Published online Dec 26, 2020. doi: 10.12998/wjcc.v8.i24.6389
Peer-review started: July 27, 2020
First decision: August 8, 2020
Revised: October 29, 2020
Accepted: November 4, 2020
Article in press: November 4, 2020
Published online: December 26, 2020
Processing time: 145 Days and 2.9 Hours
Concomitant ulcerative colitis (UC) and idiopathic thrombocytopenic purpura (ITP) is a rare phenomenon. The management of UC with ITP can be challenging, since a decreased platelet count augments UC.
A 24-year-old man with UC and steroid-resistant ITP experienced UC flare. Although continuous infusion of cyclosporine was initiated, UC did not improve. The administration of tofacitinib subsequently led to the induction of remission. The patient has maintained remission of UC and ITP for over one year on tofacitinib treatment. Whole transcriptomic sequencing was performed for inflamed rectal mucosae obtained before and after the initiation of Janus kinase (JAK) inhibitor, suggesting that distinct molecular signatures seemed to be regulated by JAK inhibitors and other conventional therapies including tumor necrosis factor lockers.
Tofacitinib should be considered in refractory cases of UC with ITP.
Core Tip: We herein report a refractory case of ulcerative colitis associated with idiopathic thrombocytopenic purpura successfully treated with tofacitinib. The relationship between these two disease entities underscore treatment implications, given their potentially shared immunological pathway and responses to similar medications. To investigate changes in gene signatures during tofacitinib therapy, whole transcriptomic sequencing was performed for inflamed rectal mucosae obtained before and after the initiation of Janus kinase (JAK) inhibitor. Distinct molecular signatures seemed to be regulated by JAK inhibition and tumor necrosis factor blockade, suggesting that the identification of gene sets may be able to predict therapeutic responses to medications.
- Citation: Komeda Y, Sakurai T, Sakai K, Morita Y, Hashimoto A, Nagai T, Hagiwara S, Matsumura I, Nishio K, Kudo M. Refractory case of ulcerative colitis with idiopathic thrombocytopenic purpura successfully treated by Janus kinase inhibitor tofacitinib: A case report. World J Clin Cases 2020; 8(24): 6389-6395
- URL: https://www.wjgnet.com/2307-8960/full/v8/i24/6389.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i24.6389
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease, is characterized by chronic relapsing inflammation of the gastrointestinal tract. The precise etiology of UC remains unknown. It has been associated with extraintestinal manifestations, including musculoskeletal diseases, mucocutaneous diseases, and hepatobiliary diseases[1,2]. The association between UC and idiopathic thrombocytopenic purpura (ITP) has also been described. The proposed pathogenesis of the concurrence of UC and ITP is antigenic mimicry between luminal antigens and platelet surface antigens[3]. The concurrence of UC with ITP adds complexity to the clinical course, since the number of bloody bowel movements is an important criterion for assessing the disease severity of UC. In addition, treatment of underlying UC flare can be challenging, since a decreased platelet count worsens the UC symptom, hematochezia. While treatment with anti-tumor necrosis factor (TNF) agents and cyclosporin as well as colectomy are acceptable options in refractory cases of UC associated with ITP[4-6], there are currently no recommended therapeutic strategies. The Janus kinase (JAK) inhibitor tofacitinib was recently introduced for the treatment of refractory UC, but its effectiveness has not been determined for UC with ITP. We herein report a refractory case of UC associated with ITP successfully treated with tofacitinib.
A 24-year-old Japanese male presented to the Gastroenterology Department of our hospital with diarrhea and hematochezia.
The patient was diagnosed with UC in 2015. At the onset, the disease was localized to the entire colon, and the patient was initially treated with mesalazine (3600 mg/d) for one year. In 2016, following a relapse, he was hospitalized. He then presented with anemia (hemoglobin 4 g/L) and thrombocytopenia (platelet count 5 × 104/μL). Further examinations, including bone marrow puncture, led to a diagnosis of concurrence of UC and ITP. The patient then started on prednisolone (30 mg/d) and achieved clinical and endoscopic remission for UC. However, since ITP was resistant to steroid and immunoglobulin treatments, he started oral cyclosporine and achieved remission for ITP. Thereafter, the patient maintained overall good health on oral mesalazine, prednisolone (5 mg/d), and cyclosporin (150 mg/d). His history was unremarkable. His family history was negative for hematological disease or inflammatory bowel disease.
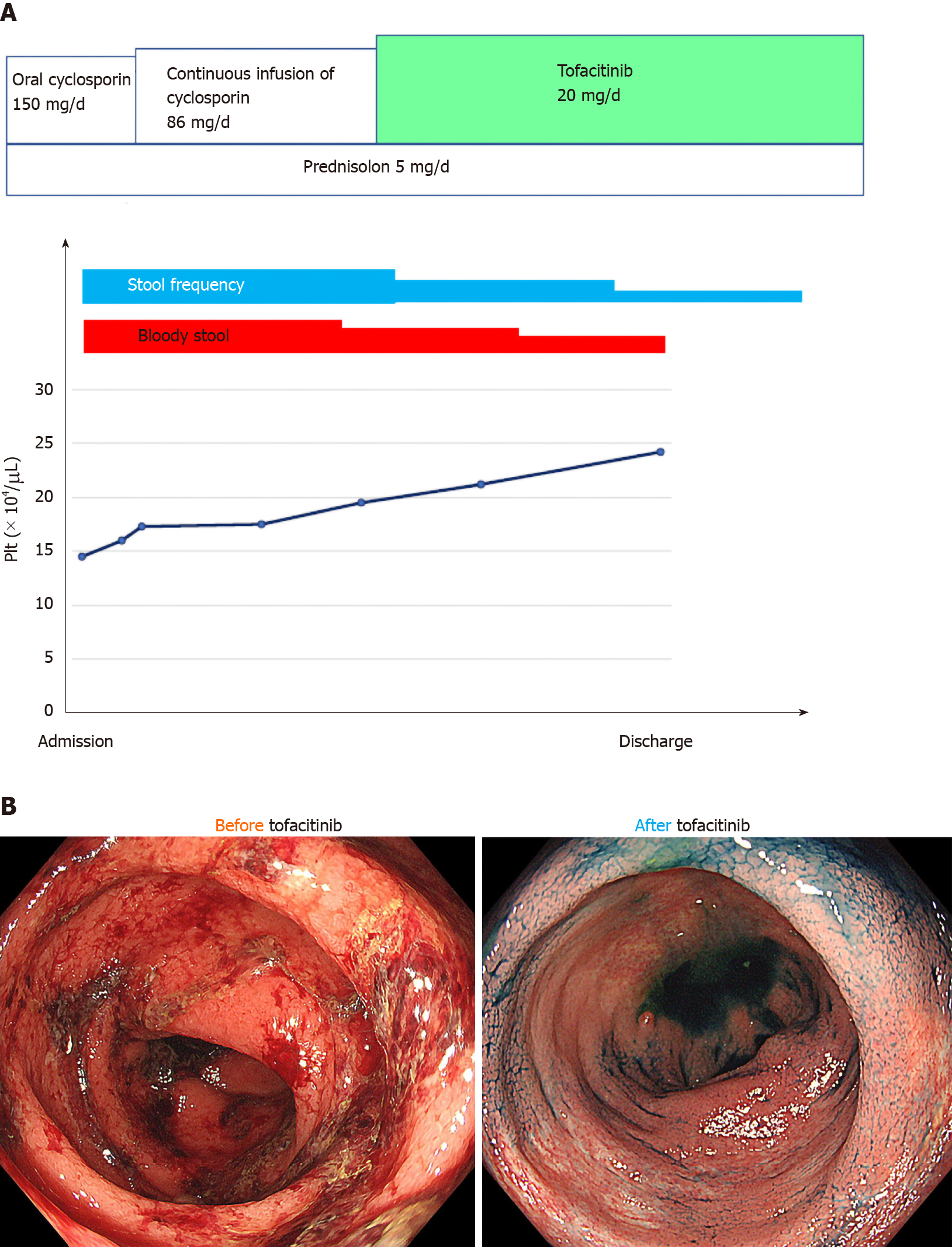
In April 2019, the patient experienced a flare of UC characterized by six bowel movements/day of liquid and bloody stool and abdominal pain. The partial Mayo index score was 7, indicating severe disease. Laboratory exams revealed anemia but not thrombocytopenia (Table 1). After the exclusion of intestinal infections, oral cyclosporine initially changed to a continuous infusion of cyclosporine to control the blood concentration strictly. The patient reported little clinical benefit. He then started tofacitinib and achieved clinical remission (Figure 1A). The patient underwent colonoscopy in September 2019, which showed endoscopic remission (Figure 1B). Prednisolone was stopped, and the patient has maintained remission in UC and ITP for over one year on mesalazine and tofacitinib.
| Laboratory test | Before tofacitinib | After tofacitinib |
| Albumin | 3.6 g/dL | 4.8 g/dL |
| C-reactive protein | 3.9 mg/dL | 0.0 mg/dL |
| White blood cell count | 11200/μL | 4500/μL |
| Erythrocyte sedimentation rate | 20 mm | 10 mm |
| Red blood cell count | 413 × 104/μL | 448 × 104/μL |
| Hemoglobin | 12.4 g/dL | 14.3 g/dL |
| Hematocrit | 36.5% | 41.7% |
| Platelet count | 14.5 × 104/μL | 25.2 × 104/μL |
Past history is unremarkable except for UC and ITP.
Personal and family history is unremarkable.
The patient did not have a fever. Physical examination showed slight tenderness in the left lower abdomen.
Laboratory exams revealed anemia but not thrombocytopenia (Table 1).
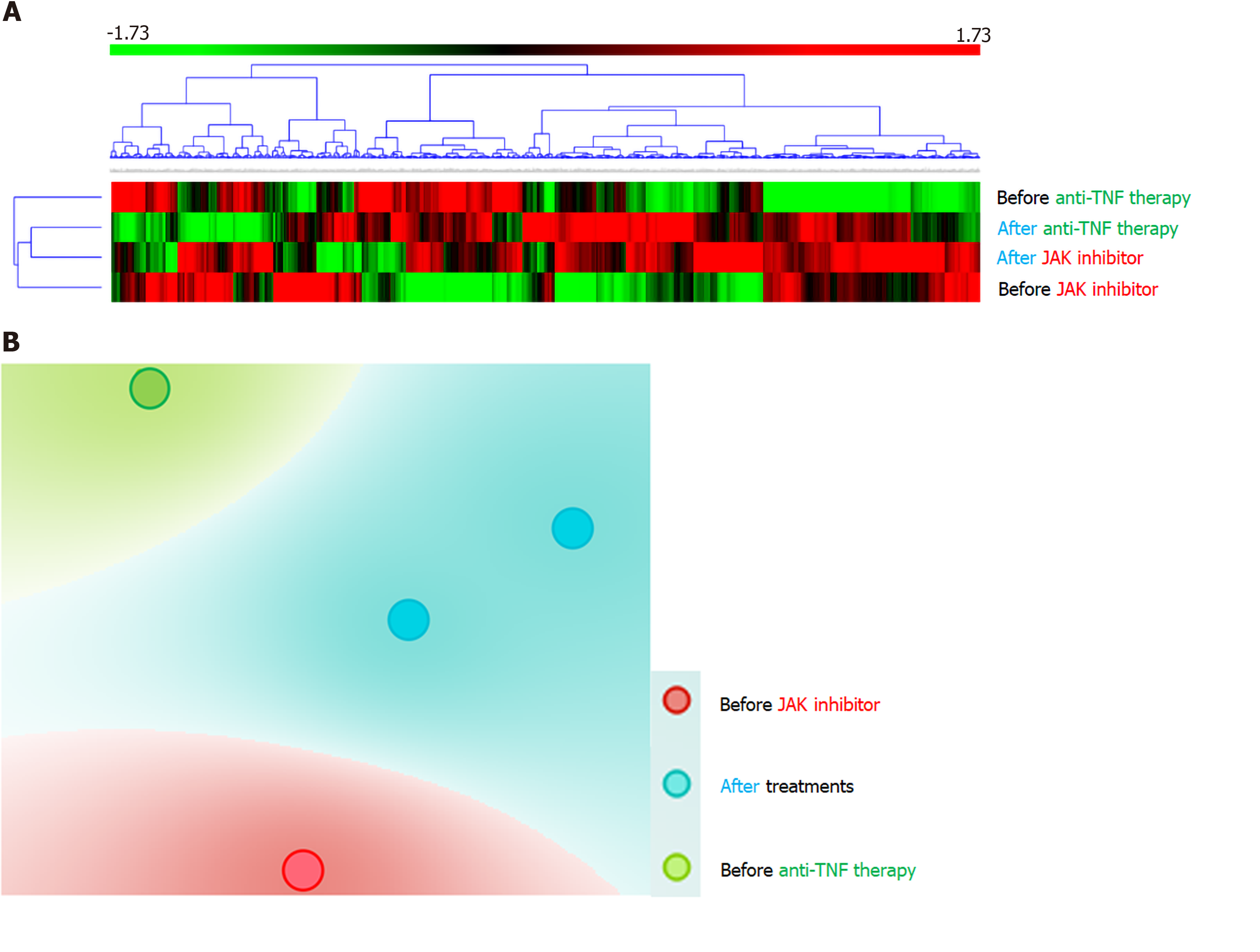
To further analyze the molecular mechanism underlying tofacitinib therapy, whole transcriptomic sequencing was performed for inflamed rectal mucosae obtained before and after the initiation of JAK inhibitor (Supplementary Material). Compared with the gene expression before tofacitinib treatment, the top 10 downregulated genes during tofacitinib treatment were REG1A, REG1B, REG3A, SPINK4, DUOXA2, TNIP3, DEFB4A, SAA2, CXCL5, and CXCL1. In contrast, the top 10 upregulated genes after the initiation of tofacitinib treatment were HMGCS2, AQP8, SLC6A19, MT1H, UGT2A3, CLDN8, MT1M, SLC51A, PRKG2, and MT1G.
A refractory case of ulcerative colitis with idiopathic thrombocytopenic purpura.
The patient was successfully treated with tofacitinib.
The patient has maintained remission in UC and ITP for over one year on mesalazine and tofacitinib.
Major concerns of tofacitinib include the risk of hematologic toxicity, such as pancytopenia, agranulocytosis, and thrombocytopenia. The patient has maintained corticosteroid-free remission for UC and ITP for one year on tofacitinib treatment. The platelet count remained within the normal range despite stopping specific treatment for ITP. The relationship between these two entities underscore treatment implications, given their potentially shared immunological pathway and responses to similar medications. To our knowledge, this study is the first to report a UC patient with ITP who was successfully treated with tofacitinib.
REG1A and REG1B are upregulated in human colonic mucosa with UC[7]. IL-22 stimulation of REG1A is based on the presence of IL-activatable elements of the REG1A promoter and may be mediated through STAT3 tyrosine phosphorylation, located downstream of the JAK signaling pathway[8]. Consistently, the expression of these genes was substantially reduced through JAK inhibition in our case. Hyams et al[9] reported that 33 genes were differentially expressed in the rectum of patients with moderate-to-severe UC who did and did not achieve corticosteroid-free remission after conventional treatments including anti-TNF therapy. The mucosal expression of DEFB4A, KRT6B, SPRR1B, TCN1, and SAA4 was high in our case, a responder to tofacitinib, while the expression was reduced in responders to conventional UC treatments, including anti-TNF therapy, compared with non-responders[9]. The expression of CHP2, GUCA2A, CA1, SLC26A2, PCK1, GLRA2, HAVCR1, and ABCG2 was low in our case that was successfully treated with tofacitinib and high in responders to conventional UC treatments[9]. In responders to tofacitinib, the expression of genes associated with resistance to conventional UC therapies was shown to be upregulated. The opposite trend in the signatures of genes related to the therapeutic response might be due to different mechanisms of action between JAK inhibitors and other therapies. Based on the findings of the present patient and another patient treated with anti-TNF antibodies who had RNA sequencing data, unsupervised hierarchal clustering of the gene panel and t-distributed Stochastic Neighbor Embedding visualization were defined (Figure 2). Consistently, distinct molecular signatures seemed to be regulated by JAK inhibition and TNF blockade, suggesting that the identification of gene sets may be able to predict therapeutic responses to medications. We have no data regarding biomarkers predicting the response to JAK inhibitors at present. Further investigations will be required to establish predictive biomarkers and personalized therapeutic strategies in IBD patients.
Tofacitinib should be considered in refractory cases of UC with ITP.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fu TL, Suppiah A S-Editor: Liu M L-Editor: A P-Editor: Ma YJ
| 1. | Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore). 1976;55:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 693] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 2. | Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 242] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Chandra S, Finn S, Obah E. Immune thrombocytopenic purpura in ulcerative colitis: a case report and systematic review. J Community Hosp Intern Med Perspect. 2014;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Hisada T, Miyamae Y, Mizuide M, Shibusawa N, Iida T, Masuo T, Okada S, Sagawa T, Ishizuka T, Kusano M, Mori M. Acute thrombocytopenia associated with preexisting ulcerative colitis successfully treated with colectomy. Intern Med. 2006;45:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Mares WG, Gerver J, Masclee AA, Pierik M. Anti-TNF treatment of ulcerative colitis associated with idiopathic thrombocytopenic purpura. Inflamm Bowel Dis. 2011;17:864-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Iwasa T, Nakamura K, Ihara E, Aso A, Ito T. The Effective Treatment with Cyclosporine of a Ulcerative Colitis Patient with Concurrent Idiopathic Thrombocytopenic Purpura Who Subsequently Developed Spontaneous Pneumomediastinum. Intern Med. 2017;56:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Tsuchida C, Sakuramoto-Tsuchida S, Taked M, Itaya-Hironaka A, Yamauchi A, Misu M, Shobatake R, Uchiyama T, Makino M, Pujol-Autonell I, Vives-Pi M, Ohbayashi C, Takasawa S. Expression of REG family genes in human inflammatory bowel diseases and its regulation. Biochem Biophys Rep. 2017;12:198-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T, Fujimori T. Involvement of the IL-22/REG Ialpha axis in ulcerative colitis. Lab Invest. 2010;90:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Hyams JS, Davis Thomas S, Gotman N, Haberman Y, Karns R, Schirmer M, Mo A, Mack DR, Boyle B, Griffiths AM, LeLeiko NS, Sauer CG, Keljo DJ, Markowitz J, Baker SS, Rosh J, Baldassano RN, Patel A, Pfefferkorn M, Otley A, Heyman M, Noe J, Oliva-Hemker M, Rufo PA, Strople J, Ziring D, Guthery SL, Sudel B, Benkov K, Wali P, Moulton D, Evans J, Kappelman MD, Marquis MA, Sylvester FA, Collins MH, Venkateswaran S, Dubinsky M, Tangpricha V, Spada KL, Saul B, Wang J, Serrano J, Hommel K, Marigorta UM, Gibson G, Xavier RJ, Kugathasan S, Walters T, Denson LA. Clinical and biological predictors of response to standardised paediatric colitis therapy (PROTECT): a multicentre inception cohort study. Lancet. 2019;393:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |