Published online Mar 26, 2020. doi: 10.12998/wjcc.v8.i6.1116
Peer-review started: December 21, 2019
First decision: January 12, 2020
Revised: February 16, 2020
Accepted: February 28, 2020
Article in press: February 28, 2020
Published online: March 26, 2020
Processing time: 95 Days and 21.3 Hours
Hepatoid carcinoma (HC) is an extremely rare neoplasm that is morphologically similar to hepatocellular carcinoma. HC has been described in various organs; however, HC of the pancreas is extremely rare. To our knowledge, only 38 cases have been reported. We present a case of HC of the pancreas in a 36-year-old male patient.
A 36-year-old cachexic man with no significant past medical history was transferred to our hospital with a history of painless jaundice, elevated blood glucose and significant weight loss. Lab tests showed elevated serum transaminases, bilirubin and alpha-fetoprotein levels. Magnetic resonance imaging of the upper abdomen showed a diffusely enlarged pancreas, appearing “sausage-shaped”. Magnetic resonance cholangiopancreatography showed upstream ductal dilation secondary to stricture of the main pancreatic duct and the common bile duct, which were not visible. Immunohistochemistry of biopsied tissue from a percutaneous pancreatic biopsy showed tumor cell positivity for HepPar1, polyclonal carcinoembryonic antigen and CK19, suggestive of HC of the pancreas. The characteristics of 39 patients with HC of the pancreas were reviewed.
HC of the pancreas is more prevalent in males, and patients have a median age of 57 years. It is most commonly asymptomatic or presents as abdominal back pain, and the pancreatic tail is the most common location. At the time of diagnosis, liver metastasis is often present.
Core tip: Hepatoid carcinoma (HC) of the pancreas is an uncommon tumor with unknown characteristics. To date, there is a lack of definitive criteria for identification, and no defined treatment strategy for patients with HC of the pancreas. This study reviews 39 cases with an emphasis on diagnostic criteria and outcome management. The possibility of HC of the pancreas should be considered for diffuse lesions throughout the pancreas.
- Citation: Zeng SX, Tan SW, Fong CJTH, Liang Q, Zhao BL, Liu K, Guo JX, Tao J. Hepatoid carcinoma of the pancreas: A case report and review of the literature. World J Clin Cases 2020; 8(6): 1116-1128
- URL: https://www.wjgnet.com/2307-8960/full/v8/i6/1116.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i6.1116
Hepatoid carcinoma (HC) is a primary rare tumor that grows outside the liver with similar serological, morphological and immunohistochemistry features to hepatocellular carcinoma (HCC). First described by Ishikura et al[1] in 1985 in the stomach, where it is most commonly found, HC may involve any part of the gastrointestinal tract[2-5], lungs[6], and genitourinary tract[7-10]. HC of the pancreas is extremely rare. The clinical features, diagnosis, management and prognosis of HC of the pancreas have yet to be clearly studied because of its rarity and the limited number of case reports in the literature. In this paper, we present a case of hepatoid carcinoma of the pancreas in a 36-year-old cachexic male patient with painless jaundice, elevated blood glucose and weight loss, as well as a review of the current literature focusing on clinical presentation, management and prognosis.
Painless jaundice and emaciation for the past 2 mo.
A 36-year-old man was transferred to the Third Affiliated Hospital of Sun Yat-Sen University with a recent history of painless jaundice, elevated blood glucose and a weight loss of approximately 10 kg for the past 2 mo with no complaints of diarrhea or vomiting.
The patient’s past medical and surgical histories were nonsignificant. He was previously diagnosed with autoimmune pancreatitis in another institution and had no response to steroid treatment.
He had a 10 pack-year history of smoking. He denied any other specific personal or family history of other diseases.
The patient appeared cachexic and was mildly jaundiced. A nontender epigastric mass of approximately 5 cm was palpable, with a soft nondistended abdomen and normal bowel sounds.
Laboratory tests showed a normal white blood cell count (9.32 × 10E9 cells/L), mild anemia (118 g/L) and an elevated platelet count (476 × 10E9 cells/L). Liver function tests showed elevated transaminases (ALT 97 U/L and AST 46 U/L), alkaline phosphatase (377 U/L), gamma-GT (337 U/L), total bilirubin (107.6 µmol/L), direct bilirubin (77.64 µmol/L), indirect bilirubin (30.2 µmol/L) and a mild decrease in albumin (31.8 g/L). Autoimmune antibodies such as ANA and rheumatoid factor were negative, and IgG4 (0.333 g/L), amylase and lipase levels were normal; the tumor marker panel showed elevated levels of alpha-fetoprotein (AFP) (475.6 ng/mL) and carbohydrate antigen 125 (77.1 U/mL) but normal serum levels of carcinoembryonic antigen (CEA) (2.2 µg/L) and carbohydrate antigen 19-9 (15.94 U/mL). Markers for hepatitis B and C and human immunodeficiency virus serology were negative.
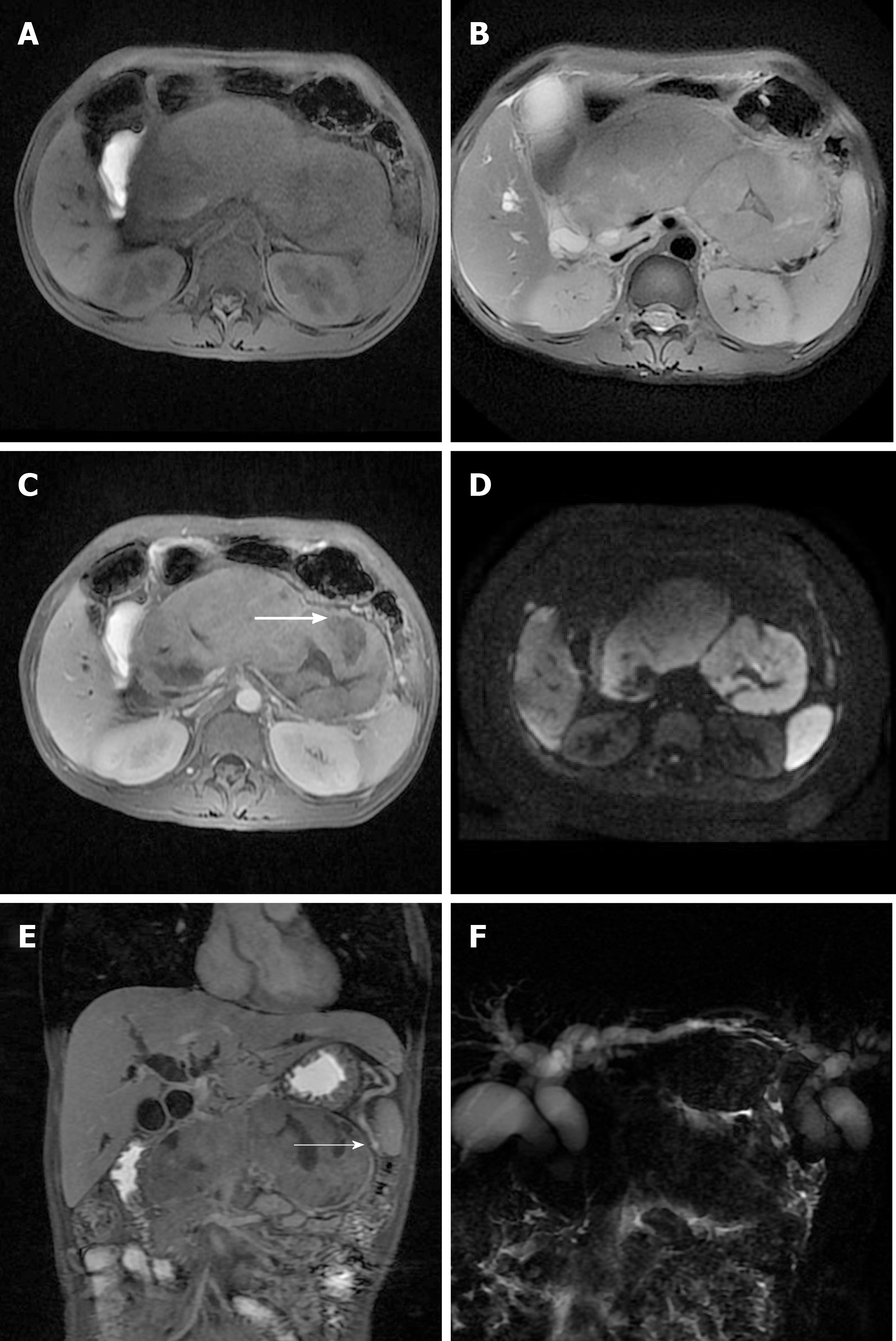
Magnetic resonance imaging of the upper abdomen showed a diffusely enlarged pancreas, appearing “sausage-shaped”, with loss of pancreatic lobular structure; the lesion was isointense to hypointense on the T1-weighted image, isointense to hyperintense on the T2-weighted image, and had a mixed signal on DWI, with central necrosis. In addition, there was a distinct hyperintense rim surrounding the mass, which demonstrated delayed enhancement and a capsule appearance. Magnetic resonance cholangiopancreatography showed upstream ductal dilation secondary to strictures of the main pancreatic duct and common bile duct, which were not visible (Figure 1).
A percutaneous pancreatic biopsy was performed under ultrasound guidance.
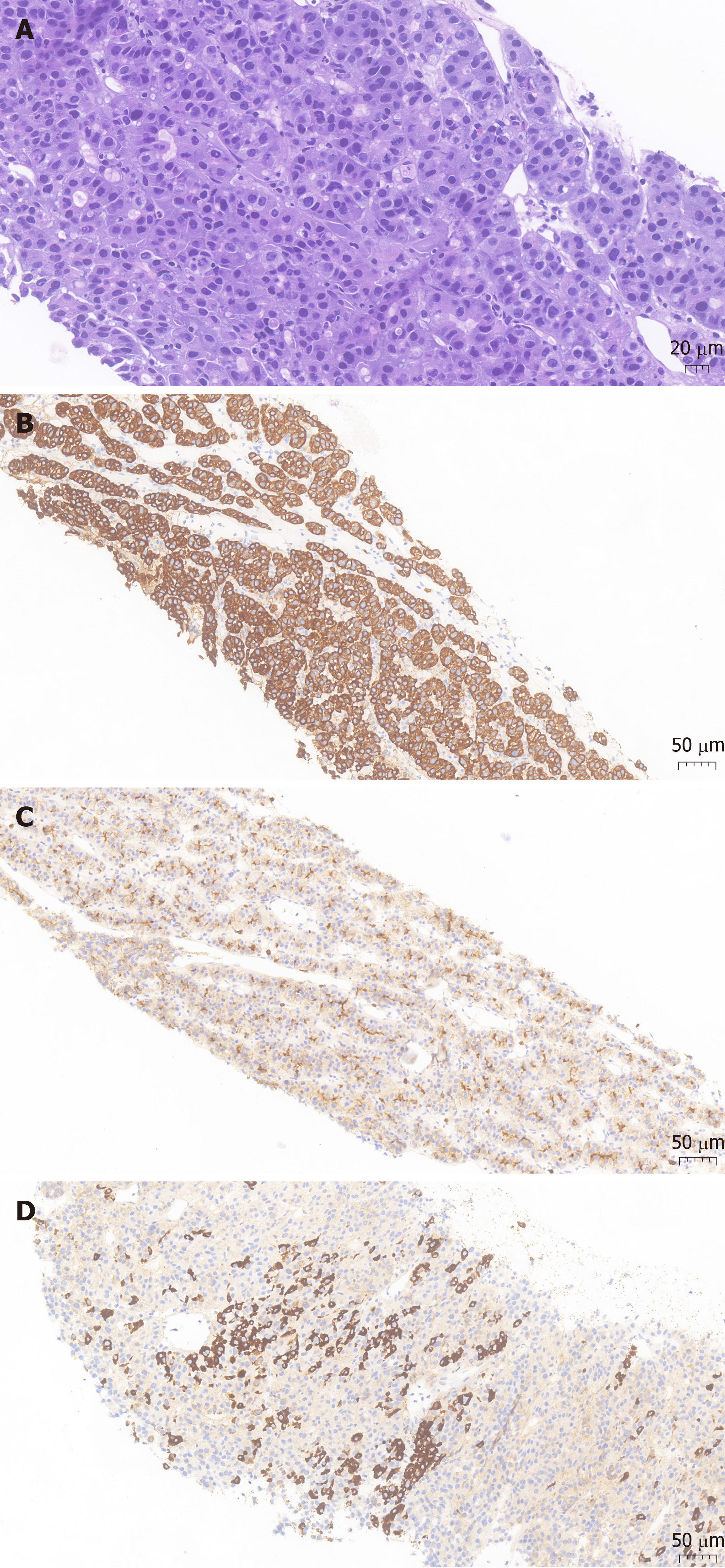
Histopathological analysis revealed heteromorphic neoplastic cells arranged in glandular, nested or striped patterns. Immunohistochemistry showed tumor cell positivity for HepPar1 (a hepatocyte-specific antigen), polyclonal CEA and CK19 (Figure 2). The morphological and immunohistochemistry features were suggestive of hepatoid carcinoma of the pancreas.
The final diagnosis of the presented case was hepatoid carcinoma of the pancreas.
The patient finally underwent exploratory laparotomy, during which a large mass of the whole pancreas, approximately 6 cm × 7 cm, was found invading the coeliac trunk, the root of the transverse mesocolon, and the upper mesojejunum. Therefore, no radical surgery was performed, and palliative jejunostomy and cholecystostomy were performed.
At 4 mo after diagnosis and refusing palliative chemotherapy, the patient died of the disease.
HC was first described in 1987 by Hruban et al[11], and we report the 39th case of hepatoid carcinoma of the pancreas diagnosed based on morphological and immunohistochemical features. The demographics and clinical presentation of the 39 cases are summarized in Table 1[11-45]. From the review, we can establish a clear male predominance (69.3%). The ages of patients range from 21 years to 83 years, with a median age of 57 years. The sizes of tumors range from 1 cm to 12 cm, with a median size of 6 cm. HC of the pancreas can be divided into either pure HCC-like (61.54%) or mixed (38.46%) forms with other histological findings, such as neuroendocrine tumors (n = 9), pancreatic ductal adenocarcinoma (n = 3), acinar cell carcinoma (n = 1) and microcytic cystadenoma (n = 2).
| Ref. | Age | Sex | Clinical presentation | Serum AFP levels at diagnosis | Serum CEA levels at diagnosis | Location | Tumor size in cm | Associated component | Site of metasta-sis | Treatment | Clinical follow-up in mo |
| Hruban et al[11], 1987 | 53 | F | Subcutaneous fat necrosis and polyarthritis | Normal | Not available | Tail | 1 | Acinar cell carcinoma | Liver | Chemotherapy (5-FU, Adriamycin) | Died of disease (2.75) |
| Tanno et al[12], 1999 | 65 | F | Epigastric and back pain, anorexia, and weight loss | Elevated | Elevated | Bodytail | 6 × 5 | Ductal adenocarcinoma | Liver, right supraclavicular, and paraaortic lymph node | Palliative care | Died of disease (6) |
| Yano et al[13], 1999 | 57 | M | Jaundice, epigastric pain, vomiting and fever | Elevated | Elevated | Head | 9 × 7 ×5 | Ductal adenocarcinoma | No | Surgery (pancreatoduodenectomy) | Died of disease (3) |
| Paner et al[14], 2000 | 28 | M | Severe abdominal and back pain | Elevated | Elevated | Multifocal | 8 × 8 × 6 | Ductal adenocarcinoma | Widespread (gastric, ileal, and colonic mucosa) | Debulking of the tumor plus chemotherapy | Died of disease (14) |
| Paner et al[14], 2000 | 57 | M | Vomiting, diarrhea, weight loss, diffuse skin rashes and diabetes mellitus | Elevated | Elevated | Tail | 6 × 4 × 3.5 | Neuroendocrine neoplasms (glucagonoma) | Liver | Surgery (distal pancreatectomy with splenectomy) plus chemotherapy | Died of disease (102) |
| Lam et al[15], 2001 | 64 | F | Hypoglycemia and recurrent nocturnal sweating | Elevated | Not available | Tail | 7 × 4 × 4 | Insulinoma | Liver | Distal pancreatectomy with splenectomy plus regional embolization and systemic chemotherapy | Died of disease (22) |
| Cuilliere et al[16], 2002 | 70 | M | Incidental (asymptomatic) | Normal | Normal | Body | 3 | Serous microcystic adenoma | No | Distal pancreatectomy with splenectomy | Alive with no evidence of recurrence (12) |
| Hughes et al[17], 2004 | 51 | M | Incidental finding | Normal | Normal | Tail | 6 × 5.5 × 5.5 | No | No | Total pancreatectomy | Alive with no evidence of recurrence (14) |
| Matsueda et al[18], 2006 | 49 | F | Weight loss | Elevated | Normal | Widespread | Not available | No | Liver (detected after 12 mo) | Surgery (total pancreatectomy), chemotherapy (gemcitabine) and liver lobectomy | Alive with no evidence of recurrence (48) |
| Shih et al[19], 2006 | 32 | M | Incidental (asympto-matic) | Normal | Elevated | Tail | 7 | No | No | Surgery (distal pancreatectomy with splenectomy) | Alive with no evidence of recurrence (18) |
| Oh et al[20], 2006 | 21 | M | Incidental (asymptomatic) | Elevated | Not available | Head | 3 × 3 × 3 | Neuroendocrine neoplasm | No | Surgery (pancreatoduodenectomy) | Alive with no evidence of recurrence (7) |
| Cardona et al[21], 2007 | 58 | M | Back and flank pain | Normal | Not available | Body | 3.3 × 2.5 × 2.5 | No | No | Surgery (distal pancreatectomy with splenectomy) | Alive with no evidence of recurrence (15) |
| Kubota et al[22], 2007 | 56 | M | Diabetes | Not available | Not available | Tail | 6.3 × 6.2 | No | No | Surgery (distal pancreatectomy with splenectomy) | Alive with no evidence of recurrence (36) |
| Hameed et al[23], 2007 | 41 | F | Gastro-esophageal reflux, jaundice, and abdominal pain | Elevated | Elevated | Head | 4.5 × 4 × 3 | Neuroendocrine neoplasm | Liver | Pancreaticoduodenectomy plus Chemotherapy with cisplatin and irinotecan | Died of disease (27) |
| Liu et al[24], 2007 | 80 | M | Nausea, diarrhea weight loss and epigastric palpable mass | Normal | Not available | Head | 5 × 5 × 6 | No | Transverse colon | Simple tumorectomy and partial transverse colon resection | Alive with no evidence of recurrence (8) |
| Zhang et al[25], 2007 | 37 | F | Upper abdominal pain, anorexia, and emaciation | Elevated | Not available | Widespread | 9 | Neuroendocrine neoplasm | No | Surgery (pancreatoduodenectomy) | Died of disease (3) |
| Jung et al[26], 2010 | 46 | M | Dyspepsia and epigastric palpable mass | Elevated | Elevated | Head | 8 × 9 | Neuroendocrine neoplasm | No | Radical pancreatoduodenectomy | Alive with no evidence of recurrence (4) |
| Petrelli et al[27], 2012 | 37 | F | Epigastric abdominal mass | Not available | Normal | Body | 11 | No | Liver, mediastinal lymph nodes, and lungs | Chemotherapy with sorafenib | Died of disease (12) |
| Kelly et al[28], 2012 | 53 | F | Severe epigastric pain | Elevated | Not available | Bodytail | Not available | No | Liver | Distal pancreatectomy and adjuvant chemotherapy (carboplatin and gemcitabine) | Alive with no evidence of recurrence (22) |
| Kai et al[29], 2012 | 79 | F | Incidental (asymptomatic) | Elevated | Elevated | Tail | 7 | No | Stomach, left adrenal gland, and liver | Distal pancreatectomy with splenectomy, combined with resection of the left adrenal gland and total gastrectomy | Died of disease (2) |
| Huang et al[30], 2012 | 52 | M | Jaundice, anorexia and epigastric pain | Not available | Elevated | Head | 0.5 nodule | Neuroendocrine tumor | No | Pancreaticoduodenectomy plus chemotherapy with sunitinib | Alive with no evidence of recurrence (16) |
| Majumder et al[31], 2013 | 69 | M | Left upper quadrant pain, jaundice and nausea | Normal | Not available | Head | 5.8 × 6.0 | No | Liver | Biliary drainage plus chemotherapy with gemcitabine | Died of disease (3) |
| Steen et al[32], 2013 | 61 | F | Incidental (asymptomatic) | Normal | Not available | Tail | 5.3 × 3.5 | No | No | Distal pancreatectomy with splenectomy | Alive with no evidence of recurrence (60) |
| Xin et al[33], 2014 | 33 | F | Incidental (asymptomatic) | Elevated | Normal | Head | 2 × 1.4 × 1.8 | Neuroendocrine neoplasm | No | Pancreaticoduodenectomy plus chemotherapy with gemcitabine | Alive with no evidence of recurrence (46) |
| Vanoli et al[34], 2015 | 57 | F | Jaundice | Elevated | Elevated | Head | 3.5 × 3 × 3 | No | No | Pancreatoduodenectomy and adjuvant chemotherapy (gemcitabine) | Alive with no evidence of recurrence (10) |
| Soofi et al[35], 2015 | 69 | M | Atypical chest pain | Elevated | Normal | Body and tail | 5.9 | No | No | Distal pancreatectomy with splenectomy | Alive with no evidence of recurrence (4) |
| Antonini et al[36], 2015 | 59 | M | Weight loss and abdominal discomfort | Normal | Normal | Body | 6 × 5 | No | No | Chemotherapy with sorafenib | Died of disease (4) |
| Kuo et al[37], 2015 | 67 | M | Incidental (asymptomatic) | Normal | Normal | Tail | 2 × 2 | No | No | Distal pancreatectomy with spleen preservation | Alive with no evidence of recurrence (6) |
| Veerankutty et al[38], 2015 | 47 | M | Incidental | Not available | Normal | Tail | 3.1 × 2.9 × 2.6 | Serous cystadenoma | No | Distal pancreatectomy with splenectomy (laparoscopic) | Alive with no evidence of recurrence (8) |
| Williams et al[39], 2015 | 71 | M | Pancreatitis and melena (oozing ulcer at the ampulla) | Normal | Normal | Head | 5 | No | Duodenal muscularis propria | Pancreaticoduodenectomy and cholecystectomy | Not available |
| Stamatova et al[40], 2016 | 78 | M | Incidental (asymptomatic) | Normal | Normal | Head | 8 × 6 | No | No | Pancreaticoduodenectomy | No evidence of recurrence but died of heart attack (2) |
| Chang et al[41], 2016 | 61 | M | Incidental (asymptomatic) | Not available | Not available | Body and tail | 1.3 | No | No | Distal pancreatectomy | Alive with no evidence of recurrence (6) |
| Akimoto et al[42], 2016 | 59 | M | Incidental (asymptomatic) | Normal | Normal | Body | 5.0 × 3.5 | No | No | Middle pancreatectomy | Alive with no evidence of recurrence (12) |
| Pellini Ferreira et al[43], 2017 | 43 | M | Jaundice, epigastric pain, and watery diarrhea | Normal | Normal | Tail | 9.0 | Neuroendocrine neoplasm | Spleen and liver | Chemotherapy with capecitabine and temozolomide | Alive with no evidence of recurrence (16) |
| Ma et al[44], 2017 | 75 | M | Weight loss | Elevated | Elevated | Tail | 7.8 | No | Liver | Chemotherapy with mFOLFIRINOX (oxaliplatin + leucovorin + irinotecan + 5-fluorouracil) plus surgery (distal pancreatectomy with splenectomy and wedge resection of the liver) | Alive with no evidence of recurrence (10) |
| Yang et al[45], 2018 | 83 | M | Abdominal pain | Not available | Not available | Body | 2.7 × 2.5 × 1.5 | No | No | Laparoscopic distal pancreatectomy and splenectomy | Alive with no evidence of recurrence (107) |
| Yang et al[45], 2018 | 72 | M | Severe back pain | Not available | Not available | Tail | 12.0 × 10.5 × 4.5 | No | No | Distal pancreatectomy with splenectomy | Died of pulmonary embolism (1) |
| Yang et al[45], 2018 | 54 | M | Incidental (asymptomatic) | Elevated | Normal | Body and tail | 10.0 × 9.0 × 9.0 | No | Adjacent transverse colon | Distal pancreatectomy along with splenectomy and left hemicolectomy | Died of disease (29) |
| Our case | 36 | M | Progressive jaundice, weight loss and epigastric palpable mass | Elevated | Normal | Widespread | 6.0 × 7.0 | No | Celiac trunk, root of transverse mesocolon, and upper mesojejunum | Palliative care | Died of disease (4) |
Table 2 outlines the main clinical features of HC of the pancreas in the reported literature, with the most common tumor site being the pancreatic tail, accounting for most of the patients who are asymptomatic or complain of abdominal/back pain.
| Variable | n (%) or median (IQR) |
| Sex | |
| Female | 12 (30.77) |
| Male | 27 (69.23) |
| Age in yr | |
| Median (range) | 57 (21-83) |
| Symptoms | |
| Asymptomatic | 13 (33.33) |
| Pain: Abdominal/back | 13 (33.33) |
| Gastrointestinal symptoms: Vomiting, diarrhea, and dyspepsia | 9 (23.08) |
| Weight loss | 8 (20.51) |
| Jaundice | 7 (17.95) |
| Epigastric mass | 3 ( 7.69 ) |
| Location | |
| Tail | 13 (33.33) |
| Head | 11 (28.21) |
| Body | 6 (15.38) |
| Body and tail | 5 (12.82) |
| Diffuse or multifocal | 4 (10.26) |
| Size of longest diameter in cm | |
| Median (range) | 6 ( 1-12 ) |
| Metastasis | 17 (43.59) |
| Liver metastasis | 12 (30.77) |
| Associated component: Mixed form | 15 (38.46) |
| Elevated AFP | 18 (46.15) |
| Elevated CEA | 11 (28.21) |
The pathogenesis of hepatoid carcinoma of the pancreas remains to be elucidated. Three theories have been proposed: The ectopic liver tissue theory, in which HC may originate from ectopic pancreatic liver tissue[21,22,46]; the pancreas-to-liver transdifferentiation theory, in which pancreatic cells can transdifferentiate into hepatocytes[47,48]; and the pancreatic multipotent/stem cell theory, in which the liver and pancreas share the same embryonic derivation - the foregut endoderm - and genes controlling hepatocytic differentiation that are normally suppressed in the pancreas may be activated during carcinogenesis[12,35].
There are currently no standard criteria to establish a diagnosis of hepatoid carcinoma of the pancreas. The differential diagnosis of HC of the pancreas includes HCC or combined hepatocellular-cholangiocarcinoma, metastatic hepatoid carcinoma and other primary pancreatic tumors with eosinophilic cell cytoplasms. Its diagnosis relies on typical morphological features and immunohistochemical staining. Histopathologically, HC consists of medium to large cords of polygonal cells with abundant eosinophilic or clear cytoplasms with centrally located and vesicular nuclei in the sheet-like or trabecular portions. The presence of bile production is a more conclusive finding and is strong evidence of hepatocyte lineage differentiation[14,49]. For immunohistochemistry findings, the hepatoid carcinoma cells show positive staining for immunoreactivity with polyclonal antibodies against AFP, CEA, glypican-3, and HepPar1 (a hepatocyte-specific antigen), as well as albumin mRNA detection by in situ hybridization[14,20]. Cytokeratin 19 positivity plays an important role in differentiating hepatoid tumors from HCC[27]. HC of the pancreas with acinar differentiation should be tested with arginase-1 to exclude acinar cell carcinoma of the pancreas, which also presents with AFP elevation. As seen in our review, serum AFP is often elevated at the time of diagnosis of HC of the pancreas (41.15%), and can be used to monitor therapeutic response and recurrence[13,15,18,24]. Serum protein induced by vitamin K absence or antagonist II, a specific marker used for early diagnosis of HCC, was elevated in some cases, aiding in early diagnosis and indicative of better prognoses[18]. Serum CEA, which was elevated in 28.21% of cases, is a less sensitive diagnostic marker for hepatoid carcinoma of the pancreas.
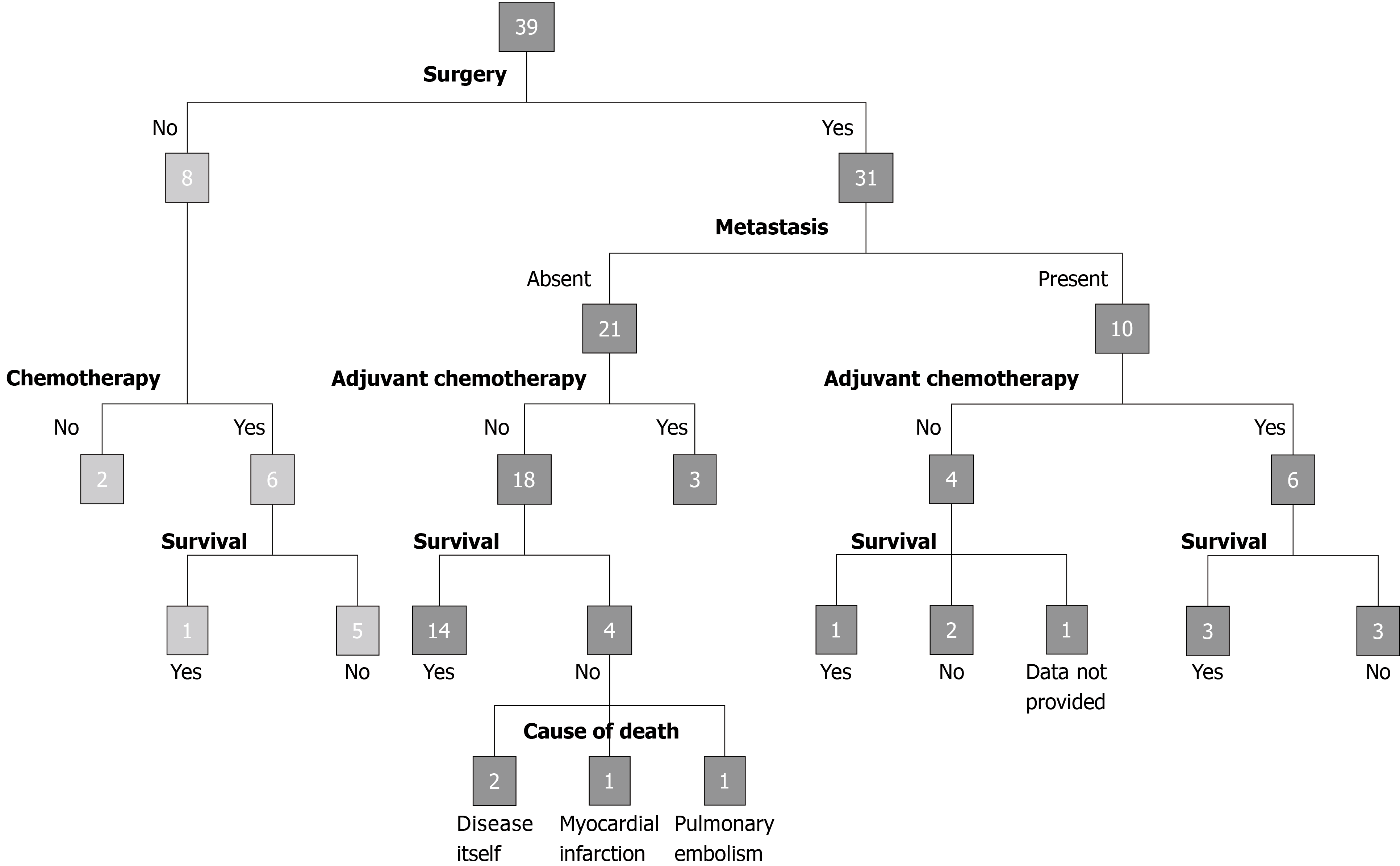
Due to its rarity, there is currently no standardized treatment for HC of the pancreas. Owing to its aggressive nature and tendency for early liver metastasis, HC of the pancreas warrants surgical resection, if possible. The effect of adjuvant therapy after surgery resection, advocated because of the metastatic potential of the tumor, is still unclear[43,44]. Survival was poor in patients treated with only chemotherapy compared to those treated with surgery and chemotherapy: 5 out of 6 patients treated with chemotherapy succumbed to the disease (after 2.75-14 mo), while 3 out of 9 patients treated with chemotherapy and surgery succumbed to the disease (after 22-102 mo). Variable survival rates of 3 locally unresectable, metastatic or recurrent cases treated with surgery and adjuvant chemotherapy with mFOLFIRINOX, di-amino triazeno-imidazol carboxamide or gemcitabine have been reported in the literature, with one patient dying of the disease at 102 mo and two patients alive at 10 mo and 48 mo[14,18,44].
The prognosis of hepatoid carcinoma of the pancreas is unclear due to its rarity and possible heterogeneity. HCs of the gastrointestinal tract are associated with an unfavorable prognosis[50] since at the time of diagnosis, liver metastasis is often already present, indicating advanced stage[37]. Survival outcomes mainly depend upon the extent of the disease and the completeness of resection, with greater survival rates after resection and adjuvant chemotherapy, as depicted in Figure 3, with the longest disease-free interval being 107 mo[45]. Owing to the limited data, further studies with long-term follow-up are needed to standardize the treatment and to predict the natural history and prognosis of HC of the pancreas compared to those of the relatively more common gastric hepatoid carcinoma.
Our case was highly challenging due to the clinical presentation of the patient that was inconsistent with the imaging that suggested autoimmune pancreatitis (AIP). AIP commonly presents with obstructive jaundice, abdominal pain, vomiting and weight loss. Type 1 AIP is associated with high serum levels of IgG4 (> 140 mg/dL), IgG4-positive plasma cell infiltration, and sclerosis, while type 2 AIP is often associated with inflammatory bowel disease[51]. The patient may have been previously diagnosed with AIP due to the “sausage-shaped” appearance of the diffusely enlarged pancreas, the presence of a capsule-like rim and ductal stricture on imaging, and the lack of biopsy. Differentiating between AIP and pancreatic malignancy has become a diagnostic challenge for modern gastroenterologists because they often share overlapping clinical and imaging features. The poor response to steroid treatment prior to admission prompted reassessment of the diagnosis. The patient was cachexic, with recent onset of diabetes mellitus (DM), no sign of systemic involvement, negative autoantibodies, and non-elevated amylase and lipase, indicating malignancy. Type 3C DM, as reported in the literature, is difficult to control, requiring at least 1 IU/kg body weight of insulin[52]. The prevalence of DM in patients with pancreatic cancer has been reported to be 40%, with half developing DM within 2 years[53]. Interestingly, DM was reported in only 6 previously reported cases (two of which had an associated neuroendocrine component and increased glucagon levels; the length of DM history was not reported in the remaining 4 cases). In our case, it was attributed to pancreatic islet destruction resulting from advanced-stage hepatoid carcinoma with the absence of a neuroendocrine component on pathology.
In summary, this review attempts to summarize the clinical characteristics, diagnostic methods, treatment and prognosis of HC based on the current literature. HC of the pancreas is an extremely rare neoplasm that resembles HCC in terms of morphology and immunohistochemistry findings. Diagnosis is mainly based on histopathological and immunohistochemical features. Elevation of serum AFP and protein induced by vitamin K absence or antagonist II may be a clue leading to the diagnosis of this tumor. Surgical resection is the mainstay of therapy and is more likely to result in long-term survival. Adjuvant chemotherapy has a role in recurrent, residual, unresectable and metastatic disease. Survival outcomes mainly depend upon the extent of the disease at diagnosis. The possibility of hepatoid carcinoma of the pancreas should be considered for diffuse lesions throughout the pancreas.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmad Z, Coskun A S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Liu MY
| 1. | Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Tanigawa H, Kida Y, Kuwao S, Uesugi H, Ojima T, Kobayashi N, Saigenji K, Okayasu I. Hepatoid adenocarcinoma in Barrett's esophagus associated with achalasia: first case report. Pathol Int. 2002;52:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Gardiner GW, Lajoie G, Keith R. Hepatoid adenocarcinoma of the papilla of Vater. Histopathology. 1992;20:541-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Yachida S, Fukushima N, Nakanishi Y, Akasu T, Kitamura H, Sakamoto M, Shimoda T. Alpha-fetoprotein-producing carcinoma of the colon: report of a case and review of the literature. Dis Colon Rectum. 2003;46:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Nakashima H, Nagafuchi K, Satoh H, Takeda K, Yamasaki T, Yonemasu H, Kishikawa H. Hepatoid adenocarcinoma of the gallbladder. J Hepatobiliary Pancreat Surg. 2000;7:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Hiroshima K, Iyoda A, Toyozaki T, Haga Y, Baba M, Fujisawa T, Ishikura H, Ohwada H. Alpha-fetoprotein-producing lung carcinoma: report of three cases. Pathol Int. 2002;52:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Ishikura H, Ishiguro T, Enatsu C, Fujii H, Kakuta Y, Kanda M, Yoshiki T. Hepatoid adenocarcinoma of the renal pelvis producing alpha-fetoprotein of hepatic type and bile pigment. Cancer. 1991;67:3051-3056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Lopez-Beltran A, Luque RJ, Quintero A, Requena MJ, Montironi R. Hepatoid adenocarcinoma of the urinary bladder. Virchows Arch. 2003;442:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Tochigi N, Kishimoto T, Supriatna Y, Nagai Y, Nikaido T, Ishikura H. Hepatoid carcinoma of the ovary: a report of three cases admixed with a common surface epithelial carcinoma. Int J Gynecol Pathol. 2003;22:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Shintaku M, Kariya M, Shime H, Ishikura H. Adenocarcinoma of the uterine cervix with choriocarcinomatous and hepatoid differentiation: report of a case. Int J Gynecol Pathol. 2000;19:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Hruban RH, Molina JM, Reddy MN, Boitnott JK. A neoplasm with pancreatic and hepatocellular differentiation presenting with subcutaneous fat necrosis. Am J Clin Pathol. 1987;88:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Tanno S, Obara T, Fujii T, Izawa T, Mizukami Y, Saitoh Y, Ura H, Kohgo Y. alpha-Fetoprotein-producing adenocarcinoma of the pancreas presenting focal hepatoid differentiation. Int J Pancreatol. 1999;26:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Yano T, Ishikura H, Wada T, Kishimoto T, Kondo S, Katoh H, Yoshiki T. Hepatoid adenocarcinoma of the pancreas. Histopathology. 1999;35:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Paner GP, Thompson KS, Reyes CV. Hepatoid carcinoma of the pancreas. Cancer. 2000;88:1582-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Lam K, Lo C, Wat M, Fan ST. Malignant insulinoma with hepatoid differentiation: a unique case with alpha-fetoprotein production. Endocr Pathol. 2001;12:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Cuilliere P, Lazure T, Bui M, Fabre M, Buffet C, Gayral F, Bedossa P. Solid adenoma with exclusive hepatocellular differentiation: a new variant among pancreatic benign neoplasms? Virchows Arch. 2002;441:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Hughes K, Kelty S, Martin R. Hepatoid carcinoma of the pancreas. Am Surg. 2004;70:1030-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Matsueda K, Yamamoto H, Yoshida Y, Notohara K. Hepatoid carcinoma of the pancreas producing protein induced by vitamin K absence or antagonist II (PIVKA-II) and alpha-fetoprotein (AFP). J Gastroenterol. 2006;41:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Shih NN, Tsung JS, Yang AH, Tsou MH, Cheng TY. A unique pancreatic tumor with exclusive hepatocytic differentiation. Ann Clin Lab Sci. 2006;36:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Oh HJ, Cheung DY, Kim TH, Kim SS, Kim MS, Kim JI, Park SH, Han JY, Han NI, Kim JK, Lee YS, Kim EK, Jung ES. [A case of hepatoid carcinoma of the pancreas]. Korean J Gastroenterol. 2006;47:389-393. [PubMed] |
| 21. | Cardona D, Grobmyer S, Crawford JM, Liu C. Hepatocellular carcinoma arising from ectopic liver tissue in the pancreas. Virchows Arch. 2007;450:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Kubota K, Kita J, Rokkaku K, Iwasaki Y, Sawada T, Imura J, Fujimori T. Ectopic hepatocellular carcinoma arising from pancreas: a case report and review of the literature. World J Gastroenterol. 2007;13:4270-4273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Hameed O, Xu H, Saddeghi S, Maluf H. Hepatoid carcinoma of the pancreas: a case report and literature review of a heterogeneous group of tumors. Am J Surg Pathol. 2007;31:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Liu CZ, Hu SY, Wang L, Zhi XT, Jin B, Zhu M, Wachtel MS, Frezza EE. Hepatoid carcinoma of the pancreas: a case report. Chin Med J (Engl). 2007;120:1850-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Zhou P, Sun Y. Hepatoid Carcinoma of the Pancreas:a Case Report[J]. Chin J Clin Oncol. 2007;6:445-447. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Jung JY, Kim YJ, Kim HM, Kim HJ, Park SW, Song SY, Chung JB, Kang CM, Pyo JY, Yang WI, Bang S. Hepatoid carcinoma of the pancreas combined with neuroendocrine carcinoma. Gut Liver. 2010;4:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Petrelli F, Ghilardi M, Colombo S, Stringhi E, Barbara C, Cabiddu M, Elia S, Corti D, Barni S. A rare case of metastatic pancreatic hepatoid carcinoma treated with sorafenib. J Gastrointest Cancer. 2012;43:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Kelly PJ, Spence R, Dasari BV, Burt AD, Taylor M, Loughrey MB. Primary hepatocellular carcinoma of the pancreas: a case report and review of the heterogeneous group of pancreatic hepatoid carcinomas. Histopathology. 2012;60:1012-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Kai K, Nakamura J, Ide T, Masuda M, Kitahara K, Miyoshi A, Noshiro H, Tokunaga O. Hepatoid carcinoma of the pancreas penetrating into the gastric cavity: a case report and literature review. Pathol Int. 2012;62:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Huang SC, Chang HC, Yeh TS, Ng KF, Chen TC. Hepatoid microcarcinoma of the pancreas: a case report and review of the literature. Chang Gung Med J. 2012;35:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Majumder S, Dasanu CA. Hepatoid variant of pancreatic cancer: insights from a case and literature review. JOP. 2013;14:442-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 32. | Steen S, Wolin E, Geller SA, Colquhoun S. Primary hepatocellular carcinoma ("hepatoid" carcinoma) of the pancreas: a case report and review of the literature. Clin Case Rep. 2013;1:66-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Xin BB, Li JA, Han X, Zhao J, Ji Y, Lou WH, Xu XF. Successful treatment of a case with pancreatic neuroendocrine carcinoma with focal hepatoid differentiation: a case report and literature review. Int J Clin Exp Med. 2014;7:3588-3594. [PubMed] |
| 34. | Vanoli A, Argenti F, Vinci A, La Rosa S, Viglio A, Riboni R, Necchi V, Pugliese L, Sessa F, Pietrabissa A, Paulli M. Hepatoid carcinoma of the pancreas with lymphoid stroma: first description of the clinical, morphological, immunohistochemical, and molecular characteristics of an unusual pancreatic carcinoma. Virchows Arch. 2015;467:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Soofi Y, Kanehira K, Abbas A, Aranez J, Bain A, Ylagan L. Pancreatic hepatoid carcinoma: a rare form of pancreatic neoplasm. Diagn Cytopathol. 2015;43:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Antonini F, Angelelli L, Rubini C, Macarri G. Endoscopic ultrasound diagnosis of a primary hepatoid carcinoma of the pancreas. Endoscopy. 2015;47:E367-E368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Kuo PC, Chen SC, Shyr YM, Kuo YJ, Lee RC, Wang SE. Hepatoid carcinoma of the pancreas. World J Surg Oncol. 2015;13:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Veerankutty FH, Yeldho V, Tu SA, Venugopal B, Manoj KS, Vidhya C. Hepatoid carcinoma of the pancreas combined with serous cystadenoma: a case report and review of the literature. Hepatobiliary Surg Nutr. 2015;4:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 39. | Williams NL, Palmer JD, Bar-Ad V, Anné PR, Sama AR, Weinstein JC, Rufail ML, Yeo CJ, Hurwitz MD. Hepatoid Carcinoma of the Pancreas: A Case Report and Review of the Literature. Case Rep Pancreat Cancer. 2015;1:3-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Stamatova D, Theilmann L, Spiegelberg C. A hepatoid carcinoma of the pancreatic head. Surg Case Rep. 2016;2:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Chang JM, Katariya NN, Lam-Himlin DM, Haakinson DJ, Ramanathan RK, Halfdanarson TR, Borad MJ, Pannala R, Faigel D, Moss AA, Mathur AK. Hepatoid Carcinoma of the Pancreas: Case Report, Next-Generation Tumor Profiling, and Literature Review. Case Rep Gastroenterol. 2016;10:605-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Akimoto Y, Kato H, Matsumoto K, Harada R, Oda S, Fushimi S, Mizukawa S, Yabe S, Uchida D, Seki H, Tomoda T, Yamamoto N, Horiguchi S, Tsutsumi K, Yagi T, Okada H. Pancreatic Hepatoid Carcinoma Mimicking a Solid Pseudopapillary Neoplasm: A Challenging Case on Endoscopic Ultrasound-guided Fine-needle Aspiration. Intern Med. 2016;55:2405-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Pellini Ferreira B, Vasquez J, Carilli A. Metastatic Hepatoid Carcinoma of the Pancreas: First Description of Treatment With Capecitabine and Temozolomide. Am J Med Sci. 2017;353:610-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Ma T, Bai X, Li G, Wei S, Liang T. Neoadjuvant modified-FOLFIRINOX followed by surgical resection of both the primary and metastatic tumors of a pancreatic hepatoid carcinoma with synchronous liver metastasis: A case report. Medicine (Baltimore). 2017;96:e8413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Yang C, Sun L, Lai JZ, Zhou L, Liu Z, Xi Y, Tao Y, Dooley E, Cao D. Primary Hepatoid Carcinoma of the Pancreas: A Clinicopathological Study of 3 Cases With Review of Additional 31 Cases in the Literature. Int J Surg Pathol. 2019;27:28-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Collan Y, Hakkiluoto A, Hästbacka J. Ectopic liver. Ann Chir Gynaecol. 1978;67:27-29. [PubMed] |
| 47. | Rao MS, Reddy JK. Hepatic transdifferentiation in the pancreas. Semin Cell Biol. 1995;6:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Shen CN, Horb ME, Slack JM, Tosh D. Transdifferentiation of pancreas to liver. Mech Dev. 2003;120:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Marchegiani G, Gareer H, Parisi A, Capelli P, Bassi C, Salvia R. Pancreatic hepatoid carcinoma: a review of the literature. Dig Surg. 2013;30:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 118] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 51. | Iida T, Wagatsuma K, Hirayama D, Yokoyama Y, Nakase H. The Etiology of Pancreatic Manifestations in Patients with Inflammatory Bowel Disease. J Clin Med. 2019;8:pii: E916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Wynne K, Devereaux B, Dornhorst A. Diabetes of the exocrine pancreas. J Gastroenterol Hepatol. 2019;34:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 53. | Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 420] [Article Influence: 24.7] [Reference Citation Analysis (0)] |