Published online Mar 6, 2020. doi: 10.12998/wjcc.v8.i5.864
Peer-review started: November 26, 2019
First decision: December 30, 2019
Revised: January 6, 2020
Accepted: February 10, 2020
Article in press: February 10, 2020
Published online: March 6, 2020
Processing time: 101 Days and 1 Hours
Gestational diabetes mellitus (GDM) is a concern due to its rapid increase in incidence in recent years.
To investigate the correlation and predictive value of serum pregnancy-associated plasma protein A (PAPP-A), triglyceride (TG), and 25-hydroxyvitamin D [25-(OH)D] with GDM in early pregnancy.
A total of 99 patients in early pregnancy admitted to Peking University International Hospital from November 2015 to September 2017 were included, and underwent a fasting glucose test and oral glucose tolerance test screening at 24-28 wk of pregnancy. Of these cases with GDM, 51 were assigned to group A and the remaining 48 cases without GDM were enrolled in group B. Serum PAPP-A, TG and 25-(OH)D in the two groups were compared and their correlation with blood sugar was analyzed. In addition, their diagnostic value in GDM was determined using receiver operating characteristic (ROC) curve analysis.
Group A had markedly lower serum PAPP-A and 25-(OH)D levels and a significantly higher serum TG level than group B, with statistical significance (P < 0.05). Furthermore, Pearson analysis identified that PAPP-A and 25-(OH)D levels were negatively correlated with fasting blood glucose (FBG) levels (r = -0.605, P < 0.001), (r = -0.597, P < 0.001), while TG and FBG levels were positively correlated (r = 0.628, P < 0.001). The sensitivity, specificity, area under the curve (AUC) and optimal cut-off value of serum PAPP-A level in the diagnosis of GDM were 72.55%, 82.35%, 0.861 and 16.340, respectively, while the sensitivity of TG in diagnosing GDM was 86.27%, the specificity was 66.67%, the AUC was 0.813, with an optimal cut-off value of 1.796. The corresponding sensitivity, specificity, AUC and optimal cut-off value of serum 25-(OH)D were 64.71%, 70.59%, 0.721 and 23.140, respectively. Moreover, multivariate logistic regression analysis revealed that FBG, vascular endothelial growth factor, Flt-1, serum PAPP-A, TG, and 25-(OH)D were related risk factors leading to GDM in patients.
Serum PAPP-A, TG, and 25-(OH)D levels are all correlated with blood glucose changes in GDM, and are independent factors affecting the occurrence of GDM and have certain value in the diagnosis of GDM.
Core tip: In the present study, the serum levels of pregnancy-associated plasma protein A, triglyceride, and 25-hydroxyvitamin D levels in early pregnancy were evaluated. The results showed that these three parameters are potential influencing factors and can provide a certain predictive value for the occurrence of gestational diabetes mellitus. These factors have high value in clinical application.
- Citation: Ren Z, Zhe D, Li Z, Sun XP, Yang K, Lin L. Study on the correlation and predictive value of serum pregnancy-associated plasma protein A, triglyceride and serum 25-hydroxyvitamin D levels with gestational diabetes mellitus. World J Clin Cases 2020; 8(5): 864-873
- URL: https://www.wjgnet.com/2307-8960/full/v8/i5/864.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i5.864
Gestational diabetes mellitus (GDM) refers to abnormal glucose metabolism of different degrees during pregnancy, while the blood glucose level is below the standard of dominant diabetes[1]. Due to improvements in people's living standards, the prevalence of GDM is increasing year by year[2]. Studies have shown that the pathogenesis of GDM is approximately the same as that of type 2 diabetes mellitus, which is accompanied by insulin resistance and islet function decline[3]. GDM may have significant adverse effects on the mother, fetus and newborn during and after pregnancy. Mothers with GDM are at high risk of developing cardiovascular disease, preeclampsia, polyhydramnios and postpartum hemorrhage[4,5], while babies born to GDM women mainly suffer from metabolic diseases, respiratory distress syndrome, jaundice and hemocytosis[6,7]. To date, interventional treatment represented by diet and exercise control and necessary medicines remain the dominant approaches for early GDM patients (1-15 wk), which have improved the majority of maternal and infant outcomes clinically. In addition, it is well established that early treatment results in a more significant effect[8]. However, there is currently a lack of indicators to predict GDM in the early stage. The screening and diagnosis of GDM is mainly carried out at the end of the second trimester of 24 to 28 wk, and the cut-off point of detection before 24 weeks is not yet clear[9].
Recent studies have demonstrated that[10] serum pregnancy-associated plasma protein A (PAPP-A) level has a high sensitivity and specificity for pregnancy complications such as spontaneous abortion and gestational hypertension in early pregnancy. Increased triglyceride (TG) level can cause insulin resistance and promote islet β cell apoptosis[11]. Serum 25-hydroxyvitamin D [25-(OH)D] has been found to have a decreasing trend in type 2 diabetes, which has the effect of enhancing insulin sensitivity and improving insulin resistance[12].
Therefore, this study aims to identify the differences in serum PAPP-A, TG and 25-(OH)D levels between GDM patients and normal subjects in early pregnancy, and to analyze their relationship with blood glucose levels, as well as their clinical value in predicting the occurrence of GDM, in order to provide direction and evidence for early intervention in the treatment of GDM.
A total of 99 patients in early pregnancy admitted to Peking University International Hospital from November 2015 to September 2017 were included in this study, and all underwent fasting glucose testing and oral glucose tolerance test (OGTT) screening at 24-28 wk. Of these cases, 51 with GDM were assigned to group A and the remaining 48 cases without GDM were enrolled in group B. There were 62 primiparas and 37 multiparas, with an average age of 28.24 ± 6.34 yr, a body mass index of 23.43 ± 2.63 kg/m2, and an average gestational age of 7-11 wk.
Inclusion criteria: Patients in early pregnancy who fulfilled any one of the following criteria were included: Fasting venous blood with 5.1 mmol/L ≤ empty abdominal blood glucose < 7. 0 mmol/L during pregnancy of 8-12 wk or those with blood glucose level ≥ 10.0 mmol/L after 1 h of 75 g OGTT, and 8.5 mmol/L ≤ OGTT 2 h blood glucose < 11.1 mmol/L at 24-28 wk of late pregnancy[13,14]. Exclusion criteria: (1) Patients with gestational hypertension; (2) Patients with other important organ dysfunction; (3) Patients with coronary heart disease; (4) Patients with glycometabolism and liver dysfunction; (5) Patients with thyroid dysfunction; and (6) Patients with incomplete pathological data. All patients and their families agreed to participate in the experiment and signed the informed consent. This experiment was approved by the Peking University International Hospital Medical Ethics Committee.
A serum separation centrifuge was purchased from Guangdong Foheng Instrument Co., Ltd., and an ELISA kit was acquired from Shanghai Yingxin Laboratory Equipment Co., Ltd. 25-(OH)D antibody was obtained from Shanghai Qincheng Biotechnology Co., Ltd., and the ELISA coloration agent from Beijing Bioss Biotechnology Co., Ltd. A micrometer was purchased from Wuhan Alilu Medical Instrument Co., Ltd., and an automatic time-resolved fluorescence immunoanalyzer from Perkin Elmer Enterprise Management (Shanghai) Co., Ltd.
Five milliliters of fasting venous blood was obtained from all subjects in the early morning, centrifuged at 3000 r/min for 6 min, and the obtained serum was then sealed in the refrigerator at -80 °C until testing. ELISA was carried out to assess 25-(OH)D level as follows: 200 µL serum samples from the two groups were added to porous plates coated with anti-25-(OH)D antibodies, and standard and blank wells were set up. The wells were then reacted at 37°C for 65 min and washed 3 times. This was followed by the addition of 100 µL coloration agent and allowed to react at room temperature for 20 min. Then, 100 µL of the mixture was removed and placed in the enzymatic labeling apparatus to measure and record the average optical density of each well. Serum PAPP-A level was determined using a fully automatic time-resolved fluorescence immunoassay analyzer, and TG level was determined by an automatic biochemical analyzer.
(1) Serum PAPP-A, TG and 25-(OH)D levels in group A and group B were compared; (2) The correlation between PAPP-A, TG and 25-(OH)D levels and fasting glucose indicators in GDM patients was analyzed; and (3) The diagnostic value of PAPP-A, TG and 25-(OH)D levels in GDM was determined using ROC curve analysis.
The experimental data were statistically analyzed using SPSS 19.0 statistical software (Beijing Network Data Times Technology Co., Ltd.). The counted data were measured by the χ2 test, and the measurement data were expressed by mean ± SD. The T test was used for inter-group comparison, and Pearson correlation analysis was used to analyze the correlation between PAPP-A, TG and 25-(OH)D levels and blood glucose. ROC curve analysis was adopted to determine the diagnostic value of PAPP-A, TG and 25-(OH)D levels in GDM, and Graphpad Prism8 was used for picture rendering in this experiment. P < 0.05 was considered statistically significant.
No significant differences in body mass index or gestational age between the two groups were observed. Significant differences in FBG, OGTT 2 h, vascular endothelial growth factor (VEGF), and vascular endothelial growth factor receptor 1 (Flt-1) expression were observed during pregnancy (P < 0.05) (Table 1).
| Groups | Group A (n = 51) | Group A (n = 48) | χ2/t | P value |
| Age (yr) | 28.54 ± 6.14 | 28.21 ± 6.31 | 0.264 | 0.793 |
| BMI (kg/m2) | 23.24 ± 2.58 | 23.53 ± 2.69 | 0.548 | 0.585 |
| Parity | 10.891 | 0.001 | ||
| Primipara | 24 (47.06) | 25 (52.08) | 0.500 | 0.480 |
| Multipara | 27 (52.94) | 23 (47.92) | ||
| Gestational age (wk) | 9.87 ± 1.83 | 9.93 ± 1.87 | 0.161 | 0.872 |
| Delivery mode (case) | 0.020 | 0.888 | ||
| Vaginal delivery | 25 (49.02) | 23 (47.92) | ||
| Cesarean delivery | 26 (50.98) | 25 (52.08) | ||
| Cholesterol (mmol/L) | 5.45 ± 1.34 | 5.25 ± 1.42 | 0.721 | 0.473 |
| Triglyceride (mmol/L) | 1.59 ± 1.01 | 1.57 ± 0.98 | 0.100 | 0.921 |
| Low density cholesterol (mmol/L) | 1.38 ± 0.14 | 1.41 ± 0.16 | 0.994 | 0.323 |
| High density cholesterol (mmol/L) | 3.98 ± 1.37 | 4.23 ± 1.42 | 0.892 | 0.375 |
| FBG during pregnancy (mmol/L) | 5.64 ± 0.42 | 4.37 ± 0.39 | 15.560 | < 0.001 |
| OGTT 2 h | 10.23 ± 1.43 | 7.34 ± 0.85 | 12.130 | < 0.001 |
| Glycosylated hemoglobin (%) | 7.24 ± 1.35 | 6.02 ± 1.02 | 5.049 | < 0.001 |
| VEGF (pg/mL) | 183.63 ± 23.24 | 129.23 ± 17.34 | 13.140 | < 0.001 |
| Flt-1 (pg/mL) | 2453.62 ± 823.24 | 1536.69 ± 782.74 | 5.672 | < 0.001 |
Serum PAPP-A and 25-(OH)D levels in group A were significantly lower than those in group B, and these differences were statistically significant (P < 0.05). The TG level in group A was significantly higher than that in group B, and the difference was statistically significant (P < 0.05) (Table 2).
| Groups | Group A (n = 51) | Group A (n = 48) | t | P value |
| PAPP-A (ng/L) | 13.84 ± 2.03 | 16.96 ± 2.45 | 4.699 | < 0.001 |
| TG (mmol/L) | 2.12 ± 0.61 | 1.32 ± 0.56 | 6.785 | < 0.001 |
| 25-(OH)D (ng/mL) | 20.34 ± 5.13 | 24.52 ± 5.42 | 3.942 | < 0.001 |
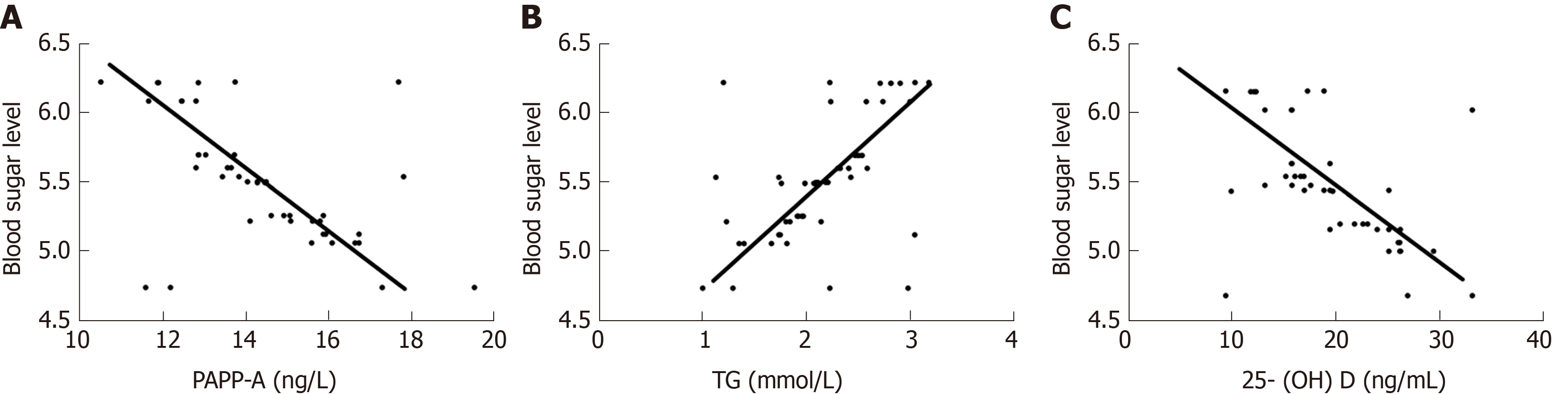
Pearson analysis showed that PAPP-A was negatively correlated with FBG (r = -0.605, P < 0.001), TG was negatively correlated with FBG (r = 0.558, P < 0.001), and 25-(OH)D was positively correlated with FBG (r = -0.629, P < 0.001) (Figure 1).
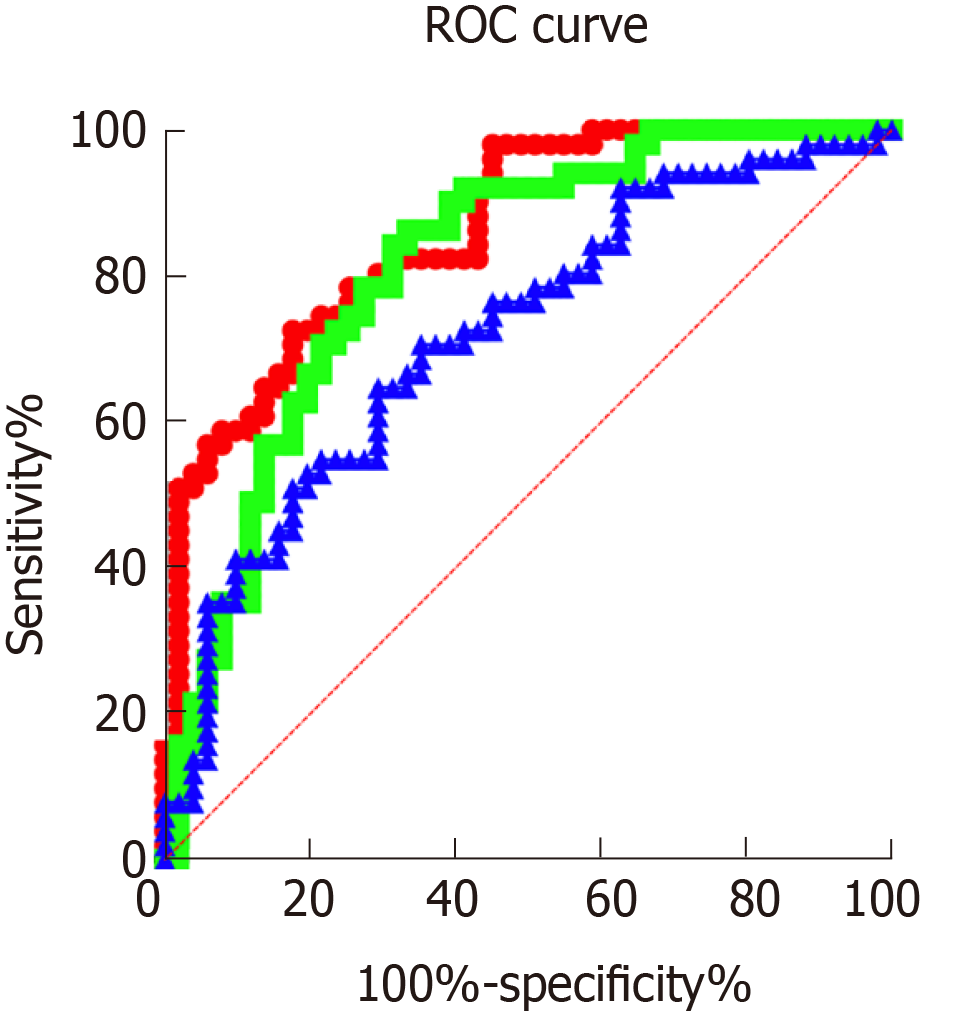
The sensitivity, specificity, AUC and optimal cut-off value of serum PAPP-A level in the diagnosis of GDM were 72.55%, 82.35%, 0.861 and 16.340, respectively, while those of TG level were 86.27%, 66.67%, 0.813 and 1.796, respectively. The corresponding sensitivity, specificity, AUC and optimal cut-off value of serum 25-(OH)D were 64.71%, 870.59%, 0.721 and 23.140, respectively (Figure 2).
The independent variables represented by the indicators with differences in Table 1 and Table 2 [FBG during pregnancy, VEGF, Flt-1, serum PAPP-A, 25-(OH)D, TG], and the dependent variable represented by the occurrence of GDM were included in the assignment (the assignment table is shown in Table 3) for multivariate logistic regression analysis. The results identified that VEGF (OR = 1.723, 95%CI: 0.874-3.013), Flt-1 (OR = 0.974, 95%CI: 0.722-1.332), FBG (OR = 1.782, 95%CI: 1.036-3.052), serum PAPP-A (OR = 2.530, 95%CI: 1.348-4.791), 25-(OH)D (OR = 1.259, 95%CI: 1.079-1.457), and TG (OR = 0.695, 95%CI: 0.508-0.964) were risk factors for GDM (Table 4).
| Factors | Assignments |
| VEGF | Raw data analysis for continuous variables |
| Flt-1 | Raw data analysis for continuous variables |
| FBG during pregnancy | Raw data analysis for continuous variables |
| Serum PAPP-A | Raw data analysis for continuous variables |
| 25-(OH)D | Raw data analysis for continuous variables |
| TG | Raw data analysis for continuous variables |
| Factors | B | SE | Wals | Sig. | Exp (B) | 95%CI for Exp (B) | |
| Lower | Upper | ||||||
| VEGF | 0.563 | 0.281 | 2.762 | 0.044 | 1.723 | 0.874 | 3.013 |
| Flt-1 | -0.023 | 0.142 | 2.642 | 0.041 | 0.974 | 0.722 | 1.332 |
| FBG during pregnancy | 0.572 | 0.279 | 4.028 | 0.042 | 1.782 | 1.036 | 3.052 |
| Serum PAPP-A | 0.937 | 0.328 | 5.028 | 0.026 | 2.530 | 1.348 | 4.791 |
| 25-(OH)D | 0.228 | 0.078 | 4.028 | 0.046 | 1.259 | 1.079 | 1.457 |
| TG | -0.362 | 0.169 | 3.978 | 0.044 | 0.695 | 0.508 | 0.964 |
As a common complication of pregnancy, GDM is caused by glucose metabolism disorder due to a decrease in insulin sensitivity in women during pregnancy, which markedly increases the risk of type 2 diabetes mellitus among pregnant women with GDM after delivery, endangering maternal and infant health[15]. The inextricable relationship between the onset of GDM and insulin resistance lies in the fact that, insulin and biological responses are lower than the lower limit of the normal prediction level, which is the main reason for the onset of GDM. All pregnant women have stronger insulin resistance during the middle and third trimester of pregnancy, but only some pregnant women develop GDM[16,17]. Increased insulin resistance results in increased demand for insulin in the body, which leads to a relative shortage of insulin, resulting in the balance of blood glucose control being disrupted and blood glucose level is elevated, increasing the possibility of GDM[18]. However, there is currently relatively little research on the early diagnosis of GDM. Therefore, this study aimed to determine the relationship between GDM and PAPP-A, TG and 25-(OH)D levels in early pregnancy, in order to provide directions for clinical prediction of early GDM occurrence and interventional treatment to improve maternal and infant health in the perinatal period.
Firstly, the PPAP-A, TG and 25-(OH)D levels in patients with early GDM and normal pregnant women were compared in this study. It was observed that group A had significantly lower serum PAPP-A and 25-(OH)D levels and a significantly higher TG level than group B. PAPP-A is a type of hydrolytic enzyme found in the plasma of pregnant women with high levels of protein, and is associated with insulin-like growth factor. During pregnancy, PAPP-A is mainly synthesized and released by placental syncytiotrophoblast and decidual cells, and is then activated in the maternal blood circulation. As the pregnancy progresses, the content of PAPP-A released gradually increases, and shows a decreasing trend in patients with insulin resistance[19,20]. 25-(OH)D is a fat-soluble steroid derivative, and its abnormal secretion may be closely related to insulin resistance or type 2 diabetes[21]. Acute elevated TG levels can cause excessive secretion of insulin in patients, and long-term maintenance of high levels will lead to increased dysfunctional secretion and increased islet β cells apoptosis, affecting disordered blood glucose levels in patients[22]. The results of our study were consistent with the literature stated above, where GDM patients had significantly lower PAPP-A and 25-(OH)D levels, and a significantly higher TG level than normal pregnant women. Furthermore, Pearson analysis of the correlation between these three factors and blood glucose in GDM patients demonstrated that PAPP-A, 25-(OH)D and FBG levels were negatively correlated, while TG and FBG levels were positively correlated. Studies have shown that PAPP-A increases insulin sensitivity by hydrolyzing insulin-like growth factor binding protein, resulting in a negative correlation between PAPP-A level and GDM[23]. Vitamin D receptors are commonly distributed in insulin cells, which can bind to insulin receptors on islet β cells to promote insulin secretion and increase the expression of insulin receptor-related genes[24]. Previous studies have shown[25] that TG concentration in the blood circulation of GDM patients is regulated by insulin metabolism and blood glucose changes, and is closely related to the incidence of GDM. Integration of the above findings with our findings further explains the relationship between these three factors and the incidence of GDM. Furthermore, according to the ROC curve, the sensitivity, specificity, AUC and optimal cut-off value of serum PAPP-A level in the diagnosis of GDM were 72.55%, 82.35%, 0.861 and 16.340, respectively, while those of TG level were 86.27%, 66.67%, 0.813 and 1.796, respectively. The corresponding sensitivity, specificity, AUC and optimal cut-off value of serum 25-(OH)D were 64.71%, 70.59%, 0.721 and 23.140, respectively. These findings indicated that PAPP-A, 25-(OH)D and TG have certain diagnostic value in GDM. Previous studies have reported[26] that PAPP-A, as a screening index in early pregnancy, can effectively regulate the occurrence of GDM in high-risk pregnant women. However, limited research has been carried out on the diagnostic value of 25-(OH)D and TG in GDM. Therefore, it was further confirmed by multiple linear regression analysis of common vascular endothelial indicators in pregnant women that FBG, VEGF, Flt-1, serum PAPP-A, TG, and 25-(OH)D during pregnancy were related risk factors leading to GDM. The placenta is an important organ for survival of the fetus, and construction of the blood circulation plays a key role in the invasion of trophoblast cells. VEGF, as a highly regulated selective dimeric glycoprotein of vascular endothelial cells, binds to Flt-1 to induce proliferation of vascular endothelial cells. When VEGF is over-expressed, its role in promoting angiogenesis and permeability is limited, which affects fetal growth and development[27-29]. These findings demonstrate that serum PAPP-A, 25-(OH)D and TG exert marked effects on the blood glucose level of GDM patients, and are related risk factors for the onset of GDM.
In summary, GDM increases short-term symptoms and signs as well as the long-term risk of disease in pregnant mothers and infants. In this study, we determined the levels of TG and 25-(OH) D in early pregnancy and concluded that these three factors are related to the occurrence of GDM, have a certain predictive value in GDM, and have high value in clinical application. In addition, this study identified that the higher the FBG before pregnancy, the higher the possibility of GDM. Therefore, the combination of PAPP-A, TG and 25-(OH)D may be more preferable in predicting the occurrence and diagnosis of GDM, which may serve as an important research direction in future studies of the early identification and timely interventions for GDM.
Gestational diabetes mellitus (GDM) refers to gestational secretory diseases that occur during pregnancy with varying degrees of abnormal glucose metabolism, but with blood glucose levels that do not meet the criteria for dominant diabetes. Early treatment is more effective, and there is currently a lack of indicators for predicting GDM. Therefore, it is very important to identify new indicators for the diagnosis and treatment of GDM.
Serum pregnancy-associated protein A (PAPP-A) levels in early pregnancy have high sensitivity and specificity for pregnancy complications such as spontaneous abortion and hypertension. Elevated triglyceride (TG) levels can increase insulin resistance and promote islet β-cell apoptosis. Serum 25-hydroxyvitamin D [25-(OH)D] has been found to show a downward trend in type 2 diabetes, and has the effect of enhancing insulin sensitivity and improving insulin resistance. However, research on the role and mechanism of these three factors in GDM is rare, and PAPP-A, TG, and 25-(OH)D may have important effects in predicting GDM.
The purpose of this investigation was to investigate the correlation and predictive value of serum PAPP-A, TG, and serum 25-(OH)D levels in GDM during early pregnancy.
Ninety-nine patients in early pregnancy were admitted to our hospital from November 2015 to September 2017 and their fasting blood glucose was determined. Group A consisted of 51 cases with GDM who were screened using the oral glucose tolerance test at 24-28 wk. Forty-eight women without GDM were included in group B. The serum PAPP-A, TG, and 25-(OH)D levels were compared between the two groups and their correlation with blood glucose was analyzed. In addition, the diagnostic value of PAPP-A, TG, and 25-(OH)D levels in GDM was determined using ROC curve analysis.
Serum PAPP-A and 25-(OH)D levels in group A were significantly lower than those in group B, and the TG levels in group A were significantly higher than those in group B. The differences were statistically significant (P < 0.05). Pearson analysis showed that PAPP-A, 25-(OH)D and fasting blood glucose levels were negatively correlated (r = -0.605, P < 0.001), (r = -0.597, P < 0.001), and TG and fasting blood glucose levels were positively correlated (r = 0.628, P < 0.001). The sensitivity of serum PAPP-A level in diagnosing GDM was 72.55%, the specificity was 82.35%, the AUC was 0.861, and the optimal cut-off was 16.340. The sensitivity of TG level in diagnosing GDM was 86.27%, the specificity was 66.67%, and the AUC was 0.813, and the best cut-off value was 1.796. 25-(OH)D level had a sensitivity of 64.71%, specificity of 70.59%, AUC of 0.721, and the best cut-off value was 23.140. Multivariate logistic regression analysis showed that fasting blood glucose during pregnancy, vascular endothelial growth factor, Flt-1, serum PAPP-A, TG, and 25-(OH)D are all related risk factors for GDM.
Serum PAPP-A, TG and 25-(OH)D levels are related to changes in GDM blood glucose, and are independent factors affecting the occurrence of GDM.
Serum PAPP-A, TG and 25-(OH)D levels were correlated with changes in GDM blood glucose, and are independent factors affecting the occurrence of GDM. The clinical determination of serum PAPP-A, TG, and 25-(OH)D levels will help to formulate a treatment plan for GDM.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabla PK S-Editor: Dou Y L-Editor: Webster JR E-Editor: Ma YJ
| 1. | American Diabetes Association. Standards of medical care in diabetes-2010. Diabetes Care. 2010;33 Suppl 1:S11-S61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2231] [Cited by in RCA: 2284] [Article Influence: 152.3] [Reference Citation Analysis (1)] |
| 2. | Meregaglia M, Dainelli L, Banks H, Benedetto C, Detzel P, Fattore G. The short-term economic burden of gestational diabetes mellitus in Italy. BMC Pregnancy Childbirth. 2018;18:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Rivas A, Landon M, Gaillard T, Schuster D, Osei K. Awareness of risk factors for type 2 diabetes in women with current and former gestational diabetes mellitus (GDM): Implications for future primary diabetes prevention. Research MSC Reviews. 2010;4:89-94. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Idris N, Wong SF, Thomae M, Gardener G, McIntyre DH. Influence of polyhydramnios on perinatal outcome in pregestational diabetic pregnancies. Ultrasound Obstet Gynecol. 2010;36:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31:1668-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 361] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 6. | Tian SX, Yang S. Study on the relationship between Gestational diabetes mellitus and neonatal jaundice. Xiandai Yufang Yixue. 2012;39:2960-2961,2963. |
| 7. | Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Schmidt L, Damm P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009;94:2464-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 8. | Sweeting AN, Ross GP, Hyett J, Molyneaux L, Constantino M, Harding AJ, Wong J. Gestational Diabetes Mellitus in Early Pregnancy: Evidence for Poor Pregnancy Outcomes Despite Treatment. Diabetes Care. 2016;39:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 9. | Ngai I, Govindappagari S, Neto N, Marji M, Landsberger E, Garry DJJO, Gynecology. Outcome of pregnancy when gestational diabetes mellitus is diagnosed before or after 24 weeks of gestation. Obstet Gynecol. 2014;123:162S-163S. |
| 10. | Talasaz ZH, Sadeghi R, Askari F, Dadgar S, Vatanchi A. First trimesters Pregnancy-Associated Plasma Protein-A levels value to Predict Gestational diabetes Mellitus: A systematic review and meta-analysis of the literature. Taiwan J Obstet Gynecol. 2018;57:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Wu H, Wang F, Ye M, Zhu H, Bu S. Long non-coding RNA MALAT1 expression in patients with gestational diabetes mellitus. Int J Gynaecol Obstet. 2018;140:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Valizadeh M, Piri Z, Mohammadian F, Kamali K, Amir Moghadami HR. The Impact of Vitamin D Supplementation on Post-Partum Glucose Tolerance and Insulin Resistance in Gestational Diabetes: A Randomized Controlled Trial. Int J Endocrinol Metab. 2016;14:e34312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2777] [Cited by in RCA: 3167] [Article Influence: 211.1] [Reference Citation Analysis (1)] |
| 14. | American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36:S11-S66. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2371] [Cited by in RCA: 2481] [Article Influence: 206.8] [Reference Citation Analysis (0)] |
| 15. | Wasalathanthri S. Attenuating type 2 diabetes with postpartum interventions following gestational diabetes mellitus. World J Diabetes. 2015;6:648-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Wang YH, Wu HH, Ding H, Li Y, Wang ZH, Li F, Zhang JP. Changes of insulin resistance and β-cell function in women with gestational diabetes mellitus and normal pregnant women during mid- and late pregnant period: a case-control study. J Obstet Gynaecol Res. 2013;39:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Gunderson EP, Hedderson MM, Chiang V, Crites Y, Walton D, Azevedo RA, Fox G, Elmasian C, Young S, Salvador N, Lum M, Quesenberry CP, Lo JC, Sternfeld B, Ferrara A, Selby JV. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care. 2012;35:50-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Katovich MJ, Reaves PY, Francis SC, Pachori AS, Wang HW, Raizada MK. Gene therapy attenuates the elevated blood pressure and glucose intolerance in an insulin-resistant model of hypertension. J Hypertens. 2001;19:1553-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Smith YE, Toomey S, Napoletano S, Kirwan G, Schadow C, Chubb AJ, Mikkelsen JH, Oxvig C, Harmey JH. Recombinant PAPP-A resistant insulin-like growth factor binding protein 4 (dBP4) inhibits angiogenesis and metastasis in a murine model of breast cancer. BMC Cancer. 2018;18:1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Tul N, Spencer K, Noble P, Chan C, Nicolaides K. Screening for trisomy 18 by fetal nuchal translucency and maternal serum free beta-hCG and PAPP-A at 10-14 weeks of gestation. Prenat Diagn. 1999;19:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | He J, Zhang R, Lu Y, Han G. Correlation study of the levels of vitamin D with rennin and angiotensin aldosterone II in type 2 diabetes. Yinanbing Zazhi. 2017;16:1233-1235. |
| 22. | Karamali M, Bahramimoghadam S, Sharifzadeh F, Asemi Z. Magnesium-zinc-calcium-vitamin D co-supplementation improves glycemic control and markers of cardiometabolic risk in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. 2018;43:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 23. | Lovati E, Beneventi F, Simonetta M, Laneri M, Quarleri L, Scudeller L, Albonico G, Locatelli E, Cavagnoli C, Tinelli C, Spinillo A, Corazza GR. Gestational diabetes mellitus: including serum pregnancy-associated plasma protein-A testing in the clinical management of primiparous women? A case-control study. Diabetes Res Clin Pract. 2013;100:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Xia S, Su X, Yu X, Zhou W, Ma J. Vitamin D and diabetes mellitus. Zhonghua Tangniaobing Zazhi. 2012;20:951-953. |
| 25. | Hertroijs DFL, Elissen AMJ, Brouwers MCGJ, Schaper NC, Köhler S, Popa MC, Asteriadis S, Hendriks SH, Bilo HJ, Ruwaard D. A risk score including body mass index, glycated haemoglobin and triglycerides predicts future glycaemic control in people with type 2 diabetes. Diabetes Obes Metab. 2018;20:681-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Kumar M, Sharma K, Singh R, Singh S, Ravi V, Singh K, Gupta U, Bhattacharya J. Role of maternal factors, PAPP-A, and Doppler in screening for early- and late-onset pregnancy hypertension in Asian population. Hypertens Pregnancy. 2016;35:382-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Pietro L, Daher S, Rudge MV, Calderon IM, Damasceno DC, Sinzato YK, Bandeira C, Bevilacqua E. Vascular endothelial growth factor (VEGF) and VEGF-receptor expression in placenta of hyperglycemic pregnant women. Placenta. 2010;31:770-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Lee ES, Oh MJ, Jung JW, Lim JE, Seol HJ, Lee KJ, Kim HJ. The levels of circulating vascular endothelial growth factor and soluble Flt-1 in pregnancies complicated by preeclampsia. J Korean Med Sci. 2007;22:94-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond). 2005;109:227-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 664] [Article Influence: 33.2] [Reference Citation Analysis (0)] |