic changes related to SA in women with preeclampsia may help optimize maternal and neonatal outcomes.
The main findings of our study were: (1) Low-dose intrathecal administration of ropivacaine produced rapid induction of anesthesia and satisfactory anesthesia in patients with preeclampsia; (2) Hemodynamics remained stable throughout each operation, with the most dramatic interval being directly after the use of oxytocin; (3) CO values were above baseline throughout surgery, and were primarily impacted by changes in SV rather than changes in HR, except after the use of oxytocin; (4) Phenylephrine intervention resulted in an increase in MAP and SVR with a decrease in HR, SV, and CO; and (5) A significant amount of preoperative fluid (> 250 mL) before SA was not required in these patients.
Compared with bupivacaine, ropivacaine is less potent in inducing motor block, but our study has shown that 12 mg of ropivacaine with no additional opioid medications can provide a satisfactory anesthetic effect in CD. In a recent study, ropivacaine given in 15 mg or 20 mg dosing regimens was recommended for SA in CD[21]. This dose is slightly higher than ours, which may be due to population differences.
In this study, the most significant moment of hemodynamic fluctuation was seen after the use of oxytocin. A profound decrease in MAP and SVR was caused immediately by oxytocin, accompanied by an increase in CO and HR. Six out of the ten patients complained of dizziness, palpitations, chest tightness, or nausea after oxytocin injection. All these symptoms were temporary and subsided spontaneously without complication. Obviously, higher doses of oxytocin (20 units) have a significant impact on the cardiovascular system, and caution should be used, especially in women with preeclampsia.
As the most commonly used uterotonic agent to prevent postpartum hemorrhage, oxytocin has no established standard dose worldwide. Current clinical opinion does not support the safety of high intravenous bolus doses (10 units) of oxytocin because of deleterious cardiovascular effects[22,23]. Recent studies suggest that the effective dose of oxytocin for prophylaxis against uterine atony during CD is significantly lower than the 5-10 units we have historically used[24]. However, higher doses of oxytocin are still widely used in mainland China, among other places.
CO values were above baseline throughout the surgery, which mainly resulted from the increase in SV rather than HR according to the trends we recorded in hemodynamic parameters (Figure 1), except for the peak CO value. CO peaked soon after delivery of the fetus, likely due to a demonstrable increase in HR caused by the injection of intrauterine oxytocin after fetal delivery. SV had a slight decrease after delivery. After delivery, the increase in CVP indicated an increase in preload, and, after oxytocin use, a significant decrease in SVR indicated a decrease in afterload. However, SV was lower after delivery, rather than increased. High-dose oxytocin can inhibit myocardial contractility[25], which could be the reason for this decrease in SV after delivery in the present study.
Two similar studies were reported by Dyer et al[26] in 2008 (n = 15) and Lavie et al[27] in 2018 (n = 17), which observed CO changes in women with preeclampsia during CD under SA. Both studies adopted the noninvasive cardiac monitoring systems and different oxytocin regimens with much lower doses than ours. The trends of hemodynamic parameters in our study were generally consistent with those found in the study by Dyer et al[26]. But in Lavie’s study, a profound increase of total peripheral resistance was observed after delivery, accompanied by decreases in SV and CO. This was different from our findings. They speculated that these changes were possibly secondary to the release of mediators from the preeclamptic placenta. Their finding requires more work to further confirm and explore these potential placental mechanisms.
Both of the studies by et al[26] and Lavie et al[27] did not perform central venous catheterization. CVP was skipped in the former, so total peripheral resistance rather than SVR was adopted in their observations, while a CVP of 5 mmHg was assumed to estimate SVR in the latter. In our study, we adopted a moderately invasive cardiac monitoring system by performing central venous and radial artery catheterizations. Our study proved that CVP values were not static, and tended to increase after delivery.
Figure 2 shows the fluctuations in individual CO using percentage changes from baseline. There were two CO values coming from two different patients that were greater than 25% below the patient’s baseline value. Around this moment in these two patients, there was no change in CVP, but an increase in SVR, a decrease in HR, and a slight decrease in SV caused by a phenylephrine injection, which was most likely responsible for the significant decrease in CO. Dyer et al[28] observed the influence of phenylephrine on CO changes in such patients undergoing CD, and their study found a rapid increase in MAP and SVR with a concomitant decrease in HR, SV, and CO. Mon et al[29] explored CO changes with phenylephrine and ephedrine infusions in healthy women during SA for CD, and concluded that ephedrine was beneficial to systolic blood pressure control and CO increase. Further work is required to compare these two drugs in patients with preeclampsia and other high-risk complications.
In our institution, obstetricians empirically advocate a strict restrictive approach to fluid management in the absence of additional blood loss when women with severe preeclampsia undergo CD in order to control the incidence of pulmonary edema after delivery. In this study, our included patients’ average stay in the operating room was 96.4 ± 7.1 min, and the average infusion volume was 510 ± 137 mL. Even with our strictly restrictive approach, hemodynamics remained stable during surgery, and vasopressor intervention was not needed in seven out of ten patients, which indicated that a large amount of fluid preload before SA was not required in these patients. However, although a restrictive approach is currently recommended in these women, there remains little evidence on the ideal fluid management strategy worldwide[30].
There were some limitations in our study. A relatively high oxytocin regimen was adopted in our study, which may have caused some of the more profound hemodynamic changes seen in our study compared to others. Second, the SA in this study was induced with ropivacaine without an adjunctive opioid, which is not common international practice. However, we believe that the hemodynamic changes we saw were mainly affected by the local anesthetic, not the opioid adjunct, so this should not have a significant impact on the generalizability of our results. Third, in our institution, large fluid preloads and prophylactic vasopressor interventions were avoided, though these are common international practices. However, our findings did confirm that this strategy brought no harm to our patients’ hemodynamics. Finally, the sample size of the current study was relatively small, and we hope to expand on it in future studies.
In conclusion, this prospective, observational study of ten women with preeclampsia demonstrated that SA with low-dose ropivacaine was linked to modest hemodynamic changes, such as a moderate decrease in MAP and SVR and a mild increase in SV and CO, and appears to be safe to use in patients with preeclampsia. Cardiovascular monitoring is required after delivery of the fetus, particularly following oxytocin administration, when our study showed that the most significant hemodynamic changes during this surgery occur. Restrictive management of volume expansion in women with preeclampsia does not seem to harm hemodynamics under SA, and a large amount of fluid and prophylactic vasopressors given before SA do not appear to be needed in these patients. Further research with larger sample sizes is warranted in order to confirm these conclusions.
Preeclampsia is a common perinatal complication and is one of the major hazards of pregnancy. Cesarean section is a common way to end pregnancy in patients with preeclampsia. Spinal anesthesia can provide satisfactory anesthesia for cesarean section, but its maternal vital sign fluctuations, such as low blood pressure, often affect maternal and fetal blood supply, which can lead to bad outcomes. Therefore, better monitoring and early treatment are the focus of anesthesia management during cesarean section for preeclampsia patients.
Ropivacaine is one of the more common local anesthetics for spinal anesthesia in cesarean section. Based on current clinical practice and research, it can provide satisfactory anesthesia for cesarean section, but the specific effects of ropivacaine on the hemodynamics of patients with preeclampsia undergoing cesarean section are unknown. Understanding the effects of ropivacaine on such patients can help improve anesthesia management, reduce related complications, and increase maternal satisfaction.
The aim of this study was to record and analyze vital sign data in women with preeclampsia undergoing cesarean delivery after spinal anesthesia using ropivacaine.
We adopted a moderately invasive monitoring system to observe the changes in cardiac output, heart rate, blood pressure, central venous pressure, and heart stroke volume of patients with preeclampsia during cesarean delivery under spinal anesthesia using ropivacaine at multiple time points. These changes were then analyzed.
In women with preeclampsia who received spinal anesthesia with ropivacaine, we found several trends. Overall changes in the above indicators were mild throughout the operation, with only three out of ten patients needing medication to raise blood pressure at any time during the procedure. At the same time, ropivacaine provided satisfactory anesthesia to ensure a comfortable and smooth operation. No maternal or neonatal complications were observed during this study, except temporary episodes of low blood pressure.
This study confirms that ropivacaine can provide satisfactory anesthesia and stable blood pressure during cesarean section in patients with preeclampsia. This study is the first to describe vital sign trends in detail in preeclamptic women receiving spinal anesthesia with ropivacaine. We hope that these data can support future perioperative monitoring and interventions.
We would like to thank Xiao-Wei Liu and Shao-Wen Wu for their help and advice during subject recruitment.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report's scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang XQ S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1596] [Cited by in RCA: 1773] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 2. | Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2100] [Cited by in RCA: 2255] [Article Influence: 150.3] [Reference Citation Analysis (0)] |
| 3. | Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011;2011:214365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 4. | Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 718] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 5. | Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1757] [Cited by in RCA: 1872] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 6. | Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4232] [Cited by in RCA: 3503] [Article Influence: 318.5] [Reference Citation Analysis (0)] |
| 7. | Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 509] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Li W, Xiao J, Chen S. The complication and mode of delivery in Chinese women with severe preeclampsia: a retrospective study. Hypertens Pregnancy. 2014;33:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Pacher J, Brix E, Lehner R. The mode of delivery in patients with preeclampsia at term subject to elective or emergency Cesarean section. Arch Gynecol Obstet. 2014;289:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Ulubaşoğlu H, Bakay K, Güven D. Relation with postpartum maternal morbidity of different types of anesthesia in preeclamptic patients. Hypertens Pregnancy. 2018;37:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Henke VG, Bateman BT, Leffert LR. Focused review: spinal anesthesia in severe preeclampsia. Anesth Analg. 2013;117:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Turner JA. Severe preeclampsia: anesthetic implications of the disease and its management. Am J Ther. 2009;16:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Aya AG, Mangin R, Vialles N, Ferrer JM, Robert C, Ripart J, de La Coussaye JE. Patients with severe preeclampsia experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients: a prospective cohort comparison. Anesth Analg. 2003;97:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Aya AG, Vialles N, Tanoubi I, Mangin R, Ferrer JM, Robert C, Ripart J, de La Coussaye JE. Spinal anesthesia-induced hypotension: a risk comparison between patients with severe preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth Analg. 2005;101:869-875, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Clark VA, Sharwood-Smith GH, Stewart AV. Ephedrine requirements are reduced during spinal anaesthesia for caesarean section in preeclampsia. Int J Obstet Anesth. 2005;14:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Robson SC, Boys RJ, Rodeck C, Morgan B. Maternal and fetal haemodynamic effects of spinal and extradural anaesthesia for elective caesarean section. Br J Anaesth. 1992;68:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1226] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Connors AF, Speroff T, Dawson NV, Thomas C, Harrell FE, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus WA. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 438] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 19. | Sibai BM. Hypertension. Philadelphia: Saunders, 2012: 779-824. |
| 20. | Pregnancy Hypertension Disease Group. Guidelines for the diagnosis and treatment of hypertensive disorders in pregnancy (2015). Zhonghua Fuchanke Zazhi. 2015;50:721-728. |
| 21. | Ateser RY, Kayacan N. Intrathecal ropivacaine in cesarean delivery. Niger J Clin Pract. 2017;20:1322-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Roach MK, Abramovici A, Tita AT. Dose and duration of oxytocin to prevent postpartum hemorrhage: a review. Am J Perinatol. 2013;30:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Yamaguchi ET, Siaulys MM, Torres ML. Oxytocin in cesarean-sections. What's new? Braz J Anesthesiol. 2016;66:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Dyer RA, Butwick AJ, Carvalho B. Oxytocin for labour and caesarean delivery: implications for the anaesthesiologist. Curr Opin Anaesthesiol. 2011;24:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Gutkowska J, Jankowski M, Antunes-Rodrigues J. The role of oxytocin in cardiovascular regulation. Braz J Med Biol Res. 2014;47:206-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Dyer RA, Piercy JL, Reed AR, Lombard CJ, Schoeman LK, James MF. Hemodynamic changes associated with spinal anesthesia for cesarean delivery in severe preeclampsia. Anesthesiology. 2008;108:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Lavie A, Ram M, Lev S, Blecher Y, Amikam U, Shulman Y, Avnon T, Weiner E, Many A. Maternal cardiovascular hemodynamics in normotensive versus preeclamptic pregnancies: a prospective longitudinal study using a noninvasive cardiac system (NICaS™). BMC Pregnancy Childbirth. 2018;18:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Dyer RA, Daniels A, Vorster A, Emmanuel A, Arcache MJ, Schulein S, Reed AR, Lombard CJ, James MF, van Dyk D. Maternal cardiac output response to colloid preload and vasopressor therapy during spinal anaesthesia for caesarean section in patients with severe pre-eclampsia: a randomised, controlled trial. Anaesthesia. 2018;73:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Mon W, Stewart A, Fernando R, Ashpole K, El-Wahab N, MacDonald S, Tamilselvan P, Columb M, Liu YM. Cardiac output changes with phenylephrine and ephedrine infusions during spinal anesthesia for cesarean section: A randomized, double-blind trial. J Clin Anesth. 2017;37:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Pretorius T, van Rensburg G, Dyer RA, Biccard BM. The influence of fluid management on outcomes in preeclampsia: a systematic review and meta-analysis. Int J Obstet Anesth. 2018;34:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |