Published online Dec 26, 2020. doi: 10.12998/wjcc.v8.i24.6408
Peer-review started: July 24, 2020
First decision: September 24, 2020
Revised: October 4, 2020
Accepted: October 13, 2020
Article in press: October 13, 2020
Published online: December 26, 2020
Processing time: 148 Days and 4.5 Hours
In this case study, a minimally invasive transalveolar approach using platelet-rich fibrin and bone substitute with simultaneous implantation was carried out in an elderly patient. We analyzed the cone-beam computed tomography (CBCT) findings to evaluate bone regeneration.
A 65-year-old female with no contraindications for dental implants and loss of maxillary bilateral molars is described. Examination by CBCT showed the available vertical bone height in the bilateral posterior maxilla was 0.5-6.8 mm in the left and 2.8-6.5 mm in the right. The patient underwent a transalveolar approach using platelet-rich fibrin and bone substitute with simulataneous placement of an implant 10 mm in length. Six months post-surgery, the implant showed excellent osseointegration with the bone graft. Thereafter, full-ceramic crowns were fitted. Follow-up at 2 years demonstrated satisfactory prognosis.
Platelet-rich fibrin and bone substitute can be used to augment the maxillary sinus with a vertical bone height less than 4 mm.
Core Tip: Insufficient height of residual bone in the posterior maxilla is commonly encountered after tooth loss in elderly people. When the vertical bone height is less than 5 mm, the lateral antrostomy approach is recommended. In this case, cone-beam computed tomography examination showed that the available vertical bone height in the bilateral posterior maxilla was 0.5-6.8 mm in the left and 2.8-6.5 mm in the right. We chose the crest approach with platelet-rich fibrin and bovine bone graft material and obtained a good outcome. This case demonstrates that minimally invasive implantation and repair in elderly patients can be achieved, with reduced surgical trauma, postoperative pain, swelling, surgical costs and significantly shortened the course of implantation and repair. Therefore, in elderly patients, based on the premise of strict mastery of surgical indications and operating skills, this minimally invasive method with simultaneous implantation in the maxillary sinus can be considered.
- Citation: Yang S, Yu W, Zhang J, Zhou Z, Meng F, Wang J, Shi R, Zhou YM, Zhao J. Minimally invasive maxillary sinus augmentation with simultaneous implantation on an elderly patient: A case report. World J Clin Cases 2020; 8(24): 6408-6417
- URL: https://www.wjgnet.com/2307-8960/full/v8/i24/6408.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i24.6408
Insufficient height of the residual bone in the posterior maxilla is commonly encountered after tooth loss in elderly people. Alveolar bone resorption and increased pneumatization of the maxillary sinus results in a decrease in vertical bone height (VBH) of the posterior alveolus in the edentulous areas. Maxillary sinus augmentation is an effective way of resolving inadequate bone height in the posterior region of the maxilla. There are two main approaches to sinus augmentation in preparation for implant placement: Transalveolar and lateral antrostomy[1]. Rosen and colleagues[2] found that the implant survival rate was 85.7% when the VBH was 4 mm or less in the posterior region of the maxilla, which improved to 96% in locations with more than 4 mm of initial bone height. When the residual bone height is less than 5 mm, the lateral antrostomy approach is recommended[3]. However, with continuous developments in implant technology, the indications for the transalveolar approach are expanding, and it is increasingly favored by oral surgeons and patients due to its advantages of simple operation, less trauma, mild postoperative response, and low cost.
A 65-year-old female complained about the loss of maxillary bilateral molars which were extracted 6 mo previously.
Maxillary bilateral molars were extracted 6 mo previously.
The patient had good general health with a history of bruxism.
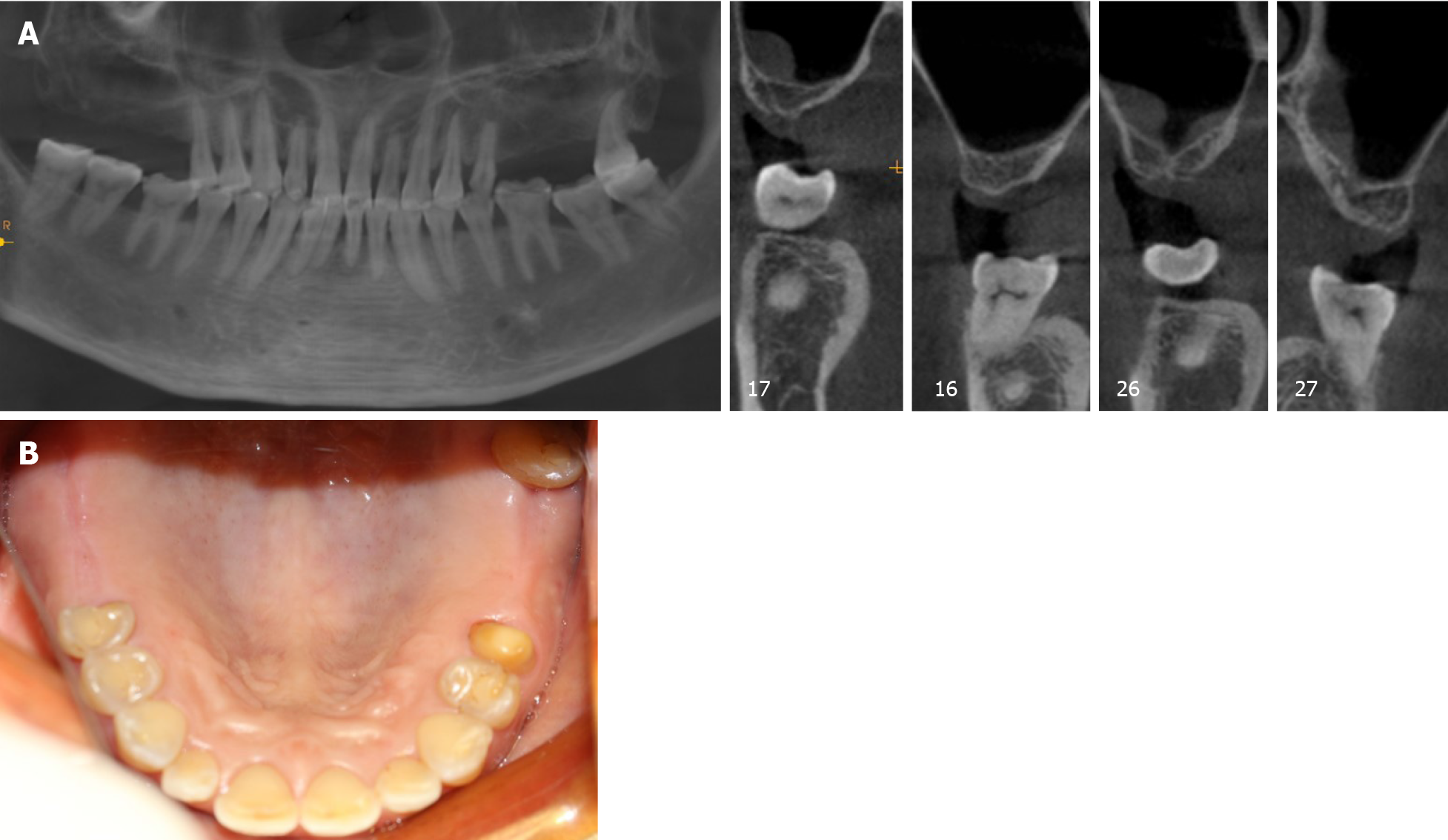
Oral examination shows that the attached gingiva was normal and the alveolar crest was less plump. There was no obvious elongation of the jaw teeth and prosthesis. #25 used to be an abutment tooth with vital pulp, which was ready for restoration. The rest of her teeth were heavily worn and she had a tight bite. Sufficient space was available for crown implants with an anatomical design (Figure 1B).
No abnormalities were observed following laboratory examinations.
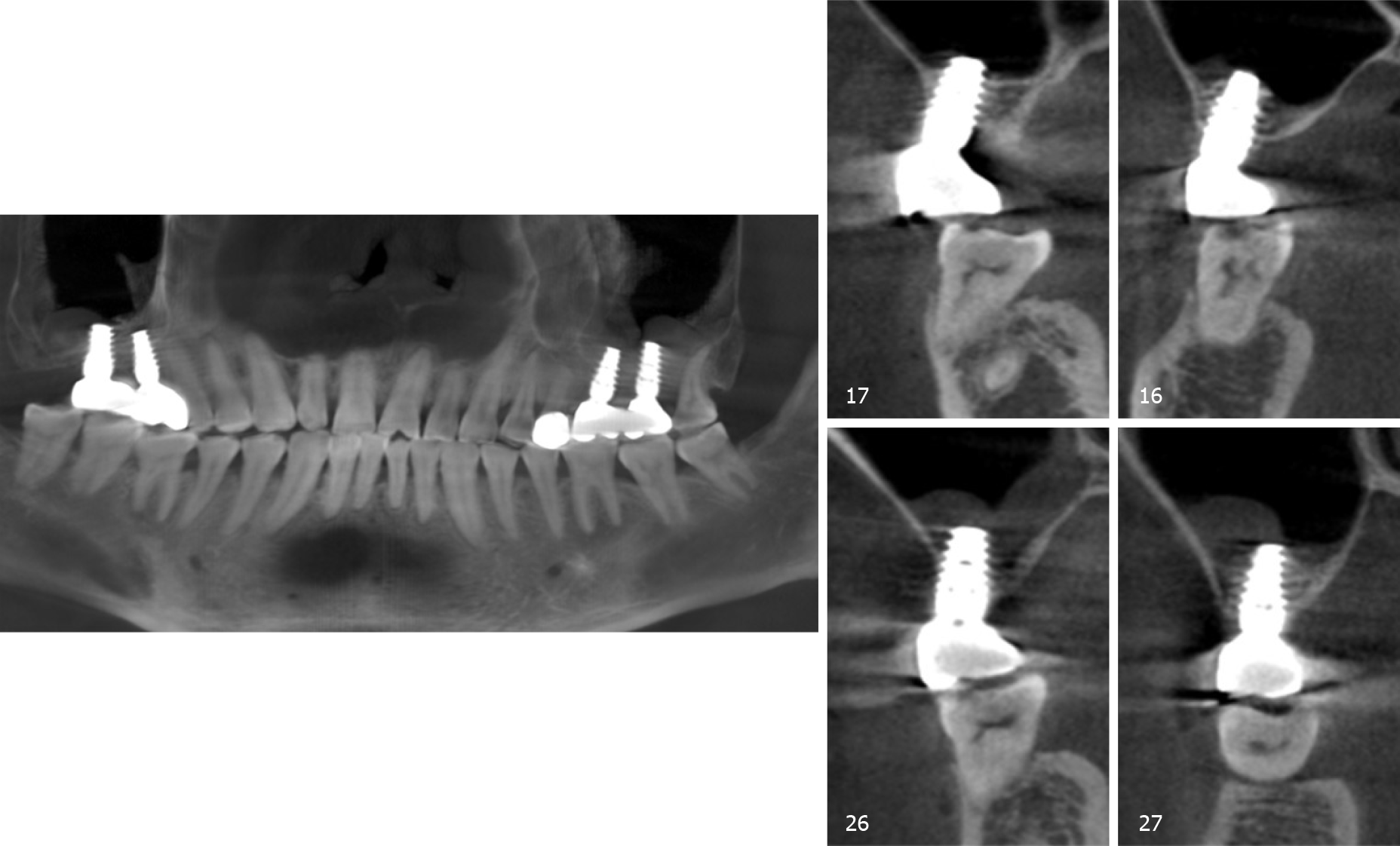
Examination by cone-beam computed tomography (CBCT, Figure 1A) showed that the available VBH was 0.5-6.8 mm in the left and 2.8-6.5 mm in the right.
The final diagnosis was Kennedy II dentition defect with a VBH less than 4 mm.
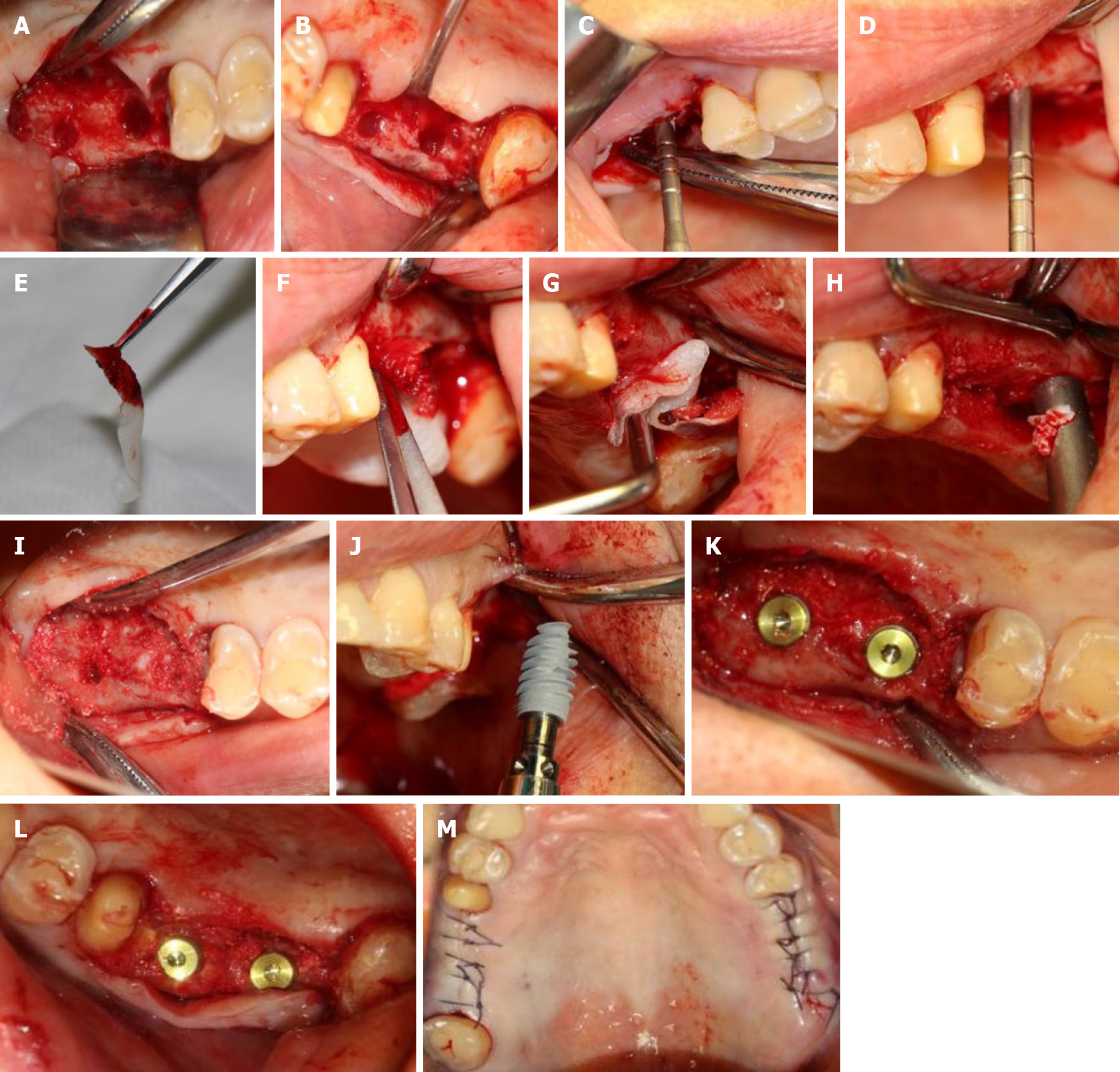
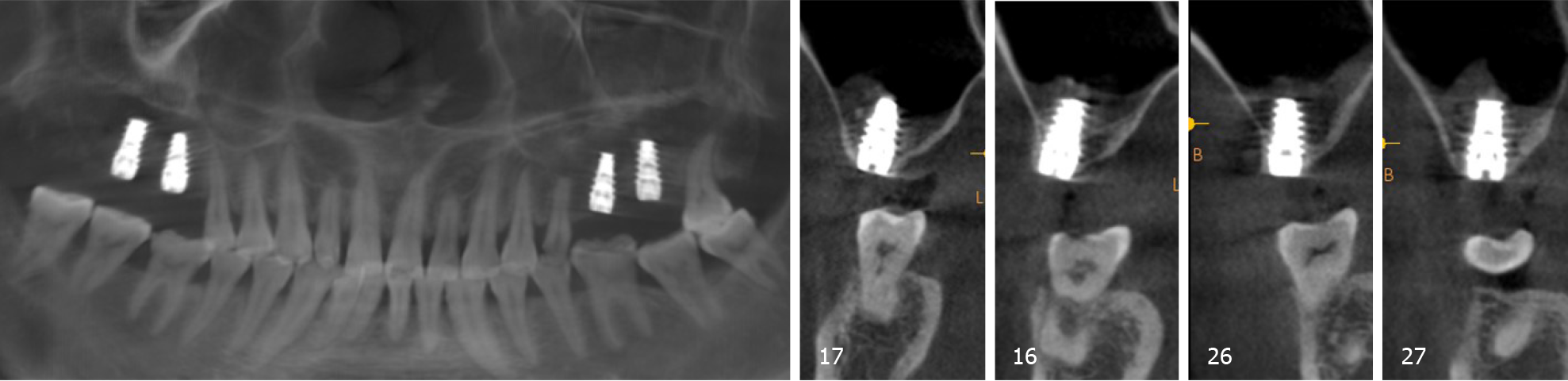
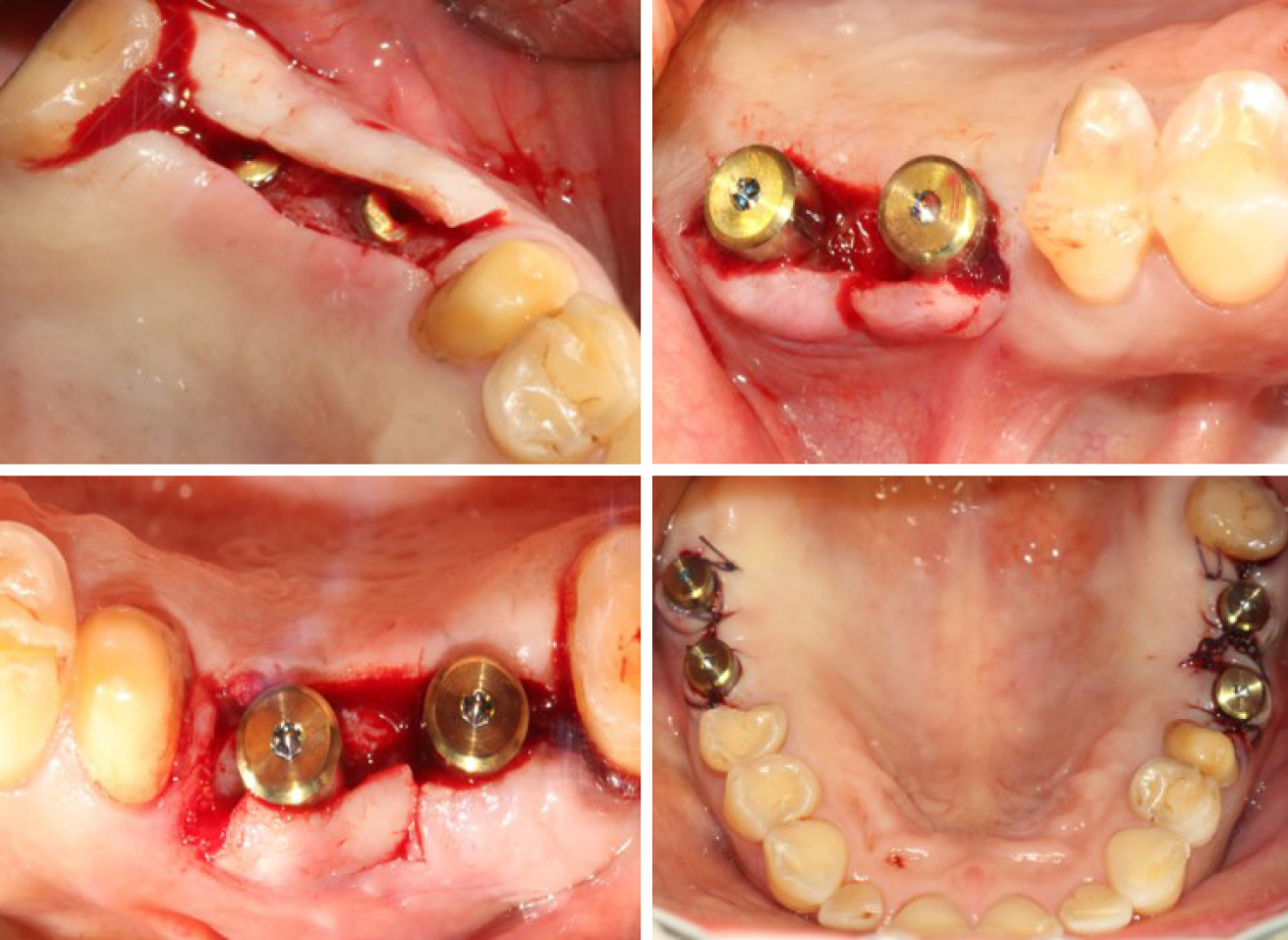
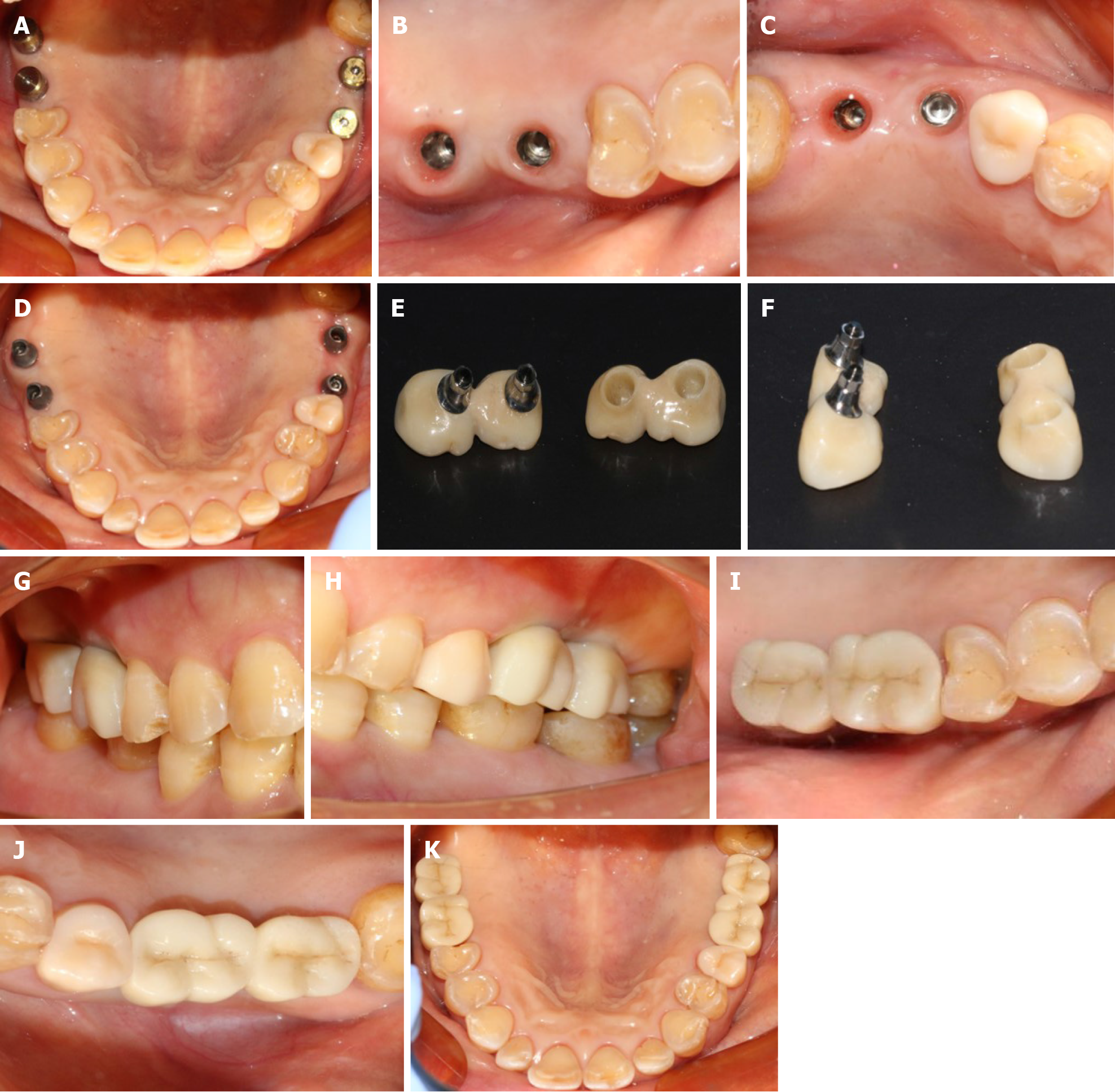
According to the patient’s condition, the treatment plan was performed using the transalveolar approach to the bilateral maxillary posterior area with four Nobel Active implants. The second stage surgery and crown repair were performed 6 mo later. Before surgery, two blood samples were centrifuged at 3000 rpm for 10 min without any anticoagulant to establish platelet-rich fibrin (PRF, Figure 2E). After coagulation, each PRF clot was prepared in membrane form (Figure 2E). In addition, 0.12% chlorhexidine mouthwash was used to rinse the mouth for 3 min/rinse which was repeated 3 times. Local infiltration of Articaine with adrenaline 1:100000 was administered to induce anesthesia. A release incision was performed to make a mucoperiosteum flap in the alveolar crest, then a pilot drill was used to prepare the implant socket leaving an approximately 1 mm gap from the maxillary sinus floor boundary (Figure 2A and B). Next, using a rounded and blunted osteotome to elevate the maxillary sinus membrane, the cortical bone of the sinus floor was up-fractured (Figure 2C and D). After that, the patient’s nose was pinched to check the integrity of the maxillary sinus. The implant, PRF and bone substitutes were simultaneously implanted (Figure 2H-J). The implants were placed (4.3 mm × 10 mm, Nobel Active, Sweden) with a torque of 35 N·cm, overlapped by a cover screw (Figure 2K and 2L). Postoperative CBCT revealed that the implants were located in the appropriate position (Figure 3).
Antibiotics, including cephalosporin and ornidazole, were administered for 3 d postoperatively.
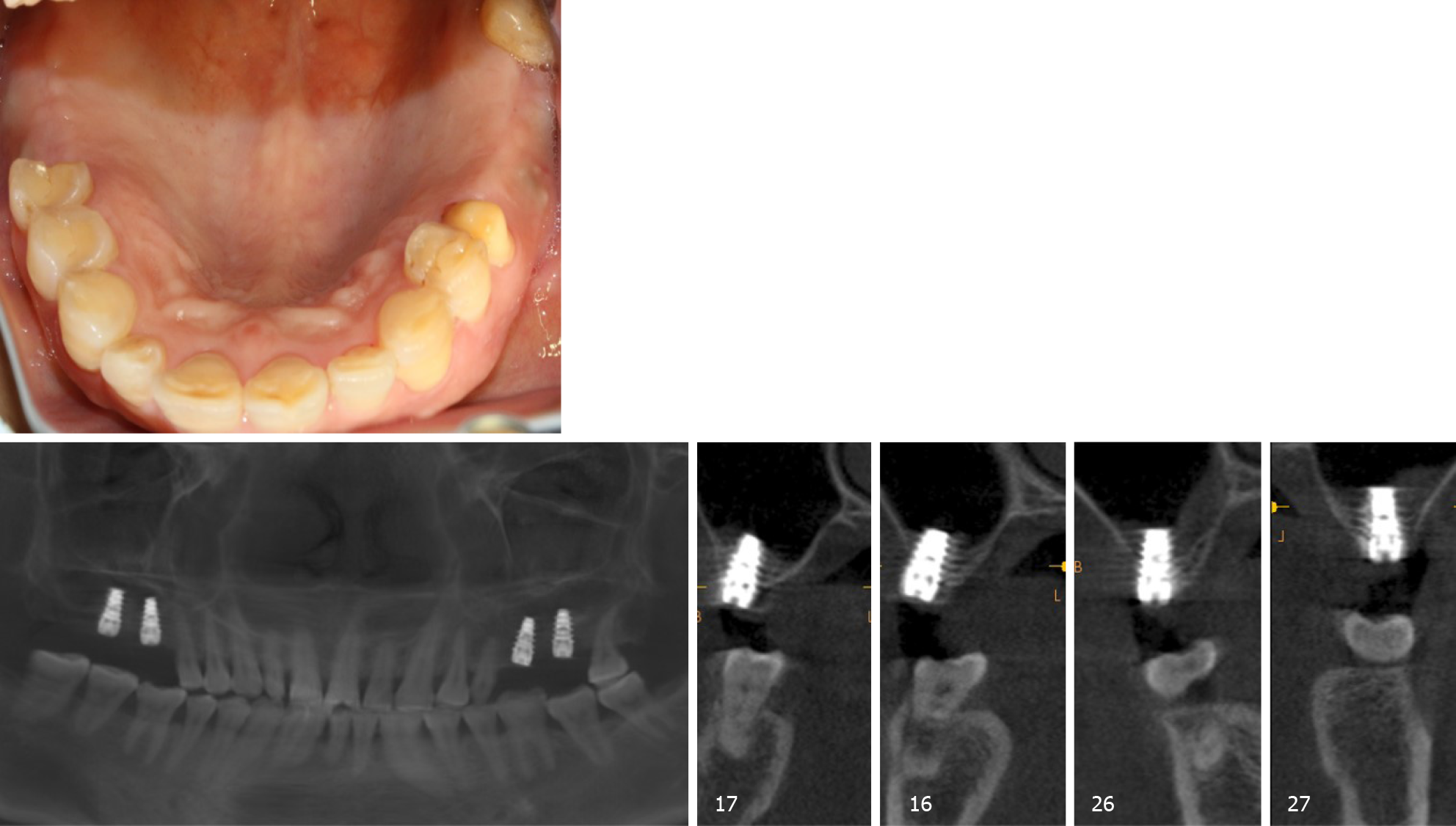
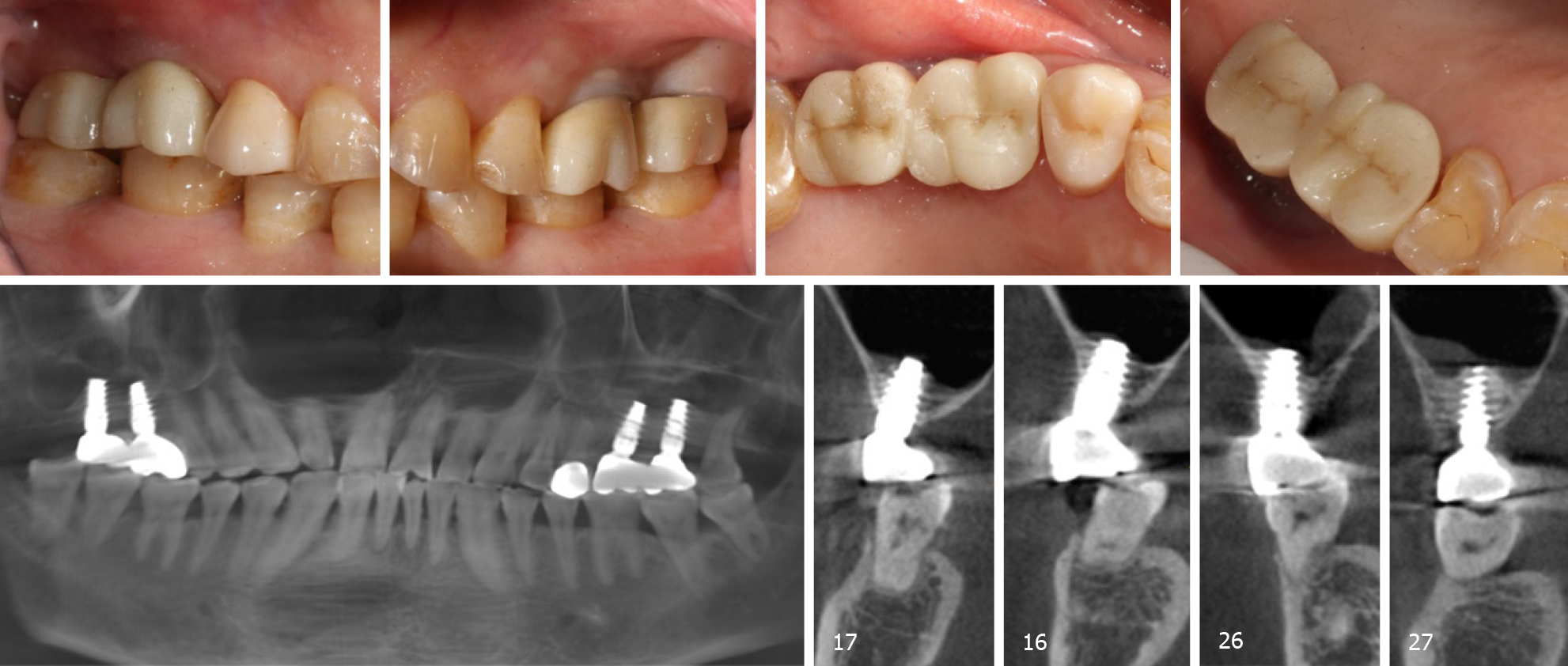
Six months later, direct contact between the bone and implant interface was observed on CBCT images (Figure 4). The cover screws were replaced with healing abutments (Figure 5). Two weeks later, a zirconia ceramic crown was constructed (Figure 6). The whole permanent abutments added torque force of 35 N·cm. At 1 mo after loading, the CBCT scan showed that the gained bone height was stable (Figure 7). The patient was followed-up for 30 mo after the first stage of surgery, with satisfactory stability, and without gingival recession (Figure 8).
Tasoulis et al[4] reviewed the literature and found that the success rate of the lateral antrostomy approach was 86%-100% while that of the transalveolar approach was 92.8%-97%. There was no significant difference in the long-term clinical effect between the two approaches. The transalveolar approach is more conservative and less invasive than the lateral approach[5]. Adequate VBH is a critical factor to avoid complications such as sinus membrane perforation. However, the VBH did not reduce the success rate of the implants and associated prostheses. Gonzalez et al[6] carried out the crestal approach with simultaneous implantation with VBH of 2-4 mm on the posterior maxillary alveolar. In this case, the elderly patient had bone rarefaction in the maxilla, and was more suitable for lateral antrostomy, but considering the patient's poor tolerance, lateral antrostomy takes longer and is more traumatic, the postoperative trauma would be greater, and recovery would be slower. Therefore, following a discussion with the patient and informed consent for treatment, we chose the crest approach. To ensure the success rate, bone substitutes, and PRF were combined to induce bone regeneration.
The combination of PRF and bovine bone graft material may be an alternative treatment option to the routinely used combination of bovine bone graft material and collagen membrane. Rodriguez et al[7] believe that the use of platelet-rich plasma in combination with bovine bone is effective for maxillary sinus augmentation with simultaneous insertion of endosseous dental implants in severely resorbed posterior maxillae. In 2001, Choukroun et al[8] developed a simpler method to concentrate platelets and fibrin without blood treatment instead of platelet-rich plasma which is more expensive and difficult to obtain. PRF belongs to the second generation of platelet concentrates, with simplified processing and without biochemical blood handling which contains a strong fibrin matrix and releases cytokines slowly, such as transforming growth factor-beta, vascular endothelial growth factor, and platelet-derived growth factor[9]. Also, fibrin is applied as a delivery system for growth factors in tissue engineering[10]. Moreover, fibrin has a significant effect on collagen synthesis in osteoblast-like cells. Thus, both cytokines and the fibrin matrix in PRF are synergistic in terms of cell migration and rapid vascularization, providing a favorable environment for the promotion of proliferation and differentiation of osteoblasts[11]. On the one hand, PRF as a sole filling material could promote natural bone regeneration when implants are placed simultaneously[12]. On the other hand, due to its good intrinsic adherence to the Schneiderian membrane, it is not only used to cover the perforation but is also used preventively to reduce the risk of perforation during the transalveolar approach[13].
Choukroun et al[14] evaluated the effectiveness of freeze-dried bone allograft (FDBA), and an FDBA and PRF mixture in sinus augmentation surgery, and found that the addition of PRF to FDBA accelerated graft maturation and decreased the healing period before implant placement[15,16]. Zhang et al[16] found that compared with PRF mixed with FDBA, the absence of precursor cells was the reason for the lack of effect of PRF mixed with bio-oss on bone formation. Tatullo et al[17] demonstrated that the combination of PRF and bovine bone graft material can promote the early stability of implants, and revealed that marked neoangiogenesis acted as good trophic support for the newly-formed bone tissue.
In this case, we placed PRF and bovine bone graft material in the floor of the maxillary sinus using the crest approach, and performed the restoration 6 mo later with the torque force at 35 N·cm. This method promotes early bone healing, and guarantees initial stability of the implant. The results in this case show that minimally invasive implantation and repair in elderly patients can be achieved, with reduced surgical trauma, postoperative pain, swelling, surgical costs and a significantly shorter implantation and repair time. Therefore, in elderly patients, based on the premise of strict mastery of surgical indications and operating skills, this minimally invasive method of simultaneous implantation in the maxillary sinus can be considered.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chhabra N S-Editor: Zhang H L-Editor: Webster JR P-Editor: Liu JH
| 1. | Zitzmann NU, Schärer P. Sinus elevation procedures in the resorbed posterior maxilla. Comparison of the crestal and lateral approaches. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 196] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Rosen PS, Summers R, Mellado JR, Salkin LM, Shanaman RH, Marks MH, Fugazzotto PA. The bone-added osteotome sinus floor elevation technique: multicenter retrospective report of consecutively treated patients. Int J Oral Maxillofac Implants. 1999;14:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Chiapasco M, Zaniboni M, Rimondini L. Dental implants placed in grafted maxillary sinuses: a retrospective analysis of clinical outcome according to the initial clinical situation and a proposal of defect classification. Clin Oral Implants Res. 2008;19:416-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Schulze-Späte U, Dietrich T, Kayal RA, Hasturk H, Dobeck J, Skobe Z, Dibart S. Analysis of bone formation after sinus augmentation using β-tricalcium phosphate. Compend Contin Educ Dent. 2012;33:364-368. [PubMed] |
| 5. | Pjetursson BE, Lang NP. Sinus floor elevation utilizing the transalveolar approach. Periodontol 2000. 2014;66:59-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Gonzalez S, Tuan MC, Ahn KM, Nowzari H. Crestal approach for maxillary sinus augmentation in patients with ≤ 4 mm of residual alveolar bone. Clin Implant Dent Relat Res. 2014;16:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Rodriguez A, Anastassov GE, Lee H, Buchbinder D, Wettan H. Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J Oral Maxillofac Surg. 2003;61:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Choukroun J, Adda F, Schoeffler C. An opportunity in perioimplantology: The PRF. Implantodontie. 2001;42:55-62. |
| 9. | Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 357] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 10. | Kawase T, Okuda K, Wolff LF, Yoshie H. Platelet-rich plasma-derived fibrin clot formation stimulates collagen synthesis in periodontal ligament and osteoblastic cells in vitro. J Periodontol. 2003;74:858-864. [PubMed] [DOI] [Full Text] |
| 11. | Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL, Chandad F, Nacopoulos C, Simonpieri A, Aalam AA, Felice P, Sammartino G, Ghanaati S, Hernandez MA, Choukroun J. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017;21:1913-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 292] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 12. | Kanayama T, Horii K, Senga Y, Shibuya Y. Crestal Approach to Sinus Floor Elevation for Atrophic Maxilla Using Platelet-Rich Fibrin as the Only Grafting Material: A 1-Year Prospective Study. Implant Dent. 2016;25:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Simonpieri A, Choukroun J, Del Corso M, Sammartino G, Dohan Ehrenfest DM. Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: a six-year experience. Implant Dent. 2011;20:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 687] [Article Influence: 36.2] [Reference Citation Analysis (1)] |
| 15. | Ali S, Bakry SA, Abd-Elhakam H. Platelet-Rich Fibrin in Maxillary Sinus Augmentation: A Systematic Review. J Oral Implantol. 2015;41:746-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, Rausch-Fan X. Effects of Choukroun's platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J Craniomaxillofac Surg. 2012;40:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Tatullo M, Marrelli M, Cassetta M, Pacifici A, Stefanelli LV, Scacco S, Dipalma G, Pacifici L, Inchingolo F. Platelet Rich Fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci. 2012;9:872-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |