Published online Mar 26, 2020. doi: 10.12998/wjcc.v8.i6.1056
Peer-review started: November 11, 2019
First decision: January 17, 2020
Revised: February 24, 2020
Accepted: March 11, 2020
Article in press: March 11, 2020
Published online: March 26, 2020
Processing time: 135 Days and 13.6 Hours
A hybrid operating room (hybrid-OR) is a surgical space that combines a conventional operating room with advanced medical imaging devices.
To explore and summarize the technical features and effectiveness of the application of a hybrid-OR in dealing with spinal dural arteriovenous fistulas (SDAVFs).
Eleven patients with SDAVFs were treated with the use of a hybrid-OR at the Department of Neurosurgery of our hospital between January 2015 and December 2018. The dual-marker localization technique was used in the hybrid-OR to locate the SDAVFs and skin incision, and the interoperative digital subtraction angiography (DSA) technique was used before and after microsurgical ligation of the fistulae in the hybrid-OR to verify the accuracy of obliteration. The patients were followed for an average of 2 years after the operation, and the preoperative American Spinal Cord Injury Association (ASIA) score and postoperative ASIA score at 6 mo after the operation were compared.
The location and skin incision of the SDAVFs were accurately obtained by using the dual-marker localization technique in the hybrid-OR in all patients, and there were no cases that required expansion of the range of the bone window in order to expose the lesions. Intraoperative error obliteration occurred and was identified in two patients by using the intraoperative DSA technique; therefore, the findings provided by the intraoperative DSA system significantly changed the surgical procedure in these two patients. With the assistance of the hybrid-OR, the feeding artery was correctly ligated in all cases, and the intraoperative error obliteration rate decreased from 18.2% (2/11) to 0%. All 11 patients were followed for an average of 2 years. The ASIA score at 6 mo after the operation was significantly improved compared with the preoperative ASIA score, and there were no patients with late recurrence during the follow-up.
Compared with intra-arterial embolization for the treatment of SDAVFs, hybrid-ORs can solve the problem of a higher incidence of initial failure and late recurrence. Compared with direct occlusion of SDAVFs in microsurgery, hybrid-ORs can take advantage of the intraoperative DSA system for locating the shunt and verifying the obliteration of fistulae in order to reduce the error obliteration rate. At this point, our experience suggests that the safety and ease of use make hybrid-ORs combined with microsurgery and intraoperative DSA systems an attractive modality for dealing with SDAVFs.
Core Tip: We retrospectively analyzed our single-institution 4-year case series study on spinal dural arteriovenous fistulas (SDAVFs) treated in a hybrid operating room (hybrid-OR). We aimed to explore and summarize the technical features and effectiveness of the application of a hybrid-OR in dealing with SDAVFs. Using the dual-marker localization technique in a hybrid-OR is highly effective and time-saving for locating SDAVFs, and the use of the interoperative digital subtraction angiography technique in hybrid-ORs can significantly reduce the error obliteration rates of the microsurgery procedure.
- Citation: Zhang N, Xin WQ. Application of hybrid operating rooms for treating spinal dural arteriovenous fistula. World J Clin Cases 2020; 8(6): 1056-1064
- URL: https://www.wjgnet.com/2307-8960/full/v8/i6/1056.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i6.1056
Vascular malformations of the spinal cord represent a rare clinical entity with profound clinical implications. Among these infrequent lesions, spinal dural arteriovenous fistulas (SDAVFs) are the most common. There is an increasing tendency to treat SDAVFs endovascularly despite the lack of clear evidence favoring embolization over surgery over the last 20 years. Recently, a large number of papers have confirmed that open surgery is associated with significantly low odds of initial treatment failure and late recurrence[1]. There are some deficits in the use of endovascular treatment for SDAVFs, such as initial treatment failure, inability to achieve total SDAVF occlusion, and late recurrence.
Therefore, it is still uncertain whether open surgery is an ideal means to treat SDAVFs. Papers have reported that the penetrating portion of SDAVFs is not readily evident on preoperative digital subtraction angiography (DSA) images in most cases, as DSA lacks information about the spinal cord or dura mater[2]. Therefore, intraoperative DSA is important for open microsurgery because the combination of microscope views and intraoperative DSA views allows surgeons to fully understand the detailed relationship between arteriovenous fistulas (AVFs) and the spinal cord or dura and to accurately assess the clipping effect.
As an intraoperative imaging modality, indocyanine green (ICG) video angiography has been introduced for spinal arteriovenous lesions, but most studies have examined spinal dural AVFs, and few studies have reported on spinal perimedullary AVFs[3,4]. The aim of the present study was to assess the influence of intraoperative ICG video angiography on the surgical strategy decision for the microsurgical treatment of spinal perimedullary AVFs. We present a series of patients with SDAVFs treated in hybrid operating rooms (Hybrid-ORs) to more fully understand the optimal approach for managing these lesions.
During the period from January 2015 to December 2018, 11 patients with SDAVFs (7 male and 4 female patients) were treated at the Department of Neurosurgery of Tianjin Medical University General Hospital, and hybrid-ORs were used for treatment. The age of these patients ranged from 43-78 years old with a mean of 59.7 years. All patients underwent spinal DSA following a spinal magnetic resonance angiography (MRA) study suggesting SDAVFs. The leading complaint was paraparesis followed by sensory impairment, sphincter dysfunction, and progressive myeloradiculopathy.
A total of four of the eleven patients were initially treated by endovascular embolization, but the treatment failed. These four patients included three initial failure patients (Cases 1, 4, and 7) and one late recurrence patient (Case 3). The remaining seven patients chose microsurgery in the hybrid-OR as the primary treatment, and in these patients, endovascular intervention was not attempted. A summary of the demographic and clinical characteristics of the 11 patients is shown in Table 1.
| Case No. | Age (yr) sex | Presentation | Corresponding artery of AV shunt | Embolization before hybrid-OR treatment | Error obliteration found by intraoperative DSA | Pre-operative ASIA score | Post-operative ASIA score at 6-mo follow-up |
| 1 | 64, F | Paraparesis | T-9 intercostal artery (L) | Initial failure | None | C | E |
| 2 | 66, F | Sensory impairment | T-5 intercostal artery (R) | None | None | C | D |
| 3 | 43, M | Paraparesis | T-6 intercostal artery (L) | Late recurrence | None | D | E |
| 4 | 54, M | Sensory impairment | T-8 intercostal artery (L) | Initial failure | None | C | D |
| 5 | 52, F | Sphincter dysfunction | T-12 subcostal artery (R) | None | None | B | D |
| 6 | 78, M | Paraparesis | T-8 intercostal artery (L) | None | None | D | D |
| 7 | 62, M | Paraparesis | T-7 intercostal artery (R) | Initial failure | None | C | D |
| 8 | 73, M | Sensory impairment | T-10 intercostal artery (L) | None | Error obliteration | C | E |
| 9 | 57, F | Myeloradiculopathy | T-8 intercostal artery (L) | None | None | C | D |
| 10 | 61, M | Sensory impairment | C-8 radicular artery (L) | None | None | D | E |
| 11 | 57, M | Sphincter dysfunction | L-1 lumbar artery (R) | None | Error obliteration | D | E |
First, hybrid-ORs were prepared preoperatively, and all of the patients were placed in the supine position after general anesthesia. Unilateral femoral puncture was performed using the Seldinger technique, and then a Cobra catheter (4-F/C2) was placed into the responsible intercostal artery with continuous flushing with 5000 U heparin per liter of normal saline solution. Neither preprocedural nor intraprocedural antiplatelet systemic heparinization agents were administered.
Second, the patients were changed to the prone position to locate the SDAVFs. Combined with the dual-marker localization technique, the localization and skin incision of SDAVFs were accurately obtained under dynamic monitoring of flat-panel fluoroscopy by using the DSA system. Regarding the dual-marker localization technique, a catheter (first marker) was deployed into the responsible intercostal artery as an imaging reference for the SDAVF, and then a paperclip (second marker) was placed on the back as a landmark for the skin incision in order to find the corresponding vertebrae with the catheter. Then, a small syringe needle was inserted into the supraspinous ligament surrounding the spinous process, and fluoroscopy was performed again. If the location was accurate, methylene blue dye was injected.
Finally, the patients underwent surgical exposure of the lesions through a spinal approach. Before microsurgical ligation of the fistulae, intraoperative DSA was administered to identify the fistulous point. If the ligation was not ideal, the temporary arterial clip was readjusted, and intraoperative DSA verification was repeated until accurate obliteration of arterial flow was achieved.
The location and skin incision of the SDAVFs were accurately obtained by using the dual-marker localization technique in the hybrid-OR in all patients, and there were no cases that required expansion of the range of the bone window in order to expose the lesions. Intraoperative error obliteration occurred and was identified in two cases by using the intraoperative DSA technique; therefore, the findings provided by the intraoperative DSA system significantly changed the surgical procedure in these two patients (Cases 8 and 11). With the assistance of the hybrid-OR, the feeding artery was ligatured correctly in all cases, and the intraoperative error obliteration rate decreased from 18.2% (2/11) to 0%. There were no neurological deficits or serious complications after surgery with the use of the hybrid-OR. The operation time was 20-30 min shorter than it was with surgery with the use of conventional intraoperative C-arm fluoroscopy.
All 11 patients were followed for an average of 2 years. The American spinal cord injury association (ASIA) score at 6 mo after the operation was significantly improved compared with the preoperative ASIA score, and there were no patients with late recurrence during the follow-up.
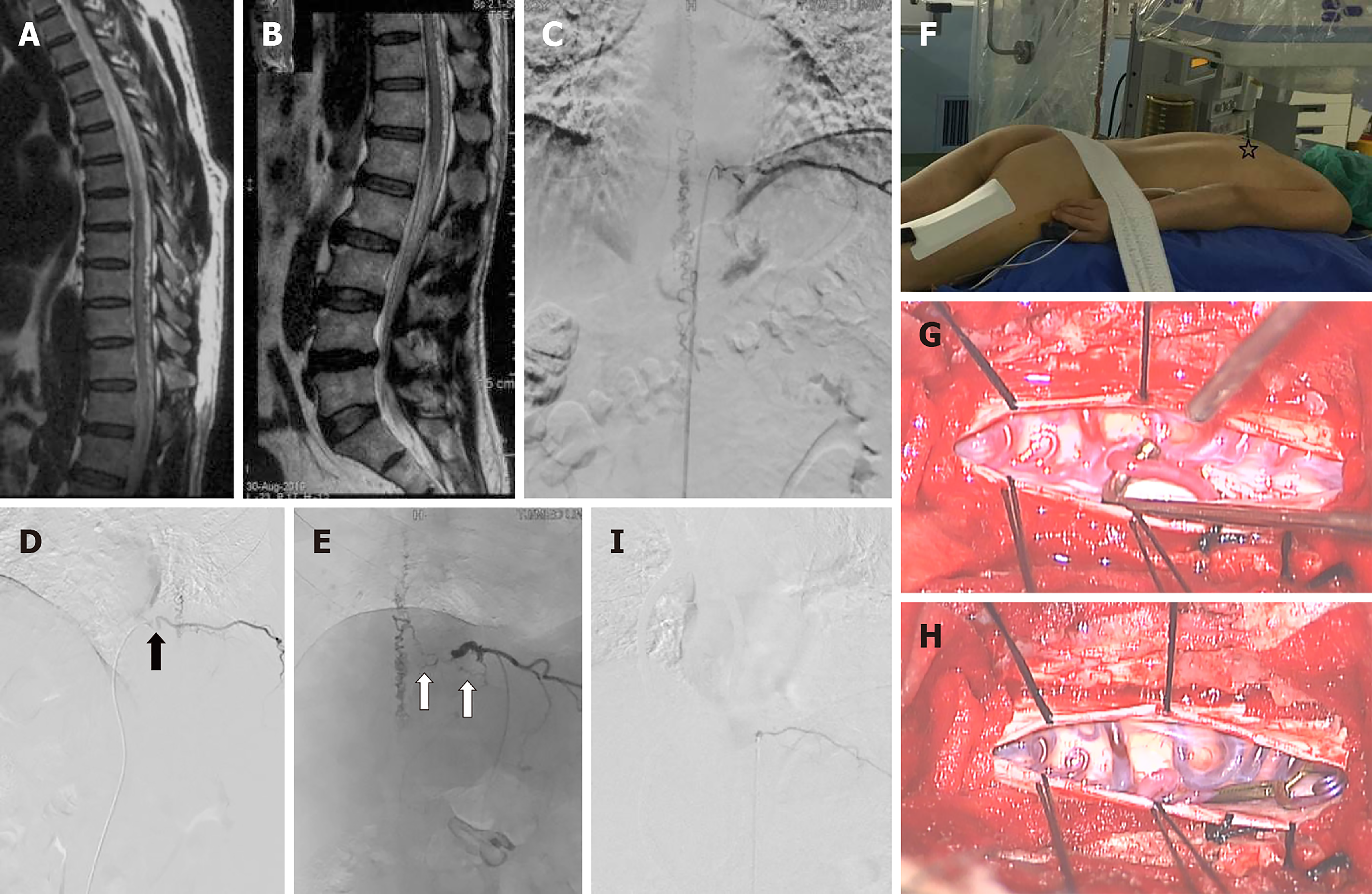
Case 1: A 52-year-old woman presented with a 6-mo history of paraparesis. Magnetic resonance imaging and DSA provided the diagnosis of a left T-9 SDAVF (Figure 1A and 1B). She was offered endovascular embolization as her primary treatment. However, endovascular embolization failed because there was S-shaped tortuosity at the beginning of the intercostal artery, and the feeding artery of the SDAVF originating from the intercostal artery was very thin and tortuous (white arrow) (Figure 1C-E). Then, she was offered microsurgery in a hybrid-OR. The localization of the SDAVF was accurately obtained using the dual-marker localization technique under dynamic flat-panel fluoroscopy (Figure 1F). During the operation, the suspected feeding artery was found and clipped temporarily under the microscope, and intraoperative DSA confirmed that the SDAVF had been completely clipped. The intraoperative findings (Figure 1G and 1H) corresponded exactly with the intraoperative DSA images (Figure 1I) in the hybrid-OR.
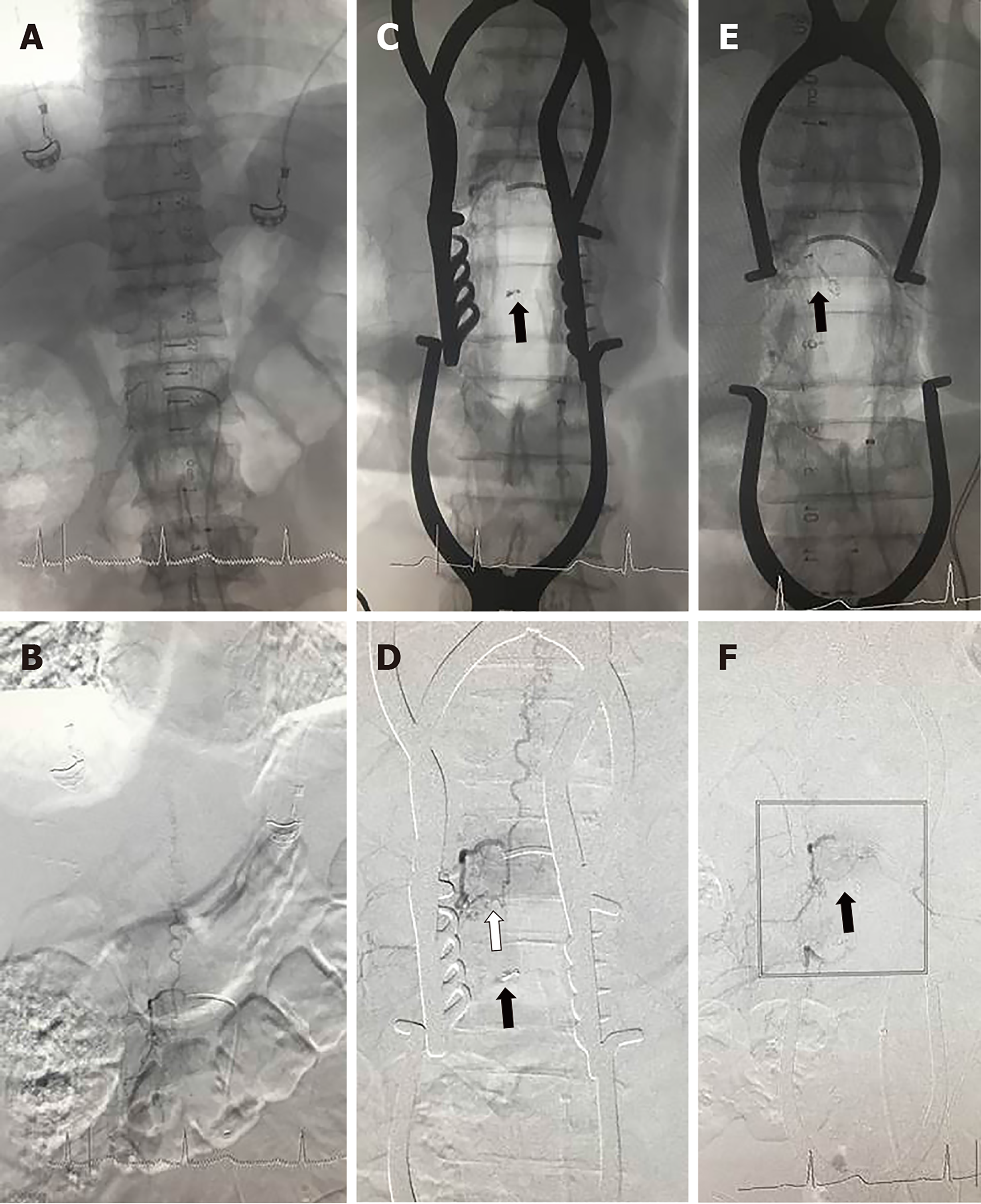
Case 11: A 57-year-old man presented with sphincter dysfunction that had progressed over 11 mo. A magnetic resonance imaging (MRI) study showed L2 signal-intensity changes at T7–L2 with surrounding flow voids. Preoperative DSA showed an arteriovenous shunt between the right L-1 intercostal artery and the intradural coronal venous plexus. He was offered both endovascular treatment options and microsurgery in the hybrid-OR. After fully considering the risks and benefits, the patient chose microsurgery in the hybrid-OR.
Based on preoperative DSA, the location of the AVF was predicted to be in the dura at the right L-1 dorsal root (Figure 2A and 2B). During the operation, the suspected feeding artery arising from the right root sleeve was found initially and then clipped temporarily under the microscope; however, the first intraoperative DSA displayed that the fistula (white arrow) was still visualized and that the microvascular clip (black arrow) was below the right fistula (Figure 2C and 2D). Then, the neurosurgeons continued to explore the lesions toward the head, found the correct responsible feeding artery, and clipped it. The second intraoperative DSA showed that the SDAVF had completely disappeared, and the microvascular clip (black arrow) was adjusted at the right fistula (Figure 2E and 2F).
Although SDAVFs are rare, they are the most common spinal vascular malformation and are responsible for 70% of spinal cord arteriovenous shunts[5]. SDAVFs are low-flow lesions and can cause venous hypertension and chronic spinal cord hypoxia. These patients present with progressive myelopathy. Paraparesis is often the initial presentation, followed by root and/or back pain, sensory impairment, and sphincter dysfunction. SDAVFs can be treated surgically or by endovascular embolization, or a combination of surgery and endovascular techniques may be used[6,7].
Endovascular intervention has developed from an adjunct to a potential alternative to surgery[8]. Improved embolic agents such as ethylene vinyl alcohol copolymer have increased the number of lesions that can be considered for endovascular intervention.
However, the technical limitations of embolic treatment have been considered in our cohort and previous literature. In some cases, access to feeding vessels is impossible with endovascular embolization[9]. Specifically, the intercostal artery in which the feeding artery originates from or the feeding artery to the fistula is so tortuous or thin that the microcatheter cannot reach the fistula. As shown in Case 1, because of the S-shaped tortuosity at the beginning of the intercostal artery, the guiding catheter could not be stabilized in the intercostal artery, and the endovascular procedure failed. In other cases, the use of an anterior spinal artery originating from the same level as SDAVF endovascular embolization was unsafe for SDAVFs[10]. The above reasons limit the generalizability of endovascular embolization to all SDAVFs.
Moreover, the efficacy and overall durability of endovascular intervention remain inferior to those of surgical occlusion. In the past several years, endovascular techniques and materials have generally improved. In addition, new liquid embolic agents such as Onyx, showing good results in the transarterial embolization of cranial SDAVFs, have become available. In recent years, three meta-analysis articles suggested that for the treatment of SDAVFs, surgery might be superior to embolization. Surgery is usually successful, and recurrence and complications are rare. These articles also suggested that endovascular intervention might be a reasonable initial option; however, this technique was associated with a high rate of initial failure and recurrence.
Previous meta-analyses by Steinmetz et al[11] in 2004 and Bakker et al[7] in 2015 showed treatment success rates of 46% and 72.2%, respectively, with endovascular embolization. Compared with these results, the updated meta-analysis by Goyal et al[1] in 2019 showed a success rate of approximately 80% with stand-alone embolization. The higher success rate observed in our review might be a reflection of advances in endovascular techniques and new endovascular agents, such as NBCA or ONYX. Even though the rate of complete occlusion with embolic treatment has progressively increased, given reported studies, the rate of initial failure is over 30%, and the rate of recurrence is over 45% with Onyx treatment and over 23% with NBCA treatment.
DSA has been regarded as the gold standard for diagnosing spinal vascular malformations[12]. No other techniques provide results comparable to DSA in terms of the sensitivity and specificity of diagnosis for these lesions. The goal of treatment of SDAVFs is permanent and complete obliteration of arterial flow through the arteriovenous shunt, so accurate localization of the fistulae and skin incision is very important for SDAVF surgery. Incorrect segment localization occurs in clinical practice at times, particularly when the lesions are small in size or located in the thoracic vertebral canal[13]. First, because there is no occupying effect on MRI, it is more difficult to accurately locate SDAVFs than the occupying lesions before surgery. Second, because preoperative DSA can only provide the predicted location of the shunt, the accurate location of the shunt can be confirmed only by probing and temporarily clipping the feeding artery and by intraoperative DSA or intraoperative ICG verification during the operation. If the preoperative location is not accurate, it may be necessary to open additional segments of the lamina to find the fistula in order to expose the lesions during the operation, which increases the incidence of perioperative complications such as cerebrospinal fluid leakage.
Conventional methods for the localization of spinal lesions involve preoperative X-ray and intraoperative continuous C-arm fluoroscopy. The localization needs to repeatedly adjust the position and projection angle of the C-arm even with intraoperative continuous C-arm fluoroscopy. With a lack of imaging references, the localization remains largely dependent on the surgeon’s experience in counting the vertebrae, beginning at either the cervical-thoracic junction or the sacrum. This practice exposes the patients and operating room personnel to significant radiation[14].
Intraoperative ICG video angiography has a significant impact on deciding the surgical strategy for the microsurgical treatment of patients with SDAVFs and spinal perimedullary AVFs (SPAVFs). In patients with SDAVFs, the surgical strategy can be changed during surgery based on intraoperative ICG video angiography findings to correctly occlude the fistula. However, it is important to note that surgeons can only visualize what neurosurgeons see under the operating microscope with ICG video angiography. It is important to fully expose the entire venous drainage of the fistula depending on the angiography results for the documentation of complete obliteration[4]. Therefore, compared with intraoperative DSA, intraoperative ICG video angiography is not convenient or direct.
Because of this technological breakthrough, current surgical limitations have been overcome with hybrid-ORs while expanding the potential of surgery. Hybrid-ORs provide neurosurgeons with a brand new, intraoperative, image-guided environment that alleviates the limitations of traditional operating rooms and DSA systems when they function individually.
First, although endovascular treatment has become the first-line treatment for most SDAVFs, open microsurgery remains the best available means of obliterating the fistula in some cases. Compared with intra-arterial embolization in the treatment of SDAVFs, microsurgery in hybrid-ORs solves the problem of a significantly higher incidence of initial failure and late recurrence. As shown in our cohort, three initial failure patients (Cases 1, 4 and 7) and one late recurrence patient (Case 3) were cured successfully by microsurgery in the hybrid-OR.
Second, compared to the C-arm system, the use of a hybrid-OR was more accurate and efficient, and it largely reduced the radiation exposure of personnel when locating the lesion using the dual-marker localization technique under dynamic flat-panel fluoroscopy. The locations of lesions were more intuitive because the lesions could be visualized by intraoperative DSA. The localization of the skin incision was more accurate, because the catheter was placed into the responsible intercostal artery as an imaging reference. Furthermore, because the receiver area of the flat panel was larger than that of the C-arm system, localization using flat-panel fluoroscopy did not require repeated adjustment of the position and projection angle and did not need to count the vertebrae such as when using the C-arm system. The location information was obtained continuously and dynamically by moving the position and projection angle of the DSA machine, which was very accurate and efficient.
Third, we only left the patients in the hybrid-OR, and the other personnel went into the monitoring room outside the hybrid-OR during the location and intraoperative DSA process. The application accuracy of the intraoperative DSA system could satisfy almost all of the lesion localization needs of spinal surgery, and it could reduce the radiation exposure of hybrid-OR personnel.
In addition, multiple adjuncts have been used in the surgical management of SDAVFs to verify their obliteration. These methods include direct visualization under an operating microscope, microvascular Doppler sonography, intraoperative ICG, or intraoperative DSA[15,16]. There has been great uncertainty in the use of direct visualization under an operating microscope or microvascular Doppler sonography, and this was the reason why many patients' symptoms could not be relieved or even worsened postoperatively. Previous studies have reported that intraoperative ICG or intraoperative DSA is a useful adjunct for the surgical management of SDAVFs in terms of localization and the confirmation of complete obliteration[4,17,18]. In 2005, Lawton et al[19] demonstrated that ICG corresponded to DSA in approximately 90% of cases, with only 2.7% surgically significant differences in aneurysm cases. In 2010, Schuette et al[4] demonstrated that the surgeon could only visualize what he or she saw under the operating microscope with intraoperative ICG. Therefore, it was important to fully expose the entire venous drainage of the fistula based on ICG for the documentation of complete obliteration. At the same time, it meant that if the shunt of the fistula or venous drainage was located in the ventral side of the spinal cord or nerve root, the effect of ICG observation would be unsatisfactory. Since intraoperative DSA is a type of fluoroscopy imaging, it is not limited by imperfect exposure or spinal cord obstruction. Therefore, it is becoming clearer and more accurate to verify the effect of shunt ligation. With the assistance of intraoperative DSA, we significantly reduced the error obliteration rate from 18.2% to 0%.
In conclusion, compared with intra-arterial embolization for the treatment of SDAVFs, hybrid-ORs can solve the problem of a higher incidence of initial failure and late recurrence. Compared with the direct occlusion of SDAVFs in microsurgery, hybrid-ORs can take advantage of the intraoperative DSA system for locating the shunt and verifying the obliteration of the fistula in order to reduce the error obliteration rate. At this point, our experience suggests that the safety and ease of use make hybrid-ORs combined with microsurgery and intraoperative DSA systems an attractive modality for dealing with SDAVFs.
A hybrid-OR is a surgical space that combines a conventional operating room with advanced medical imaging devices.
Currently, there is no standard of care of established evidence on the application of hybrid operating rooms in the treatment of spinal dural arteriovenous fistulas.
The purpose of this study was to explore and summarize the technical features and effectiveness of the application of hybrid-ORs in dealing with spinal dural arteriovenous fistulas (SDAVFs).
We treated 11 patients with SDAVFs between January 2015 and December 2018 with the use of hybrid-ORs. The dual-marker localization technique was used in the hybrid-OR to locate the SDAVFs and skin incision, and the interoperative DSA technique was used before and after microsurgical ligation of the fistulae in the hybrid-OR to verify the obliteration accuracy.
The location and skin incision of the SDAVFs were accurately obtained by using the dual-marker localization technique in the hybrid-OR in all patients, and there were no cases that required expansion of the range of the bone window in order to expose the lesions. Intraoperative error obliteration occurred and was identified in two cases by using the intraoperative DSA technique; therefore, the findings provided by the intraoperative DSA system significantly changed the surgical procedure in these two patients. With the assistance of the hybrid-OR, the feeding artery was ligatured correctly in all cases, and the intraoperative error obliteration rate decreased from 18.2% (2/11) to 0%. All 11 patients were followed for an average of 2 years. The ASIA score at 6 mo after the operation was significantly improved compared with the preoperative ASIA score, and there were no patients with late recurrence during the follow-up.
The hybrid-OR provides new ideas for the surgical treatment of SDVAFs, which effectively and greatly improves the cure rate. The safety and ease of use make hybrid-ORs an attractive modality for dealing with SDAVFs.
Although the hybrid-OR has received widespread attention for the treatment of SDVAFs, there are still no clear conclusions. This study shows that the safety and ease of use make hybrid-ORs combined with microsurgery and intraoperative DSA systems an attractive modality for dealing with SDAVFs.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chowdhury F, Velnar T S-Editor: Wang J L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Goyal A, Cesare J, Lu VM, Alvi MA, Kerezoudis P, Brinjikji W, Nasr D, Lanzino G, Bydon M. Outcomes following surgical versus endovascular treatment of spinal dural arteriovenous fistula: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2019;90:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Takai K, Kin T, Oyama H, Iijima A, Shojima M, Nishido H, Saito N. The use of 3D computer graphics in the diagnosis and treatment of spinal vascular malformations. J Neurosurg Spine. 2011;15:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Hettige S, Walsh D. Indocyanine green video-angiography as an aid to surgical treatment of spinal dural arteriovenous fistulae. Acta Neurochir (Wien). 2010;152:533-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Schuette AJ, Cawley CM, Barrow DL. Indocyanine green videoangiography in the management of dural arteriovenous fistulae. Neurosurgery. 2010;67:658-662; discussion 662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Krings T, Mull M, Gilsbach JM, Thron A. Spinal vascular malformations. Eur Radiol. 2005;15:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Afshar JK, Doppman JL, Oldfield EH. Surgical interruption of intradural draining vein as curative treatment of spinal dural arteriovenous fistulas. J Neurosurg. 1995;82:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 96] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Bakker NA, Uyttenboogaart M, Luijckx GJ, Eshghi OS, Mazuri A, Metzemaekers JD, Groen RJ, Van Dijk JM. Recurrence Rates After Surgical or Endovascular Treatment of Spinal Dural Arteriovenous Fistulas: A Meta-analysis. Neurosurgery. 2015;77:137-144; discussion 144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Narvid J, Hetts SW, Larsen D, Neuhaus J, Singh TP, McSwain H, Lawton MT, Dowd CF, Higashida RT, Halbach VV. Spinal dural arteriovenous fistulae: clinical features and long-term results. Neurosurgery. 2008;62:159-166; discussion 166-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Heldner MR, Arnold M, Nedeltchev K, Gralla J, Beck J, Fischer U. Vascular diseases of the spinal cord: a review. Curr Treat Options Neurol. 2012;14:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Koch MJ, Stapleton CJ, Agarwalla PK, Torok C, Shin JH, Coumans JV, Borges LF, Ogilvy CS, Rabinov JD, Patel AB. Open and endovascular treatment of spinal dural arteriovenous fistulas: a 10-year experience. J Neurosurg Spine. 2017;26:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Steinmetz MP, Chow MM, Krishnaney AA, Andrews-Hinders D, Benzel EC, Masaryk TJ, Mayberg MR, Rasmussen PA. Outcome after the treatment of spinal dural arteriovenous fistulae: a contemporary single-institution series and meta-analysis. Neurosurgery. 2004;55:77-87; discussion 87-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 199] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Rosenblum B, Oldfield EH, Doppman JL, Di Chiro G. Spinal arteriovenous malformations: a comparison of dural arteriovenous fistulas and intradural AVM's in 81 patients. J Neurosurg. 1987;67:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 356] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Mody MG, Nourbakhsh A, Stahl DL, Gibbs M, Alfawareh M, Garges KJ. The prevalence of wrong level surgery among spine surgeons. Spine (Phila Pa 1976). 2008;33:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Xiao X, Wu Z, Zhang L, Jia G, Zhang J, Tang J, Meng G. Using the C7-T3 spinous processes as landmarks for the localization of thoracic spinal lesions: technique notes. Neurosurg Rev. 2014;37:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Black KL, Rubin JM, Chandler WF, McGillicuddy JE. Intraoperative color-flow Doppler imaging of AVM's and aneurysms. J Neurosurg. 1988;68:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Padovani R, Farneti M, Maida G, Ghadirpour R. Spinal dural arteriovenous fistulas: the use of intraoperative microvascular Doppler monitoring. Br J Neurosurg. 2003;17:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Barrow DL, Boyer KL, Joseph GJ. Intraoperative angiography in the management of neurovascular disorders. Neurosurgery. 1992;30:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 161] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Xia Y, Ishii K, Nakamura M, Onozuka S, Ueda R, Matsumoto M, Chiba K, Toyama Y. The validity of intraoperative angiography for the treatment of spinal arteriovenous fistula. J Spinal Disord Tech. 2007;20:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Lawton MT, Sanchez-Mejia RO, Pham D, Tan J, Halbach VV. Tentorial dural arteriovenous fistulae: operative strategies and microsurgical results for six types. Neurosurgery. 2008;62:110-124; discussion 124-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |