Published online Dec 26, 2020. doi: 10.12998/wjcc.v8.i24.6487
Peer-review started: September 20, 2020
First decision: September 29, 2020
Revised: October 9, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 26, 2020
Processing time: 90 Days and 10.8 Hours
Acquired prosopagnosia is a rare condition characterized by the loss of familiarity with previously known faces and the inability to recognize new ones. It usually occurs after the onset of brain lesions such as in a stroke. The initial identification of prosopagnosia generally relies on a patient’s self-report, which can be challenging if it lacks an associated chief complaint. There were few cases of prosopagnosia presenting purely as eye symptoms in the previous literature confirmed by functional magnetic resonance imaging (MRI).
We present a case of delayed diagnosis of prosopagnosia after a right hemisphere stroke in an elderly man whose chief complaint was persistent and progressive "blurred vision" without facial recognition impairment. Ophthalmic tests revealed a homonymous left upper quadrantanopia, with normal visual acuity. He was found by accident to barely recognize familiar faces. The patient showed severe deficit in face recognition and perception tests, and mild memory loss in neuropsychological assessments. Further functional MRI revealed the visual recognition deficits were face-specific. After behavioral intervention, the patient started to rely on other cues to compensate for poor facial recognition. His prosopagnosia showed no obvious improvement eight months after the stroke, which had negative impact on his social network.
Our case demonstrates that the presentation of prosopagnosia can be atypical, and visual difficulties might be a clinical manifestation solely of prosopagnosia, which emphasizes the importance of routinely considering face recognition impairment among elderly patients with brain lesions.
Core Tip: Prosopagnosia is a rare condition characterized by the loss of familiarity with previously known faces and the inability to recognize new faces. We present a case of delayed diagnosis of prosopagnosia after a right hemisphere stroke in an elderly man who only presented with progressive "blurred vision" without any facial recognition impairment. Further functional magnetic resonance imaging revealed that the visual recognition deficits were face-specific. This case report demonstrates that the presentation of prosopagnosia can be atypical, and visual difficulties could be a clinical manifestation solely of prosopagnosia. This case highlights the importance of routinely considering prosopagnosia as a potential impairment after stroke.
- Citation: Yuan Y, Huang F, Gao ZH, Cai WC, Xiao JX, Yang YE, Zhu PL. Delayed diagnosis of prosopagnosia following a hemorrhagic stroke in an elderly man: A case report. World J Clin Cases 2020; 8(24): 6487-6498
- URL: https://www.wjgnet.com/2307-8960/full/v8/i24/6487.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i24.6487
Acquired prosopagnosia is the impaired ability to recognize faces following certain cerebral injuries. It mostly affects the recognition of familiar and new faces[1]. In contrast with developmental prosopagnosia, which has a prevalence of up to 2.5%, acquired prosopagnosia is relatively rare[2]. Extensive damage to the brain, mainly the occipital-temporal lobes[3], due to trauma, tumors, cerebrovascular disease, degenerative atrophy, or temporal lobe resection, may result in face-processing impairments[4-7]. Prosopagnosia secondary to stroke is usually accompanied by cognitive impairments, visual field defects, achromatopsia, object agnosia, or topographical disorientation[8]. The identification of prosopagnosia can be challenging if patients lack of corresponding symptoms.
This case report describes an elderly patient complaining solely of persistent and progressive “blurred vision” following a right occipital-temporal lobe stroke, and which could not be fully explained by his cerebral damage or any ophthalmic impairment. He was finally diagnosed with prosopagnosia after facial recognition tests and functional magnetic resonance imaging (MRI). We also review the available literature and summarize the characteristics of acquired prosopagnosia due to stroke.
Our patient is a 72-year-old right-handed man, and a retired local government employee with a bachelor’s degree. In October 2019, he presented with severe dizziness and was admitted to the hospital’s geriatric department.
The patient’s symptoms started with abrupt mild dizziness, which had worsened in two days. He also had moderate numbness in a left limb.
His medical history was significant for atrial fibrillation, hypertension, type 2 diabetes mellitus, and ankylosing spondylitis. His psychiatric history was unremarkable.
The patient’s family history was unremarkable.
Vital signs were normal upon admission. Neurological examination demonstrated bluntness of vibration sense in the left foot together with mild walking instability.
All clinical laboratory tests were interpreted as “within normal limits” except for the human leukocyte antigen-B27 (HLA-B27) antibody.
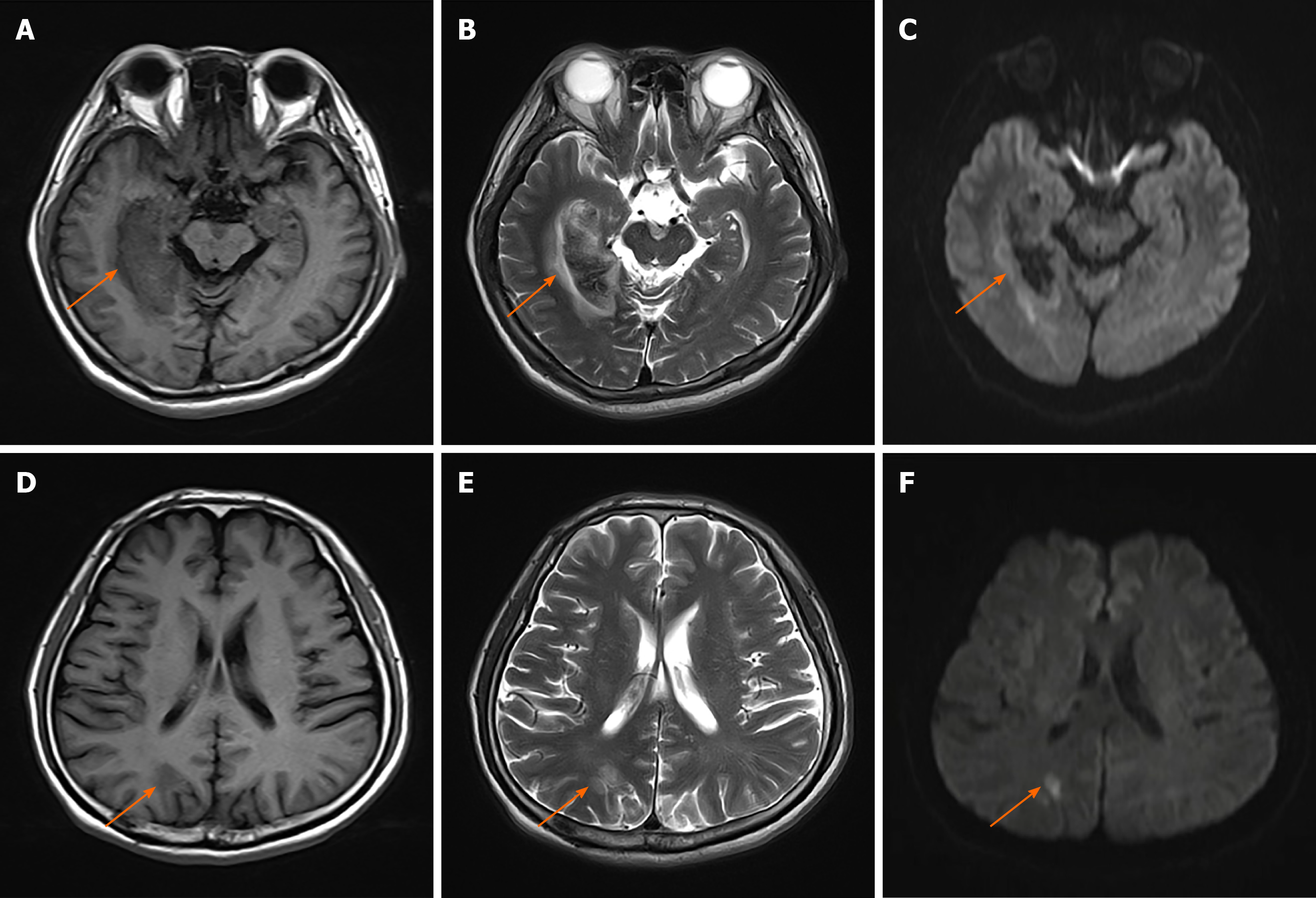
A brain MRI revealed a right occipital-temporal lobe hemorrhagic stroke and an occipital-parietal lobe ischemic stroke (Figure 1). While the symptoms of dizziness, numbness, and stagger were all improved after conservative treatment, he developed persistent "blurred vision" from 13 d after the onset of the stroke. Another structural MRI ruled out new brain lesions. Accordingly, he underwent further comprehensive ophthalmic assessments.
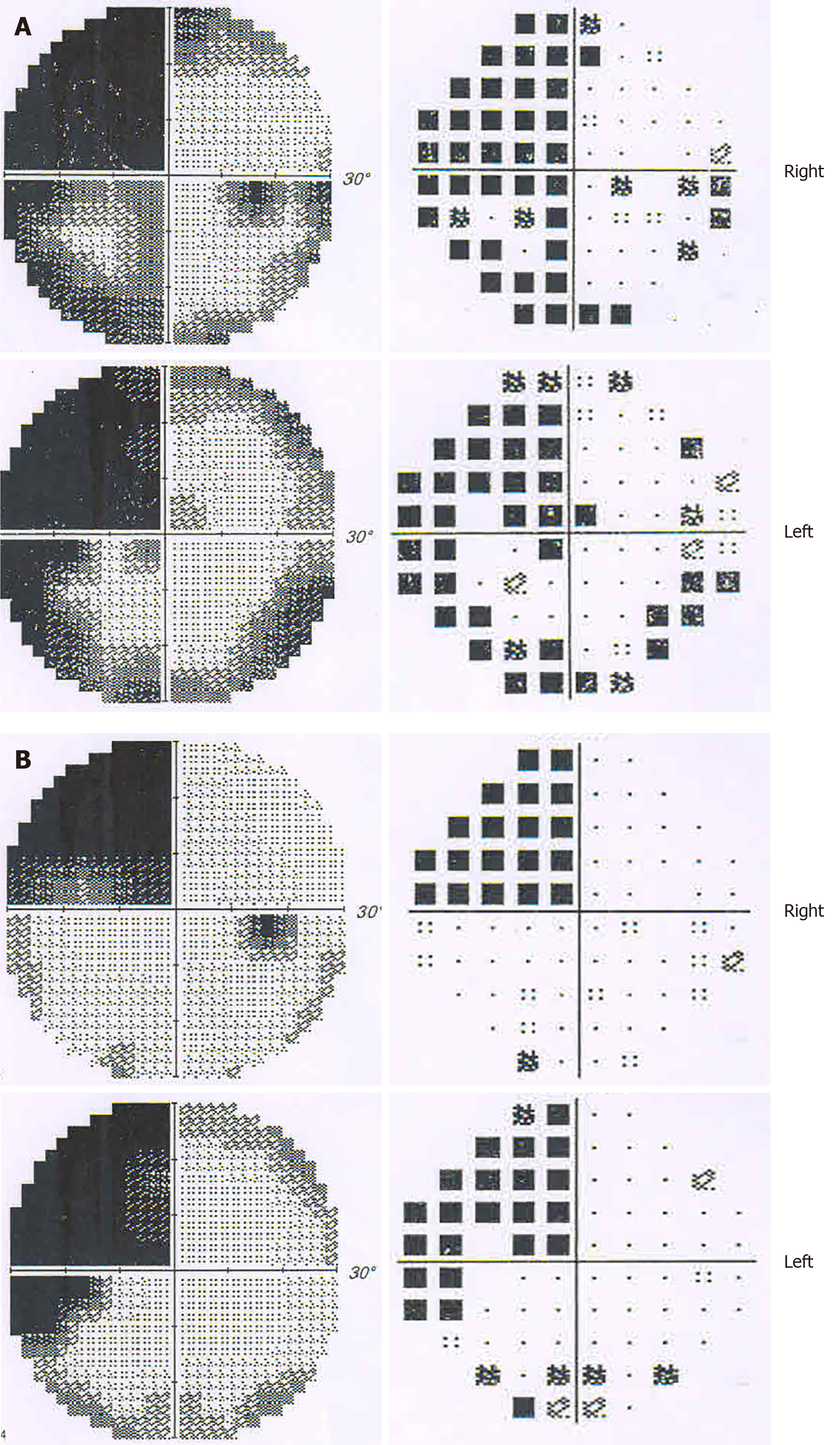
Ophthalmic tests: Although the patient suffered from ankylosing spondylitis, there were no signs of uveitis, iritis or conjunctivitis. Visual acuity (snellen chart) was 0.5 (1.0 with glasses) in the right eye, 0.6 (1.0 with glasses) in the left. Visual field testing (Humphrey field analyzer) demonstrated homonymous left upper quadrantanopia (Figure 2). The results of examinations of the cornea, anterior chamber, and iris from slit-lamp biomicroscope and fundus were unremarkable for both eyes. Diplopia and ocular motility testing was normal. Contrast sensitivity (Nicolet CS-2000) and color perception (Ishihara color plate test) were both in the upper ranges. Optical coherence tomography revealed a mild anterior macular membrane in the right eye; the left eye was clear. Visual evoked potential tests showed a prolonged incubation period of P100 waves bilaterally, especially on the right. Orbital contrast-enhanced MRI excluded abnormalities in bilateral orbits, eyeballs, optic nerves, and optic chiasms. MR diffusion tensor imaging of the optic nerve revealed an attenuated diffusivity of optic nerves and optic radiations on the right side compared with the left, which might have been a manifestation of his right occipital-temporal lobe stroke. From the above, relevant neuro-ophthalmological findings were generally consistent with his right hemispheric stroke. However, his "blurred vision” was persistent and aggravating even after the improvement of his left upper quadrantanopia (visual field index from 56% and 61% in the right and left eye respectively, to 74% in both eyes) after one month.
The symptom of prosopagnosia was first remarked upon by his younger brother, who had come to visit. His brother noticed that the patient showed no signs of recognition until he started to speak. The patient did not present face-recognition difficulties with his wife and son, but could not recognize doctors and nurses who were taking care of him. He had to check their name cards before daily conversations.
Neuropsychological assessment: To verify the hypothesis of prosopagnosia and to exclude the presence of perceptual, cognitive or memorial deficits, we started the evaluation with a selection of tests. Table 1 demonstrates the results of his neuropsychological assessments. His score on the montreal cognitive assessment (MoCA) was 26/30. One point was dropped for calculation and three for delayed recall of five words. A further rey auditory verbal learning test (RAVLT) confirmed mild memory loss in immediate and delayed recall. The results of the visual object and space perception battery and the Within-class Object Recognition-Cambridge Car Memory Test were both normal. Notably, the patient had a severe deficit in face recognition and perception tests compared with age- and sex-matched control subjects [Benton facial recognition test (BFRT): 37/54; Cambridge face memory test (CFMT): 47/72; and Famous faces test: 2/12].
| Tests | Patient’s performance | Control (n = 10, mean ± SD) | Interpretation |
| General cognition | |||
| MoCA | 26/30 | 25.1 ± 1.97 | Normal |
| Visuospatial/executive | 5/5 | 3.7 ± 0.67 | |
| Naming | 3/3 | 3.0 ± 0.00 | |
| Memory | N/A | N/A | |
| Attention and calculation | 5/6 | 4.1 ± 0.99 | |
| Language | 3/3 | 2.3 ± 0.48 | |
| Abstraction | 2/2 | 1.8 ± 0.42 | |
| Delayed recall | 2/5 | 3.6 ± 0.70 | |
| Orientation | 6/6 | 6.0 ± 0.00 | |
| Memory | Impaired | ||
| RAVLT immediate | 5/15 | 7.9 ± 1.79 | |
| RAVLT delayed | 6/15 | 8.9 ± 1.20 | |
| Object recognition | |||
| Object-spatial perception | |||
| VOSP | 118/130 | 116.5 ± 7.12 | Normal |
| Within-class object recognition | |||
| CCMT | 54/72 | 55.6 ± 7.37 | Normal |
| Face recognition | |||
| Tests of face familiarity | |||
| CFMT | 47/72 | 63.1 ± 6.84 | Impaired |
| Famous faces test | 2/12 | 11.9 ± 0.32 | Impaired |
| Tests of face perception | |||
| BFRT | 37/54 | 50.4 ± 3.03 | Impaired |
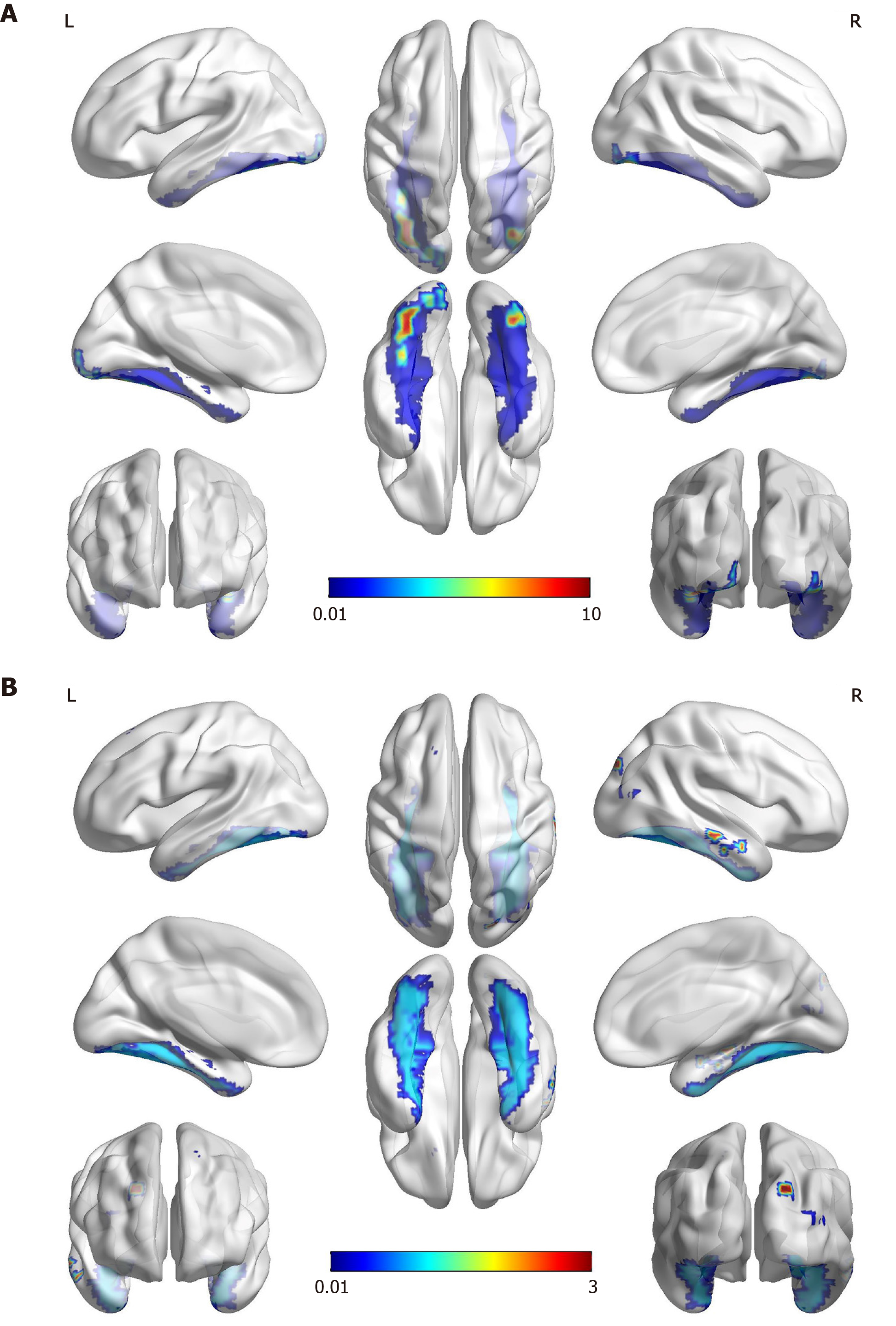
Functional MRI: We performed a further functional MRI to correlate facial recognition impairment with the core regions of the face-processing network. In the famous faces recognition task, the patient showed activations in the anterior of the bilateral fusiform gyrus, the typical location of the fusiform face area (FFA) for face recognition. The activation was more significant on the left, probably due to the compensation effect on the left side after the right occipital-temporal lobe infarction. In the test with landscapes/objects as stimuli, an increase in activation in the right occipital lobe was observed (Figure 3).
In addition to the presence of visual field impairment (homonymous left upper quadrantanopia) and mild memory loss, the patient did not show significant cognitive impairment, object agnosia, achromatopsia or topographical disorientation, which are common signs in patients with prosopagnosia. A definitive diagnosis of acquired prosopagnosia was made based on history of present illness, clinical symptoms, structural and functional MRI findings, and face recognition tests.
Behavioral intervention with compensatory strategies has been suggested by doctors for treating prosopagnosia. The patient started to rely on other cues such as voice, gait, figure, facial features (e.g., moustaches, scars, and freckles) or accessories (e.g., ear-rings and glasses) to compensate for poor facial recognition. He was also encouraged to develop mnemonic functionality and try to discriminate distance between facial features.
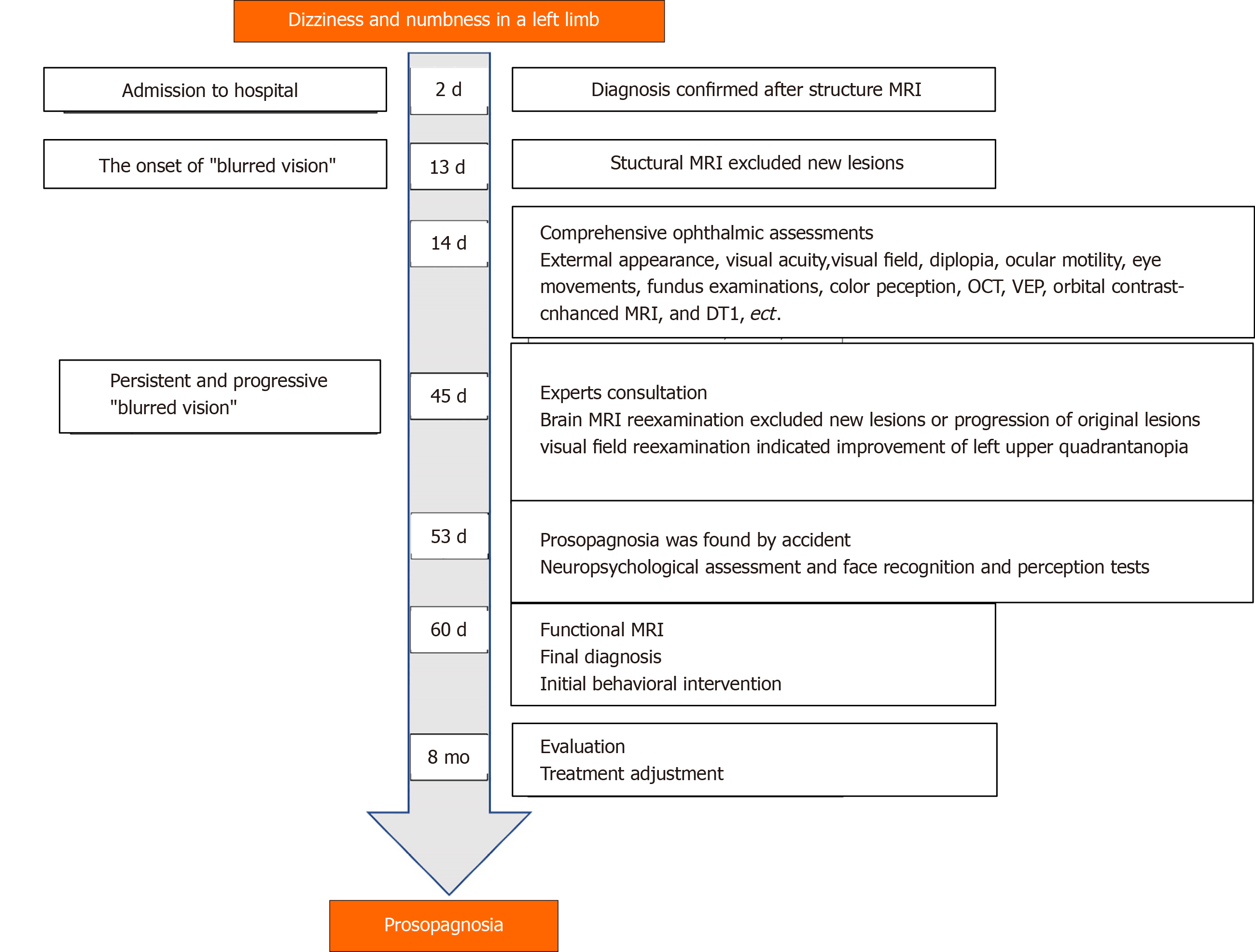
His memory loss was markedly recovered eight months after the stroke, but not his prosopagnosia. His failure to recognize faces caused him embarrassment and anxiety and traumatized his social network. The timeline of the case is displayed in Figure 4.
Subjects with prosopagnosia often present with neuro-ophthalmological symptoms other than difficulties in face recognition[3]. To better understand the characteristics of acquired prosopagnosia, we systematically searched the databases of PubMed, Embase, and CINAHL from January 1, 1980 to the present for previously reported cases. Inclusion criteria were as follows: (1) Acquired prosopagnosia was attributed to stroke; (2) Face recognition impairment was the main clinical manifestation; and (3) Case reports provided a thorough patient history and findings of auxiliary examinations. The characteristics of patients are summarized in Table 2. Forty-two subjects across 31 articles met the criteria. In general, patients with prosopagnosia secondary to stroke differ in its severity and variant, as well as in their associated visual and cognitive deficits. Acquired prosopagnosia was found predominantly in patients with right-sided stroke, and less frequently in those with bilateral or left-sided lesions. Visual field defects, including homonymous hemianopia and quadrantanopia, were detected in a significantly high percentage of patients with acquired prosopagnosia. Memory loss is the most common cognitive impairment among these patients. They also frequently presented with topographical disorientation, achromatopsia, or object agnosia.
| Clinical features | Frequency of pathological findings, patients (%) | |
| Etiologies | Ischemic stroke | 34 (81.0) |
| Hemorrhagic stroke | 7 (16.7%) | |
| Subarachnoid hemorrhage | 1 (2.4) | |
| Cerebral lesions | Unilateral cerebral lesions | Right-sided lesions: 27 (64.3); left-sided lesions: 3 (7.1%) |
| Bilateral cerebral lesions | 11 (26.2) | |
| Ocular symptoms | Visual field defects | Hemianopia: 28 (66.7); quadrantanopia: 11 (26.2%) |
| Blurred vision/deterioration of visual acuity | 6 (14.3%) | |
| Scotomas in the vision | 4 (9.5%) | |
| Dimmer/decreased brightness of vision | 3 (7.1%) | |
| Visual distortion/metamorphopsia | 4 (9.5%) | |
| Visual hallucinations | 3 (7.1%) | |
| Agnosia | Spatial agnosia/topographical disorientation | 21 (50%) |
| Color agnosia/achromatopsia | 9 (21.4%) | |
| Object agnosia | 8 (19.0%) | |
| Alexia/musical alexia | 8 (19.0) | |
| Apraxia | 3 (7.1) | |
| Simultanagnosia | 1 (2.4) | |
| Cognitive difficulties | Memory loss | 7 (16.7) |
| Perceptual impairment | 6 (14.3) | |
| Unilateral neglect | 4 (9.5) | |
| Language difficulties | 2 (4.8) | |
| Dyscalculia | 1 (2.4) | |
| Concentration impairment | 1 (2.4) | |
| Other signs/symptoms | Headache/vertigo/dizziness | 6 (14.3) |
| Tandem walking/gait instability | 5 (11.9) | |
| Hemiparesis/loss of control of limb | 4 (9.5) | |
| Hemisensory loss | 4 (9.5) | |
| Social and emotional difficulties | 4 (9.5) | |
| Hand tremors | 1 (2.4) | |
| Dressing disturbance | 1 (2.4) | |
The symptom of “blurred vision” was relatively unusual at presentation, with only six patients noting a deterioration in vision acuity[4,9-13] (Table 3). However, “blurred vision” in these patients was transient and reversible. Our patient’s main and most insistent complaint was “blurred vision” without face recognition impairment. This might explain the reason for the delayed diagnosis of prosopagnosia. The condition was not reported in any of the previous cases. Left or bilateral hemianopsia can be explained by damage to the optic radiations or striate cortex following occipitotemporal lesions, and this might cause the “dimmed vision” or “scotoma” that was occasionally perceived as “blurred vision” by patients[14]. However, our patient’s “blurred vision” was not consistent with the progression of his visual field defect, and his visual acuity was normal. We speculate that this paradox can be attributed to two reasons. Firstly, blurred vision is a non-specific symptom of various ocular diagnoses, and patients report visual symptoms despite normal eye examination results[15]. Secondly, it is possible that the patient was subconsciously reluctant to report the symptom of prosopagnosia for embarrassment or fear, and described the symptom as “blurred vision” to gain the attention of the doctors.
| Ref. | Age at diagnosis(yr), gender | Precipitating factors | Clinical presentation other than prosopagnosia | Neuro-imaging | Face recognition test | Object recognition test |
| Fadelalla et al[4], 2019 | 42, female | Acute aneurysmal subarachnoid hemorrhage | Headache, seizure, decreased level of consciousness, blurred vision in right eye, alexia, simultagnosia, impaired within-class object recognition | CT scan showed diffuse SAH, MRI (8 mo) showed presence of hemosiderin deposition around corpus callosum | WRMT: 38/501 | VOSP (part): 78/80 |
| Sugimoto et al[9], 2012 | 82, female | Cerebral infarction in the right PCA | Sudden and transient deterioration of visual acuity, left homonymous hemianopsia, left unilateral spatial agnosia | MRI showed a high signal area in the posterior region of the splenium of the corpus callosum of the right occipital lobe. PET showed decreased oxygen metabolism in the right occipital, temporal lobe | Familiar faces identification test: 0/91; discriminating unfamiliar faces: 31/401 and matching unfamiliar faces: 32/541 | N/A |
| Tohgi et al[10], 1994 | 62, male | Cerebral infarction in the territory of PCA | Left upper quadrantanopia in the right eye, metamorphopsia, blurred faces on the TV. Visual acuity was 0.5 in the right eye, 0.6 in the left | MRI showed infarctions in right occipital-temporal areas | Discriminating unfamiliar faces: Impaired | Normal |
| Ettlin et al[11], 1992 | 54, male | Stepwise succession of infarction | Left homonymous hemianopsia, reversible decreased vision, right superior quadrantanopia, visuo-perceptual and constructional difficulties. Visual acuity with correction was 20/30 in the right and 20/25 in the left eye | Infarction in the right parietal border zone territory, followed by a large occipital/parietal posterior-medial-temporal infarction, and a hemorrhage involving the left parieto-occipital area | Benton matching task: 6/271; famous faces test: 01 | Normal |
| Habib et al[12], 1986 | 71, female | Right infection in the territory of PCA | Sudden onset of vertigo, posterior cephalalgia, intense palpitations, blurred vision, left hemianopia, impairment of topographical memory, handwriting recognition impairment, emotional changes | CT showed infarction in the territory of the posterior right occipital-temporal area | Famous faces test: Poor | Partially impaired |
| Renzi et al[13], 1986 | 73, male | Ischemic stroke | Blurred vision, dense left hemianopia without macula sparing, moderate left visual neglect, topographical disorientation | CT (12 mo) showed an extensive softening involving the entire territory of the right posterior cerebral artery | LEVIN’s face recognition test: 18/271; face memory test: 26/481 | N/A |
Other than prosopagnosia, the patient retained the ability to recognize objects (including within-class objects), letters, and places. The precise mechanism of impaired perception and recognition of faces that leaves object recognition intact remains unclear. The first hypothesis is that different categories of objects, particularly faces are processed by distinct dedicated cortical regions[2]. The lesions responsible for face recognition occur in the vicinity of the right FFA and right occipital face area[16], which were also observed in our case. Furthermore, an occipital-temporal right hemisphere lesion may lead to a specific impairment in holistic perceptions, a function that appears critical for normal face recognition but not for object recognition[17]. Other hypotheses include the following: Prosopagnosic patients may be impaired in perceiving the configuration of facial features[18]; They may have problems with using information from the eye region[19]; or Their condition may be accompanied with the disconnection between facial percepts and the memory[20]. Further research is required into the proposed mechanism.
An evaluation of the facial recognition abilities of the patient indicates the presence of impairment in the (1) recognition of previously known familiar faces (Famous Faces Test); (2) ability to learn and remember new faces (CFMT); and (3) capacity to discriminate unfamiliar faces (BFRT). According to the models of face recognition proposed by Bruce et al[21], the patient was impaired not only in his recognition of familiar faces but also in his perception of the difference between faces, which made him a prosopagnosia subject with an apperceptive variant[1]. This is consistent with recent studies showing that those with occipital-temporal or fusiform lesions are more likely to have this impairment[2].
In the case of the elderly population, the term prosopagnosia refers to a state of selective deficit in face recognition that cannot be explained by general impairment in perception, cognition, or memory[1,5]. Approximately 65% of stroke survivors manifest new or increased cognitive impairments[8]. Therefore, the diagnosis of prosopagnosia requires neuropsychological evaluation of these functions and exclusion of Alzheimer’s dementia[22], mild cognitive impairment[23], Parkinson’s disease[24], and ocular diseases such as macular degeneration[25]. In our patient, perception and cognitive function were generally intact. The results of the MoCA and the RAVLT indicated mild memory loss, which was significantly improved after eight months, suggesting a relative reversible process of cognitive impairment after the stroke[8]. In addition, the symptom of “facial agnosia” was never mentioned by him. We assume that prosopagnosia can be obscured by insensitivity and poor verbal expression in older adults.
The patient underwent comprehensive ophthalmic tests and neuropsychological assessments. The diagnosis of acquired prosopagnosia was made based on well-established face recognition tests and functional MRI. But we have not yet thoroughly evaluated the effectiveness of compensatory strategies on the remission of the patient’s prosopagnosia or on his quality of life.
There are some important lessons to be drawn from our case. Firstly, the possibility of acquired prosopagnosia was underestimated, and clinicians did not routinely consider whether there may be face-processing impairments after stroke. In the elderly population, symptoms of prosopagnosia may be atypical, or masked by other impairments, leaving patients without proper rehabilitation and intervention. Secondly, those with acquired prosopagnosia often report depressed emotions, personal distress, or negative effects on their social life. It is therefore absolutely crucial to understand their feelings and offer emotional support and psycho-education[26]. Thirdly, there is no consensus with regard to the diagnostic criteria for prosopagnosia, the identification of which still relies mainly on patient-report. However, stroke survivors, especially the elderly, may not be able to explain their symptoms properly. This raises the question of how many stroke patients with prosopagnosia remain undetected. Comprehensive screening should be implemented to identify various aspects of stroke patients’ disabilities.
We have presented a case of delayed diagnosis of prosopagnosia after a right hemisphere stroke manifesting as progressive “blurred vision.” It demonstrates that visual difficulties can be a presentation solely of prosopagnosia in some instances. We have reported this case to highlight the awareness in clinical practice of screening for and identifying prosopagnosia after stroke or other cerebral injury.
We thank the patient and his family for their collaboration.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Scicchitano P S-Editor: Fan JR L-Editor: MedE-Ma JY P-Editor: Li JH
| 1. | Davies-Thompson J, Pancaroglu R, Barton J. Acquired prosopagnosia: structural basis and processing impairments. Front Biosci (Elite Ed). 2014;6:159-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Albonico A, Barton J. Progress in perceptual research: the case of prosopagnosia. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Schmidt D. Neuro-ophthalmological findings in patients with acquired prosopagnosia. Graefes Arch Clin Exp Ophthalmol. 2015;253:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Fadelalla M, Kanodia A, Elsheikh M, Ellis J, Smith V, Hossain-Ibrahim K. A case of aneurysmal subarchnoid haemorrhage and superficial siderosis complicated by prospagnosia, simultagnosia and alexia without agraphia. Br J Neurosurg. 2019;1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Monti C, Sozzi M, Bossi F, Corbo M, Rivolta D. Atypical holistic processing of facial identity and expression in a case of acquired prosopagnosia. Cogn Neuropsychol. 2019;36:358-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Susilo T, Wright V, Tree JJ, Duchaine B. Acquired prosopagnosia without word recognition deficits. Cogn Neuropsychol. 2015;32:321-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Moriyama Y, Muramatsu T, Kato M, Mimura M, Akiyama T, Kashima H. Frégoli syndrome accompanied with prosopagnosia in a woman with a 40-year history of schizophrenia. Keio J Med. 2007;56:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Cousins R. Prosopagnosia after stroke: potentials for impairment and treatment. Top Stroke Rehabil. 2013;20:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Sugimoto A, Miller MW, Kawai Y, Shiota J, Kawamura M. Another piece in the jigsaw: a case report of prosopagnosia with symptomatological, imaging and post mortem anatomical evidence. Cortex. 2012;48:641-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Tohgi H, Watanabe K, Takahashi H, Yonezawa H, Hatano K, Sasaki T. Prosopagnosia without topographagnosia and object agnosia associated with a lesion confined to the right occipitotemporal region. J Neurol. 1994;241:470-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Ettlin TM, Beckson M, Benson DF, Langfitt JT, Amos EC, Pineda GS. Prosopagnosia: a bihemispheric disorder. Cortex. 1992;28:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Habib M. Visual hypoemotionality and prosopagnosia associated with right temporal lobe isolation. Neuropsychologia. 1986;24:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | De Renzi E. Prosopagnosia in two patients with CT scan evidence of damage confined to the right hemisphere. Neuropsychologia. 1986;24:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 142] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Zhou S, Carroll E, Nicholson S, Vize CJ. Blurred vision. BMJ. 2020;368:m569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Rowe FJ; VIS writing Group. Vision In Stroke cohort: Profile overview of visual impairment. Brain Behav. 2017;7:e00771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Kanwisher N. The Quest for the FFA and Where It Led. J Neurosci. 2017;37:1056-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Busigny T, Joubert S, Felician O, Ceccaldi M, Rossion B. Holistic perception of the individual face is specific and necessary: evidence from an extensive case study of acquired prosopagnosia. Neuropsychologia. 2010;48:4057-4092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Barton JJ. Structure and function in acquired prosopagnosia: lessons from a series of 10 patients with brain damage. J Neuropsychol. 2008;2:197-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 19. | Pancaroglu R, Hills CS, Sekunova A, Viswanathan J, Duchaine B, Barton JJ. Seeing the eyes in acquired prosopagnosia. Cortex. 2016;81:251-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Fox CJ, Iaria G, Barton JJ. Disconnection in prosopagnosia and face processing. Cortex. 2008;44:996-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Bruce V, Young A. Understanding face recognition. Br J Psychol. 1986;77:305-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2523] [Cited by in RCA: 2357] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 22. | Lenoir H, Siéroff É. Visual perceptual disorders in Alzheimer's disease. Geriatr Psychol Neuropsychiatr Vieil. 2019;17:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Kawagoe T, Matsushita M, Hashimoto M, Ikeda M, Sekiyama K. Face-specific memory deficits and changes in eye scanning patterns among patients with amnestic mild cognitive impairment. Sci Rep. 2017;7:14344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Villa-Bonomo C, Pagonabarraga J, Martínez-Horta S, Fernandez de Bobadilla R, Garcia-Sanchez C, Campolongo A, Kulisevsky J. Short-lasting episodes of prosopagnosia in Parkinson's disease. Parkinsonism Relat Disord. 2013;19:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Tejeria L, Harper RA, Artes PH, Dickinson CM. Face recognition in age related macular degeneration: perceived disability, measured disability, and performance with a bioptic device. Br J Ophthalmol. 2002;86:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Bala A, Iwański S, Żyłkowski J, Jaworski M, Seniów J, Marchel A. Visual disorders, the prosopometamorphopsia and prosopagnosia type in the early days after the onset of brain hemorrhagic stroke--a case report. Neurocase. 2015;21:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |