Published online Apr 26, 2020. doi: 10.12998/wjcc.v8.i8.1343
Peer-review started: March 4, 2020
First decision: March 27, 2020
Revised: March 30, 2020
Accepted: April 4, 2020
Article in press: April 4, 2020
Published online: April 26, 2020
Processing time: 51 Days and 3.2 Hours
The pneumonia caused by the coronavirus disease-2019 (COVID-19) outbreak in Wuhan, China constitutes a public health emergency of international concern. The gastrointestinal symptoms of vomiting, diarrhea and abdominal pain and the detection of COVID-19 nucleic acid from fecal specimens in a small number of patients suggest the possibility of transmission via the gastrointestinal tract. People of all ages are vulnerable to this virus, including children. Digestive endoscopy is an invasive procedure during which children cannot wear masks; therefore, they have higher risks of exposure to COVID-19, and the digestive endoscopy center is a relatively high-risk area for COVID-19 infection. Based on these factors and in combination with related policies and regulations, a prevention and control program for the COVID-19 pneumonia in a children's digestive endoscopy center was established to prevent the COVID-19 nosocomial infection.
Core tip: We established a prevention and control program for the novel coronavirus infection in a children’s digestive endoscopy center to prevent nosocomial infection. The program is based on the characteristics of pediatric patients, the severity of the epidemic, and a relatively high risk of infection due to invasive procedures, according to the related policies and regulations. The program is expected to be useful for pediatric digestive endoscopists as a guideline.
- Citation: Ma XP, Wang H, Bai DM, Zou Y, Zhou SM, Wen FQ, Dai DL. Prevention program for the COVID-19 in a children’s digestive endoscopy center. World J Clin Cases 2020; 8(8): 1343-1349
- URL: https://www.wjgnet.com/2307-8960/full/v8/i8/1343.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i8.1343
In mid-December 2019, a cluster of pneumonia associated with the coronavirus disease-2019 (COVID-19) emerged in Wuhan, China, and rapidly spread to other areas globally[1]. The World Health Organization declared the pneumonia outbreak caused by the novel coronavirus a public health emergency of international concern[2]. The confirmed patients are the main source of infection; however, patients in the latent period and asymptomatic patients can be potential sources of infection[1]. People are generally vulnerable, including children[1,3-6]. Most patients have symptoms of fever, cough, and myalgia or fatigue while a small number of patients have symptoms of vomiting, diarrhea and abdominal pain[1,6-8]. A minority of patients seek medical care with gastrointestinal symptoms as the first manifestation[5]. The detection of COVID-19 nucleic acid from fecal specimens suggests the possibility of transmission via the gastrointestinal tract[1,5,9], although this possibility remains to be further clarified. Digestive endoscopy is an invasive procedure during which children cannot wear masks; therefore, they have higher risks of exposure to COVID-19, and the digestive endoscopy center is a relatively high-risk area for COVID-19 infection. Based on the above factors and in combination with related policies, regulations and norms[1,10], a prevention and control program for the COVID-19 pneumonia in a children’s digestive endoscopy center was established to prevent the COVID-19 nosocomial infection and provide guidelines for endoscopists[11-17]. After being approved by the director of Shenzhen Children's Hospital, this program was initiated from February 1, 2020. It was also put into practice in Shenzhen Baoan Maternal and Child Health Hospital as suggested by the Pediatric Committee of Shenzhen Medical Association. The case detection rate and the nosocomial infection rate were used to evaluate the effectiveness of the program.
During the COVID-19 epidemic, it is recommended that ordinary gastroscopy, colonoscopy, and enteroscopy should be suspended; only endoscopy for emergency diagnosis and treatment is kept in normal operation and is required to proceed as follows: (1) The clinical physician should determine whether the child needs emergency endoscopic diagnosis or treatment. If it is not urgent or the child’s condition is stable, it is recommended to make an appointment after the epidemic. When emergency endoscopy is necessary, the physician should rigorously evaluate the child's condition according to the indications for emergency endoscopy and informed the parents of the possible risks. (2) Indications for selective, confined and emergency endoscopy are defined as follows: (A) Indications for selective endoscopy: common gastrointestinal symptoms such as recurrent abdominal pain, vomiting, abdominal distension and diarrhea; a small amount of hematemesis and hematochezia. The illness is less severe, and the patient's condition is good; (B) Indications for confined endoscopy: foreign body in digestive tract (blunt, noncorrosive, nonobstructive); mild gastrointestinal tract bleeding; acute abdominal pain (suspected Henoch-Schonlein purpura); upper gastrointestinal obstruction; endoscopic feeding tube placement; dilatation of esophageal stenosis; chronic diarrhea (suspected inflammatory bowel disease); chronic pain that is unrelieved after routine treatment; frequent vomiting with unknown causes; (C) Indications for emergency endoscopy: Button buttery, magnets, sharp or toxic foreign bodies in the digestive tract; gastrointestinal tract obstruction caused by foreign bodies; endoscopic diagnosis and treatment for massive gastrointestinal bleeding; and (D) For special cases, endoscopists’ consultation is needed to determine whether digestive endoscopy is necessary. (3) The epidemiological history of the children and their family members is screened: (A) A history of travel or residence in Wuhan or surrounding cities, or in other communities where confirmed cases were reported within 14 d; (B) A history of contact with patients with fever or respiratory symptoms who are from Wuhan or surrounding cities, or from other communities where confirmed cases were reported within 14 d; (C) Cluster onset of disease; and (D) A history of contact with diagnosed patients with COVID-19 infection, which refers to positive detection of COVID-19 nucleic acid. In the case of any of the above epidemiological history and any two of the following clinical manifestations, the screening procedure should be carried out as suspected casesbased on fever, imaging findings of pneumonia, and normal or reduced white blood cell count or reduced lymphocyte count[1]. (4) Emergency endoscopy will be arranged if the patient has a history of the above epidemiology but no fever, no imaging manifestation, or no leukocyte decline or lymphocyte decline. (5) On the day of examination, the staff should take the temperature of all children and their family members who enter the digestive endoscopy center. If the children's temperature exceeds 37.3 °C, they should be immediately sent to the fever clinic for further assessment; if their family member’s temperature exceeds 37.3 °C, they should be referred to a designated fever clinic for consultation. If the child's temperature is normal, endoscopy will be arranged directly. (6) Children and their family members are required to wear masks before they arrive at the endoscopy center. After confirming that the children and their family members have no fever, epidemiological exposure or any symptoms, the staff of the front desk should ask the parents to sign the informed consent form. (7) The endoscopists should carefully review the documents, including informed consent, chest computed tomographic findings and routine blood results, before beginning endoscopy. (8) Anesthesiologists should strictly implement disposable oxygen delivery catheters and oxygen masks and closely monitor vital signs during anesthesia to avoid choking. Quarantine measures should be taken to avoid cross-infection in the anesthesia recovery room. And (9) The transport and disinfection protection of children should be supervised by professional nurses.
During the epidemic of COVID-19 pneumonia, medical personnel should strictly implement standard precautions during endoscopy[11,18-20] and enforce preventive measures against the transmission of COVID-19 pneumonia to ensure the safety of personnel in digestive endoscopy centers. We suggest the following rules:
All staff in the center, including doctors, nurses, anesthesiologists, training staff, technicians, receptionists and health care workers, should report the following on a daily basis: (1) Any symptoms of fever, fatigue and cough; and (2) History of contact with a diagnosed or suspected COVID-19 pneumonia patient. In the case of any of the above, patients should be immediately sent to the relevant department for investigation or home isolation.
If any staff member in the center experiences unprotected contact with a child suspected to have COVID-19, medical isolation will be required. This staff member can return to work after the child is excluded for infection; if any staff member in the center happens to have unprotected contact with a child diagnosed with COVID-19 infection, the medical isolation period will be at least 14 d.
All staff members should take their temperature before and after work every day. In case of any abnormality, they should stop work immediately and receive medical interventions or isolation when necessary.
All staff should wash their hands in accordance with the “six-step washing method” for 2 min or disinfect hands using quick-drying hand sanitizer during the working period.
(1) Staff at the front desk and in charge of equipment cleaning and disinfection should adhere to universal protection principles; (2) Medical workers who have direct contact with children should take first-level protective measures. They must wear disposable gowns, N95 masks, goggles/face shields, medical protective caps and shoe covers. Companion is prohibited from entering the operating room during endoscopy without wearing protective gear; (3) During tracheal intubation, airway care and sputum suction with the possibility of droplets or spray, the staff should wear N95 masks, protective face shields, latex gloves, impermeable isolation clothing, protective clothing and respiratory protective hoods if necessary and replace them quickly in case of contamination; (4) After endoscopy, endoscopists should assign other doctors or training staff to help with the report to avoid contamination of the reporting area and repeatedly taking off and putting on isolation clothes; and (5) The staff will take off the isolation gowns after finishing the relevant work, followed by hand hygiene, and change the mask before entering the rest area to avoid cross infection.
During non-working days, the staff should stay at home and avoid gathering with others for meals to reduce the possibility of epidemic transmission.
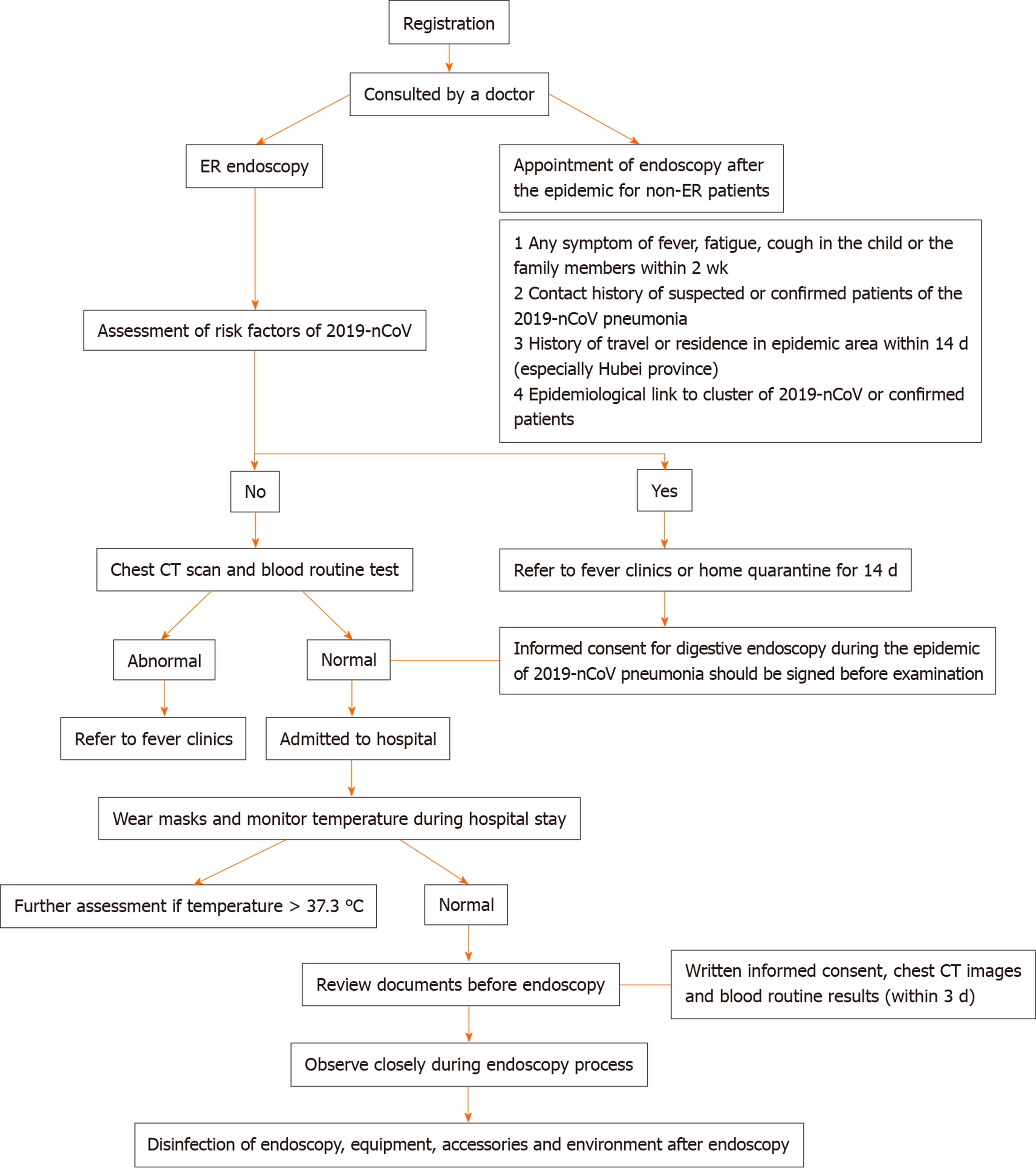
The channel shunt system is implemented in children's digestive endoscopy centers, that is, the medical staff go through the medical channel, and the children and their accompanying family members go through the patient channel. (1) When children enter the center, they must follow the arrangement of the staff, voluntarily cooperate with the temperature measurement, and minimize the number of accompanying family members. The nurses should disinfect the children's hands with quick-drying hand disinfectant and give the children an age-appropriate protective mask before entering the operating area. After endoscopy, the children also need to wear masks. Accompanying family members should take the same disinfection precautions as well. Children, family members and other accompanying persons are prohibited from entering the operating area of the center without disinfection and protective equipment. (2) The children and their families should provide their personal information truthfully, including any symptoms of fever, fatigue and cough, the result of total white blood cell count and lymphocyte count, the epidemiological history of novel coronavirus pneumonia, and radiographic imaging findings of the lungs. (3) Some screening measures, such as chest computed tomography and routine blood tests, are necessary. Family members should cooperate with the staff. Endoscopy should not be performed if required results are not available. and (4) The children will be hospitalized according to the workflow if necessary (Figure 1).
Common children without epidemiological history or related symptoms: (1) At the key stage of epidemic prevention and control, endoscopy should be performed in an isolated room. Endoscopists should be protected according to the first-level protection requirements. Endoscopy should not be performed without protection; and (2) The number of children waiting in a general waiting area should be minimized. The distance between each waiting child should be more than 1 meter apart. The children and their accompanying family members are instructed to wear masks correctly.
Children excluded from suspected novel coronavirus infection, in convalescence (released from quarantine for negative virus nucleic acid tests 2 or more times), or in full recovery from COVID-19 infection: (1) The indications for digestive endoscopy must be strictly followed; (2) Endoscopy should be performed in an isolated room. Medical personnel should be protected according to the second-level protection requirements; and (3) It is recommended to perform digestive endoscopy with the child under sober sedation or intravenous anesthesia.
(1) The indications for digestive endoscopy should be followed strictly, and the purpose and urgency of endoscopy should be clearly defined. Endoscopy is not recommended if it is not an emergency or not for life-saving purposes; (2) Endoscopy should not be performed for children with suspected or confirmed COVID-19 infection in the digestive endoscopy center; and (3) If necessary, endoscopy should be performed in a standard isolation ward for children suspected to have or diagnosed with COVID-19 infection. Second-level protection measures should be taken during consultation, and third-level protection measures should be taken during endoscopy. Protective equipment should be in a mode of “single person for single use” and disposable, and rooms and equipment should be disinfected in a timely manner according to the requirements of infectious diseases.
After endoscopy or contact with children’s saliva, gastric juice or other secretions, hands should be washed with flowing water or sanitized with fast-drying hand disinfectant immediately, and hand hygiene should be strictly implemented in accordance with hand hygiene requirements of the hand hygiene code for medical personnel (WS/T313.2019)[19] during daily work. Wearing gloves is not a substitute for hand hygiene. Hand hygiene should be implemented after removing gloves, and not to touch public goods with gloves on.
(1) Sterilization of all endoscopes and accessories should be conducted strictly in accordance with the technical specifications for disinfection in medical institutions[12] and the technical specifications for cleaning and disinfection of soft endoscopes (WS 507-2016)[13]; (2) When there is no stain on the surface of the endoscope equipment (processor, endoscope transfer vehicle, treatment vehicle, resection knife, monitor, anesthesia machine, etc.), it should be wiped with 1000 mg/L chlorine-containing disinfectant after endoscopy and rinsed with water 30 min later[11]; (3) If contaminated by blood, body fluids, secretions and other pollutants from patients with suspected or confirmed COVID-19 infection, all medical instruments, objects, processor surface and ground should be disinfected using 2000-5000 mg/L chlorine-containing disinfectant and then rinsed with water 30 min later; and (4) Staff who are responsible for equipment cleaning and disinfection should wear disposable medical caps, surgical masks, medical isolating eye shields or face shields, medical disposable gowns, and double-layer disposable rubber gloves to cover the sleeves of the gowns.
Strict terminal disinfection must be implemented after endoscopy. The examining bed should be wiped with 500 mg/L chlorine-containing disinfectant, gloves should be disposed of after removing the contaminated bedspread, and then, hands should be washed in accordance with the “six- step washing method”.
(1) Air disinfection: Air disinfection should be performed twice a day for 2 h each time in accordance with the requirements of “Code for management of hospital air purification” if using the air disinfection machine[14]. It should be more than 30 min each time if using ultraviolet disinfection. Windows should be opened twice a day for more than 30 min each time to ensure air circulation in the operating room of the center. (2) Cleaning and disinfection of object surfaces: The processor table, instrument vehicles, equipment and other objects, as well as resuscitation areas, the front desk, operating areas, cleaning areas, and office areas should be disinfected at least twice a day according to conventional disinfection methods, that is, wiping with chlorine-containing disinfectant with effective chlorine of 1000 mg/L, then wiping with water 30 min later. If stained by children's saliva, body fluids, blood and other pollutants, the surfaces should be disinfected with 2000 mg/L chlorine-containing disinfectant directly. (3) The waiting area should be disinfected regularly as required. (4) Floor disinfection: The floor should be mopped with 500 mg/L chlorine-containing disinfectant and then wiped with water 30 min later; this process should be repeated at least twice a day. and (5) Disinfection of cleaning tools: Items should be soaked in 500 mg/L chlorine-containing disinfectant for disinfection. Rags and dishcloths should be sealed in water-soluble fabric bags and sent to the cleaning and disinfection center for further disinfection.
Medical waste generated during digestive endoscopy, such as disposable biopsy forceps and gastric juice collectors, should be disposed of and managed in accordance with the Regulations on the Management of Medical Waste[15] and the Regulations on the Management of Medical Waste in Medical and Health Institutions[16]. Wastes from children with suspected or confirmed novel coronavirus pneumonia should be placed in double-layer yellow medical waste bags, tied up, labeled separately, and treated according to the Regulations on Medical Waste Management during the novel coronavirus infection epidemic[17].
Medical staff in the center should have their work shift reasonably arranged to avoid overwork; and they should pay attention to their health condition and monitor their temperature and respiratory symptoms every day.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Pediatric Committee of Shenzhen Medical Association.
Specialty type: Medicine, Research and Experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aydin M, Wang R S-Editor: Dou Y L-Editor: MedE- Ma JY E-Editor: Liu JH
| 1. | National Health Commission of the People's Republic of China. Diagnosis and treatment project for pneumonia caused by novel coronavirus infection. Trial version 5. 2020; Available from: http://www.gov.cn/zhengce/zhengceku/2020-02/05/5474791/files/de44557832ad4be1929091dcbcfca891.pdf. |
| 2. | World Health Organization. New coronavirus an international public health emergency, WHO declares. 2020;. |
| 3. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17646] [Article Influence: 3529.2] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. 2020; Available from: https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117. |
| 5. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9319] [Article Influence: 1863.8] [Reference Citation Analysis (0)] |
| 6. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30116] [Article Influence: 6023.2] [Reference Citation Analysis (3)] |
| 7. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 8. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12976] [Article Influence: 2595.2] [Reference Citation Analysis (1)] |
| 9. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK, Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4155] [Cited by in RCA: 3822] [Article Influence: 764.4] [Reference Citation Analysis (1)] |
| 10. | National Health Commission Medical Administration Bureau. Management standards for clinical application of pediatric digestive endoscopy. 2019; Available from: http://www.nhc.gov.cn/yzygj/s3585/201912/994f74193202417e957adbc1fc601fb5/files/23d5f5367810472baae250ec91895ead.pdf. |
| 11. | National Health Commission Medical Administration Bureau. Technical guidelines for novel coronavirus infection prevention and control in medical institutions (First Edition). 2020; Available from: http://www.nhc.gov.cn/yzygj/s7659/202001/b91fdab7c304431eb082d67847d27e14.shtml. |
| 12. | Ministry of Health of the People's Republic of China. Technical code for disinfection in medical institutions. Notice (2012)6. 2012; Available from: http://www.nhc.gov.cn/wjw/s9496/201204/54510.shtml. |
| 13. | National Health and Family Planning Commission of the People's Republic of China. Technical code for cleaning and disinfection of flexible endoscopes (WS 507-2016). 2016; Available from: http://www.nhc.gov.cn/wjw/s9496/201701/491ec38efc884531801549cfb90d865d.shtml. |
| 14. | Ministry of health of the people's Republic of China. Code for management of hospital air purification. 2012; Available from: http://www.nhc.gov.cn/wjw/s9496/201204/54511.shtml. |
| 15. | Order of the State Council of the People's Republic of China (No. 380). Regulations on the management of medical waste. 2003; Available from: http://www.nhc.gov.cn/wjw/flfg/200804/31d39591e46447cab6fa9e3884c9aa26.shtml. |
| 16. | Order of the Ministry of Health of the People's Republic of China (No. 36). Regulations on the management of medical waste in medical and health institutions. 2003; Available from: http://www.nhc.gov.cn/wjw/bmgz/200804/133efb6d99cd47d4ac6765a16874161c.shtml. |
| 17. | Department of General Administration of National Health Committee. Regulations on medical waste management during novel coronavirus infection epidemic (National Health Office medical letter [2020]81). 2020; Available from: http://www.nhc.gov.cn/yzygj/s7659/202001/6b7bc23a44624ab2846b127d146be758.shtml. |
| 18. | Ministry of Health of the People's Republic of China. Technical code for hospital isolation (WS/T311.2009). 2009; Available from: http://www.nhc.gov.cn/cmsresources/mohyzs/cmsrsdocument/doc5841.pdf. |
| 19. | Ministry of health of the people's Republic of China. Code for hand hygiene of medical staff (WS/T313.2019). 2019; Available from: http://www.nhc.gov.cn/wjw/s9496/202002/dbd143c44abd4de8b59a235feef7d75e/files/6a3e2bf3d82b4ee8a718dbfc3cde8338.pdf. |
| 20. | National Health Commission. Guidelines for the scope of use of common medical protective products in novel coronavirus infection prevention and control (Trial). 2020; Available from: http://www.nhc.gov.cn/yzygj/s7659/202001/e71c5de925a64eafbe1ce790debab5c6.shtml. |