Published online Mar 26, 2020. doi: 10.12998/wjcc.v8.i6.1164
Peer-review started: December 23, 2019
First decision: February 20, 2020
Revised: March 9, 2020
Accepted: March 14, 2020
Article in press: March 14, 2020
Published online: March 26, 2020
Processing time: 93 Days and 11.6 Hours
The aim of the present study was to examine the clinical characteristics of hepatoid adenocarcinoma of the stomach (HAS) and its diagnosis, treatment, and prognosis.
A retrospective analysis of 13 HAS cases was performed. The mean age of the 13 patients was 66.08 years, and 10 of the 13 patients were male. Prior to treatment, the alpha-fetoprotein levels in the serum were elevated in 7 patients, the tumour was located in the distal or gastric body in 11 patients, and the gastroscopy pathological results showed that 3 patients had poorly differentiated tumours and that 8 patients had moderately/poorly differentiated tumours. Abdominal CT scans showed local stomach wall thickening, and enlarged lymph nodes were visible around the stomach in 8 patients. Of the 13 patients, 11 underwent radical surgery. The clinical pathological staging was as follows: Stage II in 2 cases; stage III in 8 cases; and stage IV in 1 case. A total of 3 patients were lost to follow-up. Otherwise, as of the last follow-up, 3 patients had survived for 56 mo, and the other 7 patients failed to achieve long-term survival (survival period of 1-56 mo).
HAS is a special type of gastric cancer, and the prognosis of HAS has improved compared with past prognoses. Measurement of alpha-fetoprotein, early diagnosis, active surgical treatment, and application of new diagnostic and treatment techniques are conducive to improving the prognosis of HAS.
Core tip: Hepatoid adenocarcinoma of the stomach (HAS) is a rare special type of gastric cancer that is prone to lymph node and liver metastases and has a poor prognosis. In the majority of HAS cases, the ability to produce the alpha-fetoprotein and the serum levels of alpha-fetoprotein may be elevated during the early stages. In this study, we evaluated the clinical data, pathological features, treatment results, and prognosis of HAS patients.
- Citation: Zhang ZR, Wu J, Li HW, Wang T. Hepatoid adenocarcinoma of the stomach: Thirteen case reports and review of literature. World J Clin Cases 2020; 8(6): 1164-1171
- URL: https://www.wjgnet.com/2307-8960/full/v8/i6/1164.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i6.1164
In clinical practice, alpha-fetoprotein (AFP) is a useful tumour marker for screening or monitoring hepatocellular carcinoma or yolk sac tumours. However, other human tumours can also produce AFP. The complexity of the disease makes the differential diagnosis challenging, especially when the primary tumour is unknown and a hepatic mass is the only initial finding. Currently, it is easy for these tumours to be misdiagnosed as primary liver cancer because of the elevated AFP levels. Hepatoid adenocarcinoma (HAC), a typical histologic subtype of hepatocellular carcinoma, has been described in various organs, such as the stomach, ovary, renal pelvis, papilla of Vater, lung, and pancreas. The stomach is one of the organs in which HAC has been most frequently identified. HAC that originates in the stomach is termed hepatoid adenocarcinoma of the stomach (HAS)[1].
At present, the underlying mechanisms regarding the development and progression of HAS are unknown. HAS is frequently characterized by the presence of both adenocarcinoma and hepatocellular carcinoid differentiation at the same time. The majority of patients present with elevated serum AFP levels. Compared with gastric cancer without components of HAC, HAS has a higher degree of malignancy and a poorer prognosis.
HAS is rare in clinical practice. Although various cases have been reported in recent years, there is still a lack of systematic research regarding HAS. HAS is a relatively rare type of HAC. Previous case reports or case analyses focused on the diagnostic or pathological features rather than treatment outcomes. In this study, we evaluated the clinical data, pathological features, treatment results, and prognosis of gastric cancer patients. The present study analysed the data of 13 patients diagnosed with HAS who underwent surgery at The First Affiliated Hospital of Wannan Medical College between January 2014 and December 2016 to improve our understanding of HAS.
Patients with pathologically diagnosed HAS were enrolled at The First Affiliated Hospital of Wannan Medical College between January 2014 and December 2016 and were retrospectively analysed. Among 2343 patients with gastric cancer, 13 (0.55%) were diagnosed with HAS. Among them, 10 were male, and 3 were female. The patients were aged 55-85 years, with an average age of 66.08 years. The tumour stage at diagnosis was stage I in 1 patient, stage II in 3 patients, and stage III in 9 patients.
After admission, a detailed medical history of all patients was collected, a physical examination, gastroscopy examination of the abdomen, and coaxial tomography (CT) were performed, and the AFP levels and other tumour markers were measured. The surgical method (radical or palliative surgery) was selected according to the extent of tumour invasion, and routine pathological and immunohistochemical examinations were performed on the surgical specimens. The majority of the patients received postoperative chemotherapy, regular CT examinations, tumour marker level detection, and long-term follow-up.
Of the 13 patients, 10 had symptoms such as abdominal discomfort and postprandial aggravation, 2 complained of haematemesis and black stool, and 1 was admitted to the hospital due to elevated levels of AFP detected during a physical examination.
None of the 13 patients had received relevant treatment and were treated for the first time at our hospital.
Additionally, 1 patient presented as positive for hepatitis B surface antigen, 1 patient had a history of treatment for gastric ulcers 8 years ago, 1 patient had a history of type 2 diabetes, and 3 patients had a history of hypertension.
Three of these patients had hypertension, two of them had simultaneous cerebral infarction, and two of these patients had diabetes. None of the patients had any other family members with similar diseases.
None of the patients had typical symptoms on physical examination, 6 patients had mild upper abdominal tenderness, and 5 patients had anaemia manifestations of different severities. The other tests showed no obvious abnormal manifestations.
The 13 patients had anaemia of varying degrees. The serum AFP levels in 7 patients were elevated. The carcinogenic embryonic antigen levels of 7 patients were elevated, and carbohydrate antigen 199 levels were increased in 3 patients. There were no cases in which CA-125 and other tumour marker levels were elevated (Table 1).
| Serial number | Sex | Age | Preoperative AFP (0.89-8.78 ng/mL) | Preoperative CEA (0-5 ng/mL) |
| 1 | Male | 68 | 186.31 | |
| 2 | Male | 63 | 43.19 | 51.28 |
| 3 | Male | 58 | 3.25 | 1.08 |
| 4 | Male | 66 | > 700 | 2.56 |
| 5 | Male | 59 | 5.93 | 3.02 |
| 6 | Female | 55 | 1.41 | 2.54 |
| 7 | Male | 60 | 779.91 | 3.65 |
| 8 | Female | 85 | 1085.75 | 26.73 |
| 9 | Male | 70 | 1.79 | 2.45 |
| 10 | Male | 74 | 41.34 | |
| 11 | Male | 71 | 39.87 | 21.47 |
| 12 | Female | 66 | 8.8 | 262.67 |
| 13 | Male | 64 | 508.69 | 12.81 |
Abdominal CT revealed abnormal thickening of the gastric wall in 10 patients, and the examination revealed enlarged lymph nodes around the stomachs of 8 patients. None of the patients had liver metastases before surgery (Table 2).
| Serial number | Tumour location | Gastric wall thickening | Enlarged lymph nodes | Liver metastases |
| 1 | Gastric sinus | Yes | No | No |
| 2 | Cardia | Yes | Yes | No |
| 3 | Gastric body | Yes | Yes | No |
| 4 | Gastric body | No | No | No |
| 5 | Gastric sinus | Yes | Yes | No |
| 6 | Gastric sinus | Yes | No | No |
| 7 | Gastric sinus | Yes | Yes | No |
| 8 | Gastric sinus | No | No | No |
| 9 | Gastric sinus | Yes | Yes | No |
| 10 | Gastric sinus | Yes | Yes | No |
| 11 | Gastric sinus | Yes | Yes | No |
| 12 | Gastric body | Yes | Yes | No |
| 13 | Cardia | Yes | No | No |
The diagnostic criteria for HAS were based on the pathological and morphological characteristics. The diagnosis of HAS was defined as the pathological detection of gastric cancer containing hepatoid differentiation, regardless of whether there were elevated levels of serum AFP. All 13 patients were diagnosed with HAS.
A total of 13 patients were hospitalized and underwent surgical treatment, among whom 11 underwent D2 radical gastrectomy. One patient was found to have colon cancer during surgery, and thus, a radical resection was performed at the same time. One patient was found to have peritoneal metastasis during surgery and thus underwent palliative surgery. Cerebral haemorrhage occurred in 1 patient on the first day after surgery. No other complications occurred in the other patients during the perioperative period.
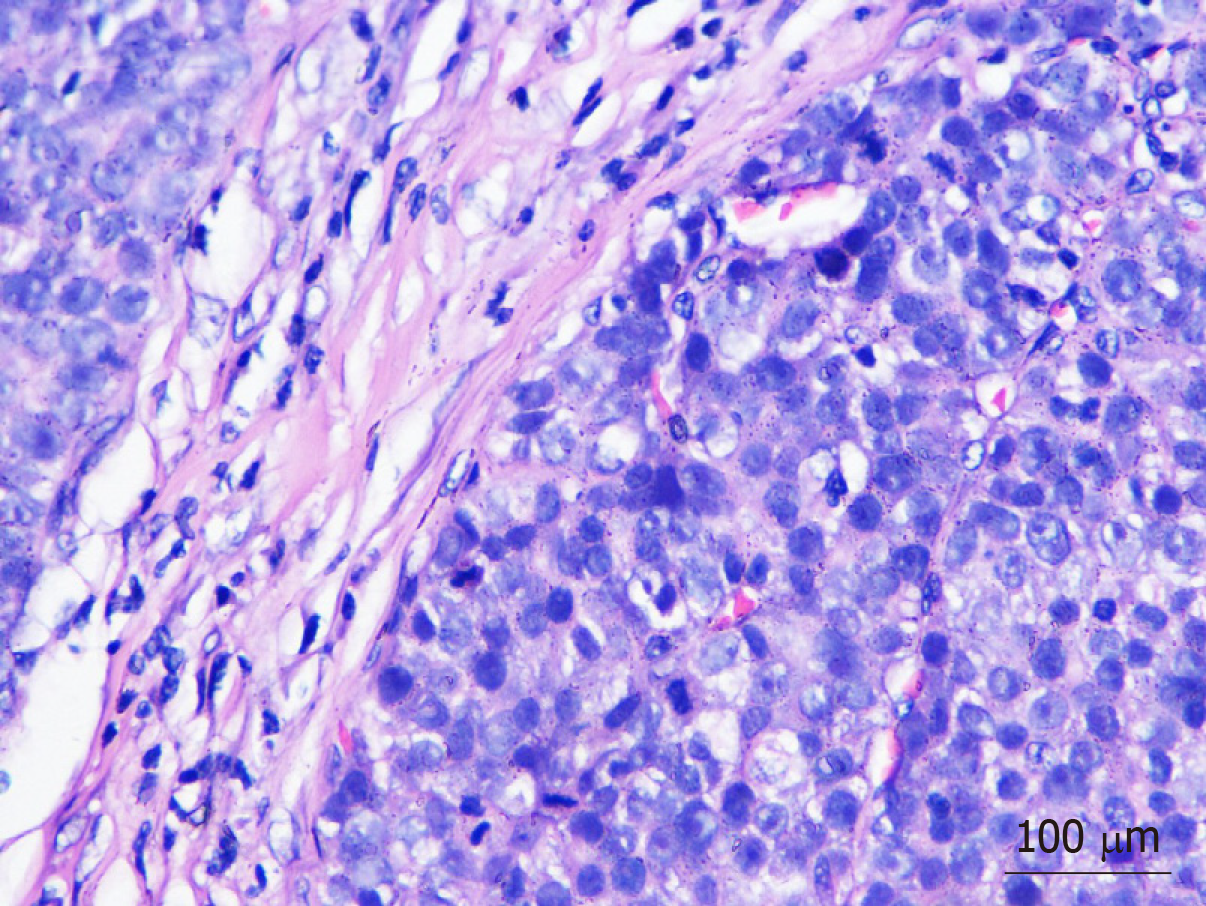
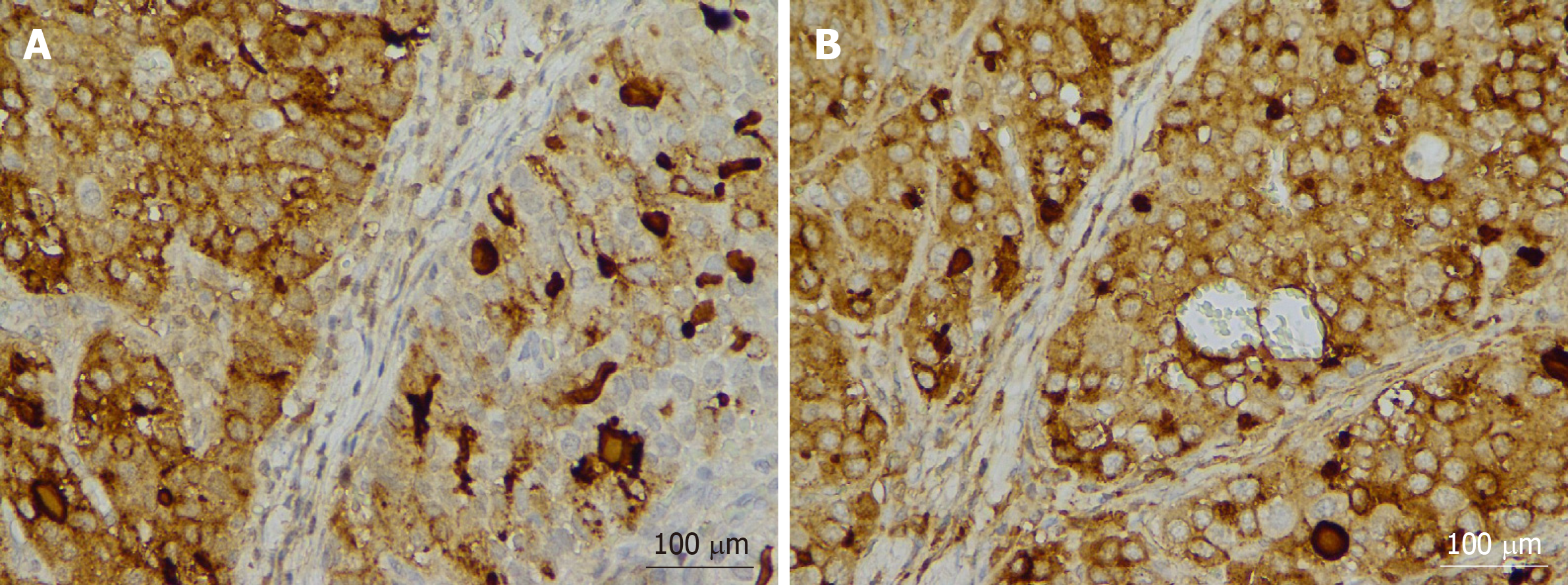
Postoperative pathology revealed lymph node metastasis in 10 patients. According to the Tumour-Node-Metastasis staging system for gastric cancer (8th edition) published by the American Joint Committee on Cancer, the postoperative clinicopathological staging of the 13 patients was as follows: 1 patient as stage I; 3 patients as stage II; 8 patients as stage III; and 1 patient as stage IV. All patients were pathologically confirmed to have HAS that was detected by immunohistochemistry (IHC) (Figures 1 and 2). Postoperatively, 10 patients underwent different courses of chemotherapy based on fluorouracil.
After surgery and chemotherapy, 3 patients were lost to follow-up, and the other 10 patients underwent follow-up until January 2019, including regular physical examination, tumour index examination, and imaging examination. By the end of the last follow-up, 1 patient had died of postoperative cerebral haemorrhage, and 4 patients died due to tumour recurrence. Three patients survived, although they showed evidence of disease progression (Table 3).
| Serial number | TNM | Clinicopathologic stage | Lymph node | AFP | Hepatocyte | Adjuvant chemotherapy | Follow-up | Progression | Progression-free survival (mo) |
| 1 | T4aN3aM0 | IIIB | (8/13) | + | - | Yes | Survival | No | 56 |
| 2 | T4aN2M0 | IIIA | (5/15) | + | + | Yes | Death | Yes | 28 |
| 3 | T2N0M0 | IB | (0/35) | - | + | Yes | Survival | No | 56 |
| 4 | T4N0M0 | IIB | (0/14) | + | Unknown | Yes | Death | Yes | 27 |
| 5 | T4N1M0 | IIIB | (6/22) | + | + | Yes | Lost | No | Unknown |
| 6 | T4N0M0 | IIB | (0/18) | - | + | Yes | Survival | Yes | 56 |
| 7 | T4N3bM1 | IV | (18/27) | + | + | Yes | Death | Yes | 32 |
| 8 | T4aN3aM0 | IIIB | (7/18) | + | Unknown | No | Death | Yes | 6 |
| 9 | T4N3bM0 | IIIC | (15/18) | - | + | Yes | Survival | Yes | 23 |
| 10 | T4bN2M0 | IIIB | (7/23) | + | + | No | Death | Yes | 1 |
| 11 | T4bN1M0 | IIIB | (3/17) | - | Unknown | Yes | Lost | Unknown | Unknown |
| 12 | T3N1M0 | IIB | (3/21) | + | Unknown | No | Lost | Unknown | Unknown |
| 13 | T3N3bM0 | IIIC | (16/19) | + | + | Yes | Survival | Yes | 11 |
Ishikura et al[2] reported a gastric adenocarcinoma that produced AFP and termed it HAC. HAC is considered relatively rare worldwide. Certain cases of HAC originate in the colon, oesophagus, lungs, urinary reproductive system, or other parts of the body; however, HAS accounts for 83.9% of the total number of HAC cases. The antrum of the stomach is the most common site of HAS[3]. HAS accounts for a very low proportion of malignant gastric tumours and 0.3%-1% of all cases of gastric adenocarcinoma[4,5], which is in agreement with the rate of occurrence detected in the present study (0.5%).
The development of gastric cancer rarely originates directly from the normal gastric mucosal epithelium, instead typically undergoing a rather long evolutionary process before being considered cancerous. Dysplasia of the gastric mucosa and intestinal metaplasia are considered precancerous lesions of gastric cancer.
The development of HAS is not completely understood. A possible explanation is based on the fact that the stomach and liver originate from the endoderm and from the primitive foregut during embryonic development. Therefore, these two organs are closely related in histology and embryology. In the process of tumour development, some primary gastric cancer cells are abnormally differentiated into hepatocytes, which develop into HAS and produce AFP[6], thus fully reflecting the heterogeneous characteristics of tumour growth. The AFP levels in the serum in a few patients with HAS were not elevated. The medical records showed that the serum AFP levels of 7 patients were significantly elevated, while the levels of 4 patients were normal.
HAS predominantly occurs in elderly men, with men of all ages accounting for approximately 70% of all cases. The average age of onset was 63.5 years old, similar to previous reports[7]. HAS is frequently present in the gastric antrum and body (76.9%) but rarely in the cardia and gastric fundus (23.1%). Infiltrating ulcer type was the most frequent tumour type, accounting for 68.5% of all cases[8]. The primary clinical symptoms were abdominal pain, abdominal distension, black stool, and other upper gastrointestinal symptoms, which lacked specificity.
The majority of the patients were male (76.8%), with an average age of 66.1 years. The tumours of 11 patients were located in the gastric antrum and gastric body (84.6%). The statistical results of the 13 patients in the present report were similar[9].
Currently, the preoperative diagnosis of gastric HAC is difficult. Statistics show that the positive rate of endoscopic biopsy is very low at only 9.3%[10]. In clinical practice, most patients need to rely on pathological and immunohistochemical results after specimen resection. As the tissues clipped by the endoscopic biopsy forceps are thin and shallow, the area of HAS hepatoid differentiation accounts for a small proportion of the cancerous tissue frequently located in the deeper parts of the tumour. Preoperative endoscopic biopsy did not reveal the presence of HAS; instead, all cases were identified by postoperative pathology.
Although the increase in the serum levels of AFP was a significant factor in diagnosing HAS, there were still patients with HAS whose serum AFP levels were not increased despite pathological results that demonstrated the presence of hepatic differentiation. Inagawa et al[3] reported that 70%-80% of patients with HAS had elevated serum AFP levels, which was hypothesized to be associated with the degree of tumour differentiation. Certain patients had normal serum AFP levels, suggesting a low degree of tumour differentiation. In addition, Nagai et al[10] reported that 46% of gastric HACs were AFP-negative, and irrespective of whether AFP staining was positive, the presence of microscopic hepatoid features was deemed highly indicative of prognosis.
Therefore, the level of serum AFP should not be used as the basis for diagnosis. Instead, areas of liver differentiation should be diagnosed as HAS when pathological examination is performed.
In immunohistochemical staining, 85%-95% of cases were AFP-positive, and 30%-83.3% were hepatocyte-positive[11]. Among the 13 patients confirmed with HAS in the present case report, 10 were AFP-positive (76.9%), 8 were hepatocyte-positive (88.9%), and 4 were hepatocyte-unknown. The pathological morphology of all patients was consistent with HAS, and the immunohistochemical staining results were as expected.
Recent studies have pointed to the observations that Sal-like protein 4, hepatocyte paraffin 1, and glypican 3 are significantly more positive in HAS[12]. Some novel technologies, such as liquid biopsies, may increase the positive rate of diagnosis[13]. The combination of hepatocyte paraffin 1 and Sal-like protein 4 could serve as a reliable prognostic factor in HAS.
HAS is primarily treated by surgery, which is supplemented with chemotherapy and other comprehensive treatment modes. Radical surgery is the preferred treatment for HAS, and its surgical principles are similar to those of general gastric cancer. D2 radical surgery should be performed, and comprehensive treatment, such as chemotherapy, should be administered or continued following the operation. Oxaliplatin combined with capecitabine can be used as the main chemotherapy regimen[14]. Patients who are unable to undergo radical surgery at the time of treatment should be treated with fluorouracil, platinum, and other chemotherapeutic regimens, although the effect of chemotherapy is limited, and the reported effective rate is only 15.4%-53.8%[15]. Among the 13 patients in the present case report, 10 received postoperative chemotherapy. The chemotherapy regimen was primarily oxaliplatin and fluorouracil. By the end of the last follow-up, 2 patients were lost to follow-up, 3 patients had no evidence of disease progression, 5 patients had disease progression or had died, and the effective rate of chemotherapy was 37.5%, which is similar to previous reports.
Liver metastasis often occurs in HAS, and interventional therapy is also necessary for the treatment of HAS. Peritoneal arterial stem perfusion is primarily used to increase the effective local drug concentration in the liver, which is conducive to preventing liver metastasis.
Due to the lack of specificity, a high degree of malignancy, and the difficulty of preoperative diagnosis during the early stages of HAS, patients often present with middle- or late-stage HAS.
HAS is highly invasive. The literature reports show that the proportions of vascular invasion, liver metastasis, and lymph node metastasis of HAS are typically greater than those of poorly differentiated gastric adenocarcinoma and primary liver cancer. Liver metastases of HAS were present at the time of detection, with lymph node metastases being identified in 43.8%-100% of cases[16], and vascular invasion was present in 71% of patients[17]. Preoperative CT and intraoperative examinations of the 13 patients showed no evidence of liver metastases, which was a lower rate than the statistical proportion. Postoperative pathology confirmed lymph node metastases in 10 (76.9%) patients. Additionally, there was vascular invasion in 5 (38.4%) patients, which was a lower rate than the statistical proportion.
The prognosis of patients with HAS is poor, and the literature reports that the average survival period is 10-18 mo and that the 1-, 3-, and 5-year survival rates are 30%-37.5%, 7%-13%, and 8.3%-9%, respectively[7,18,19]. Studies have pointed out that preoperative serum elevation of AFP level ≥ 500 ng/mL is associated with a low overall survival rate[20]. The serum AFP level plays an important role in predicting the prognosis of HAS and can be used to monitor the recurrence and metastasis of HAS. If the AFP level of postoperative patients increases, tumour recurrence or metastasis should be considered. Patients with HAS should be closely followed, and follow-up should include imaging examinations and serum AFP measurements. Among the patients in the present case report, by the end of the last follow-up, the survival period was 1-56 mo, with an average survival time of 29.6 mo. The 1-year survival rate was 70% (7/10), and the 3-year survival rate was 30% (3/10); these rates were higher than those in previous studies. Meanwhile, a report found that the 5-year overall survival rate for the disease was 34%, and the authors believe that the prognosis is not as bad as previously thought[21]. This may be due to a better understanding of gastric HAC, more effective examinations and treatment, and more active inpatient consultations than before.
HAS is a rare and special type of gastric cancer that is prone to lymph node and liver metastasis and has a poor prognosis. Serum AFP measurement is conducive to the early detection of HAS. The treatment of HAS should mainly involve radical surgery, supplemented by chemotherapy and/or interventional therapy. Serum AFP levels play an important role in predicting the prognosis of HAS and can be used to monitor the recurrence and metastasis of HAS. Close follow-up should be conducted after treatment, including imaging examination and serum AFP measurement. At present, the prognosis of the disease has improved compared with the past. Measurement of AFP, early diagnosis, active surgical treatment, and application of new diagnostic and treatment techniques are conducive to improving the prognosis of HAS.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Azzam A, Tchilikidi KY, Yoshida H S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Kashani A, Ellis JC, Kahn M, Jamil LH. Liver metastasis from hepatoid adenocarcinoma of the esophagus mimicking hepatocellular carcinoma. Gastroenterol Rep (Oxf). 2017;5:67-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Inagawa S, Shimazaki J, Hori M, Yoshimi F, Adachi S, Kawamoto T, Fukao K, Itabashi M. Hepatoid adenocarcinoma of the stomach. Gastric Cancer. 2001;4:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Yoshizawa J, Ishizone S, Ikeyama M, Nakayama J. Gastric hepatoid adenocarcinoma resulting in a spontaneous gastric perforation: a case report and review of the literature. BMC Cancer. 2017;17:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Liu X, Cheng Y, Sheng W, Lu H, Xu X, Xu Y, Long Z, Zhu H, Wang Y. Analysis of clinicopathologic features and prognostic factors in hepatoid adenocarcinoma of the stomach. Am J Surg Pathol. 2010;34:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Kinjo T, Taniguchi H, Kushima R, Sekine S, Oda I, Saka M, Gotoda T, Kinjo F, Fujita J, Shimoda T. Histologic and immunohistochemical analyses of α-fetoprotein--producing cancer of the stomach. Am J Surg Pathol. 2012;36:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Qu BG, Bi WM, Qu BT, Qu T, Han XH, Wang H, Liu YX, Jia YG. PRISMA-Compliant Article: Clinical Characteristics and Factors Influencing Prognosis of Patients With Hepatoid Adenocarcinoma of the Stomach in China. Medicine (Baltimore). 2016;95:e3399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Zeng XY, Yin YP, Xiao H, Zhang P, He J, Liu WZ, Gao JB, Shuai XM, Wang GB, Wu XL, Tao KX. Clinicopathological Characteristics and Prognosis of Hepatoid Adenocarcinoma of the Stomach: Evaluation of a Pooled Case Series. Curr Med Sci. 2018;38:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Søreide JA. Therapeutic Approaches to Gastric Hepatoid Adenocarcinoma: Current Perspectives. Ther Clin Risk Manag. 2019;15:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Metzgeroth G, Ströbel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie. 2010;33:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Osada M, Aishima S, Hirahashi M, Takizawa N, Takahashi S, Nakamura K, Tanaka M, Maehara Y, Takayanagi R, Oda Y. Combination of hepatocellular markers is useful for prognostication in gastric hepatoid adenocarcinoma. Hum Pathol. 2014;45:1243-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Siravegna G, Mussolin B, Venesio T, Marsoni S, Seoane J, Dive C, Papadopoulos N, Kopetz S, Corcoran RB, Siu LL, Bardelli A. How liquid biopsies can change clinical practice in oncology. Ann Oncol. 2019;30:1580-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 14. | Zou M, Li Y, Dai Y, Sun L, Huang T, Yuan X, Qiu H. AFP-producing hepatoid adenocarcinoma (HAC) of peritoneum and omentum: a case report and literature review. Onco Targets Ther. 2019;12:7649-7654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Yang J, Wang R, Zhang W, Zhuang W, Wang M, Tang C. Clinicopathological and prognostic characteristics of hepatoid adenocarcinoma of the stomach. Gastroenterol Res Pract. 2014;2014:140587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Bazeries P, Barral M, Labrife M, Soyer P, Aubé C. Hepatic metastases from gastric hepatoid adenocarcinoma: An unusual cause of capsular retraction of the liver. Diagn Interv Imaging. 2016;97:931-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Baek SK, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic characteristics and treatment outcomes of hepatoid adenocarcinoma of the stomach, a rare but unique subtype of gastric cancer. BMC Gastroenterol. 2011;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Liu X, Sheng W, Wang Y. An analysis of clinicopathological features and prognosis by comparing hepatoid adenocarcinoma of the stomach with AFP-producing gastric cancer. J Surg Oncol. 2012;106:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Yao LT, Xu HM. Clinic pathological features, immunohistochemisty and prognosis of gastric hepatoid adenocarcinoma. Zhonghua Changwei Waike Zazhi. 2014;17:196-200. [DOI] [Full Text] |
| 20. | Wang Y, Sun L, Li Z, Gao J, Ge S, Zhang C, Yuan J, Wang X, Li J, Lu Z, Gong J, Lu M, Zhou J, Peng Z, Shen L, Zhang X. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer. 2019;22:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | Inoue M, Sano T, Kuchiba A, Taniguchi H, Fukagawa T, Katai H. Long-term results of gastrectomy for alpha-fetoprotein-producing gastric cancer. Br J Surg. 2010;97:1056-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |