Published online Dec 26, 2020. doi: 10.12998/wjcc.v8.i24.6364
Peer-review started: July 2, 2020
First decision: September 12, 2020
Revised: September 24, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: December 26, 2020
Processing time: 169 Days and 22.8 Hours
Sclerosing stromal tumor (SST) is an extremely rare sex cord stromal tumor of the ovary. It was first reported and named in 1973. These tumors typically present with pelvic/abdominal pain and tenderness, a mass, and/or abnormal menses, but rarely present with masculinity in children and adolescents. Only 2 cases of these tumors have been reported in premenarchal girls, who demonstrated hormonal activity, with a history of the development of a virilizing female due to hyperandrogenism. Here, we report a case of a giant SST with obvious masculinity combined with Meig’s syndrome and CA125 elevation.
A 17-year-old female presented with a 7-year history of the development of masculinity and a 2-year history of amenorrhea. She had hirsutism, acne, obvious laryngeal prominence, and voice deepening. Physical examination showed a male suprapubic hair pattern and a 4.0 cm × 1.5 cm enlarged clitoris. Laboratory tests showed that the testosterone level was > 15.00 ng/mL (normal range: 0.14-0.76 ng/mL), and androstenedione level was > 10.00 ng/mL (normal range: 0.3-3.3 ng/mL). A computed tomography scan of the abdomen and pelvis was carried out and showed a large, solid and cystic, partly calcified pelvic mass in the right ovary measuring 27.1 cm × 20.0 cm × 11.0 cm, 15 cm above the umbilicus (to the level of the upper part of L1). Intraoperative findings at laparotomy revealed a large tumor arising from the right ovary. Approximately, 500 mL of pale-yellow clear liquid was found in the pelvic cavity. A right salpingo-oophorectomy was performed. Microscopic examination and immunohistochemical staining of the surgical specimen showed an SST of the ovary.
This report is remarkable as our patient was not only diagnosed with an SST of the ovary, which is extremely rare in this age group, but was the largest and most obvious reported patient with this tumor who presented with virilization. Therefore, gynecologists should be aware of this potential complication in adolescent girls with a mass in the ovary.
Core Tip: Sclerosing stromal tumor (SST) of the ovary is a rare benign subtype of a sex cord stromal tumor, which occurs early in life, with an average age of 28 years. However, we report a case of a giant SST with obvious masculinity in an adolescent girl. Gynecologists should be aware of this potential complication in adolescent girls with a mass in the ovary.
- Citation: Chen Q, Chen YH, Tang HY, Shen YM, Tan X. Sclerosing stromal tumor of the ovary with masculinization, Meig’s syndrome and CA125 elevation in an adolescent girl: A case report. World J Clin Cases 2020; 8(24): 6364-6372
- URL: https://www.wjgnet.com/2307-8960/full/v8/i24/6364.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i24.6364
Sclerosing stromal tumor (SST) of the ovary is a rare benign subtype of a sex cord stromal tumor. It was first reported and named by Chalvardjian et al[1] in 1973. The tumor occurs early in life (80% occurring in the second and third decades), with an average age of 28 years, in contrast to other stromal tumors that commonly occur during the fifth and sixth decades[1]. It rarely occurs in children, adolescents and postmenopausal women[1]. These tumors typically present with pelvic/abdominal pain and tenderness, a mass, and/or abnormal menses, but rarely present with masculinity. However, we report a case of a giant SST with obvious masculinity combined with Meig’s syndrome and CA-125 elevation.
A 17-year-old female presented with a 7-year history of the development of masculinity and a 2-year history of amenorrhea.
A 17-year-old female was admitted to our hospital for investigation of virilization syndrome for 7 years and amenorrhea for 2 years. The patient had normal growth and development during childhood, the secondary sexual signs of masculinity gradually appeared from 10-year-old, with the development of pubic and axillary hair, thick hair on the upper lip, facial acne, deepening of the voice, and clitoromegaly. Menarche occurred at the age of 15 years, and she then developed amenorrhea. She complained of a feeling of lower abdominal distention occasionally in the past month, without other clinical abnormalities.
The patient had no significant history of past illness.
The patient does not smoke or drink, and she denied a history of drug allergy.
The patient had hirsutism, acne, obvious laryngeal prominence, and deepening of the voice. Subcutaneous tissues were few, the skeleton was thick, and muscles showed a masculine distribution. Tanner stage was II for breast and V for pubic hair development, abdominal distention was obvious, palpation of a hard mass approximately 30 cm in size, up to the xiphoid process, was observed with a clear boundary, poor activity, and no tenderness. Gynecological examination showed a male suprapubic hair pattern and a 4.0 cm × 1.5 cm enlarged clitoris (Figure 1). The hymen was seen at the vaginal orifice, and the uterus and accessories were not obvious on anal examination.
Laboratory testing showed that the testosterone level was elevated to more than 15.00 ng/mL (normal range: 0.14-0.76 ng/mL), androstenedione level was more than 10.00 ng/mL (0.3-3.3 ng/mL), dehydroepiandrosterone-sulfate (DHEA-S) level was 309.00 μg/dL (35-430 μg/dL) and CA-125 was 49.7 U/mL (0-35 U/mL). Normal levels of beta human chorionic gonadotropin < 2.0 mIU/mL (0-10 mIU/mL), alpha fetoprotein 6.0 ng/mL (< 8.1 ng/mL), CA-199 19.3 U/mL (< 30.9 U/mL), and carcinoembryonic antigen (CEA) of 1.0 ng/mL (< 2.5 ng/mL) were observed. Additional testing showed luteinizing hormone of < 0.1 IU/L, follicle stimulating hormone of 0.2 IU/L, and estradiol of 115.0 pg/mL. The chromosome evaluation revealed a 46, XX female karyotype.
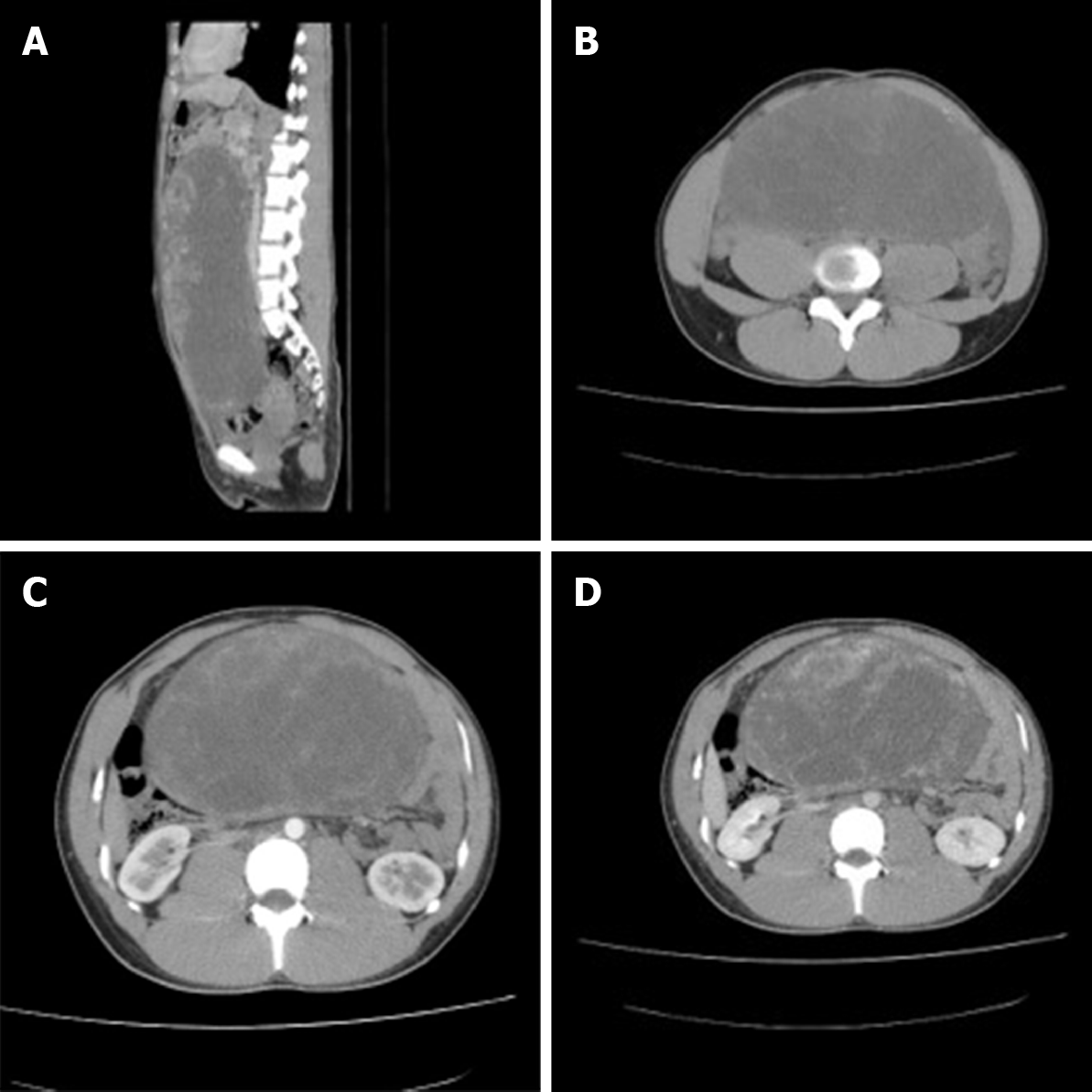
Pelvic ultrasound identified a 30.0 cm × 20.0 cm mass in the pelvic cavity. For further evaluation of the mass and adnexa, a computed tomography (CT) scan of the abdomen and pelvis was carried out and showed a large, solid and cystic, partly calcified pelvic mass in the right ovary measuring 27.1 cm × 20.0 cm × 11.0 cm, 15 cm above the umbilicus (to the level of the upper part of L1), and located in the bladder rectum depression, pushing the intestinal tube (Figure 2A). The tumor had clear boundaries and showed non-homogeneous density with solid tissue at the periphery (Figure 2B). The clitoris was approximately 4.3 cm × 1.5 cm in size, and there was a small amount of fluid in the abdominal cavity. Intravenous bolus injection of iodinated contrast medium yielded early ring enhancement of the peripheral portion of the mass (Figure 2C). In the venous phase, the degree of enhancement was decreased, but the area of enhancement increased with centripetal progression (Figure 2D). The cystic components of the inner region of the lesion were not enhanced in these phases. No discrete adrenal masses or evidence of mesenteric, retroperitoneal, or pelvic lymphadenopathy were noted. The patient was diagnosed with an ovarian tumor suspected to be a sex cord-stromal tumor.
SST of the ovary with masculinization.
Intraoperative findings at laparotomy revealed a large tumor arising from the right ovary, and approximately 500 mL of pale-yellow clear liquid in the pelvic cavity. A right salpingo-oophorectomy was performed. Evaluation of the peritoneum revealed a normal uterus, left ovary, and fallopian tubes. There was no palpable lymphadenopathy.
On gross inspection, the right ovarian mass measured 27 cm × 21 cm × 5.5 cm and weighed 4150 g, the mass was yellow to pink in color and had a smooth and well-encapsulated surface. The mass was cystic and solid in sections with a rubbery consistency, and included yellow cysts with a diameter of 0.4-5.5 cm. The solid area was gray-white, and most areas showed soft edema and necrosis, and hemorrhage was seen particularly in the subcapsular area.
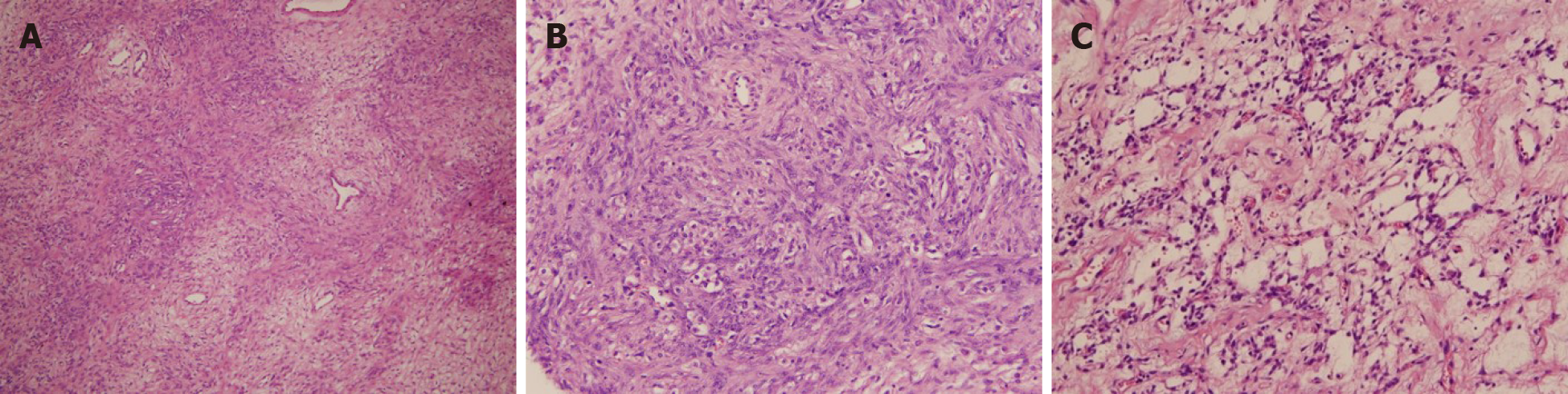
On microscopic examination, the tumor had pseudo-lobular structures of different sizes and irregular shapes (Figure 3A). The lobular architecture of the tumor was separated by thick cord-like or filamentous collagen fiber regions and loose edema regions, alternated with hypo-and hyper-cellular areas. Within the hypercellular areas, round and short spindle cells were predominant. Some of the tumor cells were rich in cytoplasm or contained vacuoles, the nucleus was oval, centered or deviated, and looked like signet-ring-like cells (Figure 3B). No cytologic atypia and rare to absent mitotic activity were noted. The background stroma, especially in the hypo-cellular areas, contained abundant collagen and blood vessels, some with “staghorn” (branching) morphology, scattered throughout the tumor (Figure 3C). Immunohistochemical staining was strongly positive for α-inhibin (Figure 4A), calretinin (Figure 4B), and multifocally positive CD99, but negative for epithelial membrane antigen (EMA), smooth muscle actin, desmin and cytokeratin (CK). Special staining showed that reticulocytes surrounded individual tumor cells (Figure 4C). The Ki67 positive rate was approximately 3%. These findings were consistent with an SST of the ovary.
Following surgery, the hormone levels returned to the normal range at 1 d, testosterone was 0.27 ng/mL, androstenedione was 2.1 ng/mL, and DHEA-S was 90.1 μg/dL. Twenty-seven days after surgery, the patient had spontaneous menarche, which showed a regular pattern. A review of hormones and B ultrasound were normal. Skin acne was significantly reduced, breast development was normal, and the clitoris was shortened compared with that before surgery, but there was no significant change in her voice and throat at the 22-mo follow-up.
Ovarian tumors have complex tissue components and are classified into epithelial tumors, germ cell tumors, sex cord-stromal tumors, and metastatic tumors according to their histological types. Ovary tumors in children and adolescents are mainly germ cell tumors, accounting for approximately 67.5%[2], 16.2% are epithelial ovarian tumors, and sex cord-stromal tumors account for only 11.1%. An SST is rare in children and adolescents. A study of ovarian cord-stromal tumors in children and adolescents showed that ovarian SSTs account for only 0.8% of ovarian tumors[3].
SSTs of the ovary are extremely rare benign neoplasms that occur early in life (80% occurring in the second and third decades), with an average age of 28 years, in contrast to other stromal tumors that commonly occur during the fifth and sixth decades[1]. They rarely occur in children, adolescents and postmenopausal women[1]. In 1999, the World Health Organization (WHO) classified SST as a subtype of follicular membrane-fibroma in ovarian cord-stromal cell tumors. In 2014, it was classified by the WHO as a pure cord-stromal tumor subtype of stromal tumors. At present, most scholars believe that SST originates from undifferentiated mesenchymal cells with multiple differentiation potentials in the ovarian cortex and can differentiate into smooth muscle. Immunohistochemistry and ultrastructural observation by electron microscopy support this view[4].
Most SSTs are hormonally inactive, the typical clinical presentation is pelvic pain, a palpable pelvic mass, menstrual disorders, precocious puberty, infertility, virilization, etc. It was initially reported as a nonfunctional benign ovarian tumor[5,6], and it was confirmed in 1975 that SST cells can produce steroid hormones[7], which usually increase patients’ estrogen levels, causing irregular menstruation, amenorrhea, and infertility. The youngest patient reported is a 7-month-old infant presenting with vaginal bleeding due to hyperestrogenism caused by SST[8]. SST can also produce androgens, and many cases of SST[9-13] combined with androgen elevation have been reported during pregnancy, but rarely in children, adolescents and postmenopausal women[14] (Table 1). Among pregnant women, two cases of SST occurred during oral clomiphene treatment for ovulation induction. Therefore, some scholars have put forward the hypothesis that the etiology of SST is related to the use of clomiphene[9]. Hormonal effects such as masculinization are extremely rare, and to date, 8 cases (Table 1) have been reported of which 3 cases occurred in pregnancy, 2 cases in premenarchal girls, 1 case in a postmenopausal woman, 1 case in a patient with McCune Albright syndrome and 1 case in a 34-year-old woman. Only two cases of SST with virilization have been reported in premenarchal girls, with the age at presentation of 9 years[15] and 11 years[16]. Following removal of the tumor, the patient’s hormone levels can quickly recover to the normal range[17,18].
| Ref. | Age (yr) | Position | Size | Clinical presentation | Course of disease | Hormone levels | Tumor marker | Type of operation | Follow-up |
| Ismail et al[10] | 29 | Bilateral ovary | The right: 9 cm, the left: 4 cm | Profuse facial and abdominal hair growth, acne and deepening of her voice | 7 wk | Testosterone, andostenedione, and DHEA-S levels were elevated | No | Bilateral salpingo-oophorectomy at 17 wk gestation | Peripheral venous testosterone and DHEA-S levels returned to normal. The patient delivered a live boy at 27 wk |
| Cashell et al[13] | 27 | Left ovary | 3 cm | Facial hair development between 14 and 16 wk of pregnancy | 2 wk | Testosterone, andostenedione, and DHEA-S levels were elevated | No | Left salpingo-oophorectomy at 19 wk gestation | The hormone levels returned to normal and hirsutism resolved |
| Huang et al[12] | 31 | Right ovary | 7 cm | The voice was deep, abdominal distention and shortness of breath occurred at 8 wk of pregnancy, and ascites was found | Unknown | Testosterone, andostenedione, and DHEA-S levels were elevated | CA-125 elevated | Right ovariectomy | Hormone levels returned to normal, ascites disappeared, term delivery, no recurrence |
| Kuscu et al[17] | 34 | Left ovary | 7 cm | Hirsute, hypomenorrhea | 3 mo | Testosterone, andostenedione, and DHEA-S levels were elevated | Normal | Left ovariectomy | Hormone levels returned to normal without recurrence |
| Park et al[16] | 11 | Left ovary | 9 cm | Deepening of the voice and hirsutism, a male suprapubic hair pattern and 1 cm sized enlarged clitoris. Tanner II for breast development, Tanner V for pubic hair | 1 yr | Andostenedione, DHEA-S and 17-OHP levels were elevated | No | Left oophorectomy | The hormone levels recovered to the normal range, the patient had menarche three months after surgery |
| Boussaïd et al[18] | 24 | Left ovary | 4.5 cm | Hirsutism, acne, deepening of voice, amenorrhea, and clitoromegaly | 5 yr | Testosterone, and 17-OHP levels were elevated | No | Left salpingo-oophorectomy | The menstrual cycle returned to normal 2 mo after surgery, the patient became pregnant shortly after |
| Yen et al[15] | 9 | Left ovary | 15 cm | Pubic and axillary hair, axillary odor, and facial acne, associated with rapid linear growth, thick hair on the upper lip, and deepening of the voice. Tanner I for breast development, Tanner IV for pubic hair, clitoromegaly measuring 4 cm × 1.5 cm | 6 mo | Testosterone, andostenedione, DHEA-S and 17-OHP levels were elevated | Normal | Left salpingo-oophorectomy | Normalization of androgen and precursor levels |
| Özdemir et al[14] | 78 | Left ovary | 10 cm | Hair growth, deepening voice, and a receding hairline, clitoromegaly measuring 2 cm | 1 yr | Testosterone, andostenedione, DHEA-S and 17-OHP levels were elevated | Normal | Total abdominal hysterectomy and bilateral salpingo-oophorectomy | The hormone levels returned to normal and virilization resolved |
A few patients may be complicated by Gorlin-Goltz syndrome[9] or Meig’s syndrome, and abnormal tumor markers[19]. Compared with the previously reported SSTs, this case had the following characteristics: (1) The tumor occurred during puberty with a long disease duration; (2) The patient had high testosterone levels and obvious male secondary sexual characteristics; (3) The patient also had Meig’s syndrome and elevated CA-125; and (4) The tumor was extremely large, with a maximum diameter of 27 cm.
A CT scan of the SST showed solid or cystic lesions. As the tumor cells were scattered in the area where the tissues were loose and edema present, the CT scan displayed a solid mass with an irregular hypodense area, and light uneven enhancement during the delayed phase and venous phase. The hypercellular areas were markedly enhanced following contrast administration, while no enhancement was seen in the cystic region and mucoid region. These imaging features have certain characteristics and differential diagnostic value[20]. The diagnosis of SST mainly depends on pathology. It typically shows a solid or cystic mass with an intact membrane. The mass can be single, multicystic or have a honeycomb-like structure. The size of the tumor varies and has a smooth surface. In section, the mass is mostly solid and greyish-white, with focal yellow areas and areas of edema. On microscopic examination, SSTs show pseudolobulation with cellular areas composed of spindle-, round- or ovoid-shaped cells, occasional mitoses, and signet-ring-like cells. These tumors also show a prominent vascular network.
The differential diagnosis of SSTs of the ovary includes ovarian metastatic signet ring cell carcinoma (Krukenberg tumor) and other sex cord-stromal tumors, such as fibroma, thecoma, and ovarian granulosa cell tumors.
In this case, the patient usually has ascites, signet ring-like cells can be seen on histology of the tumor, and this tumor is easy to misdiagnose. However, most Krukenberg tumors are bilateral, the tumor marker CA19-9 in blood is often elevated, and gastrointestinal symptoms may be present. There is no pseudolobular structure under the microscope, and the tumor cells are highly heterogeneous, showing a single scattered or cord-like distribution in the ovarian stroma. In addition, small clumps of myxoid cancer cells, and even signet ring cells, and pathological mitotic figures are easily seen. Periodic acid-Schiff (PAS) staining is positive in cancer cells, and immunohistochemical staining is positive for CK, EMA, and CEA. while SST hollow vesicles or signet ring-like tumor cells do not contain mucus and lipid; thus, PAS staining is negative, and immunohistochemical staining is negative for CK, EMA, and CEA.
All three tumors belong to the lineage of ovarian cord mesenchymal tumors, with some features overlapping, but SST nuclear deviated signet ring cells, and tumor mesenchymal blood vessels are abundant, and cell-rich areas of pseudolobular-like structures are regionally distributed with oligocellular areas, while ovarian fibroma and thecoma do not have the above characteristics.
Adult granulosa cell tumors mostly occur in perimenopausal or postmenopausal patients. The microscopic morphology of the tissue is diverse, with common nuclear grooves and Call-Exner bodies. Juvenile granulocytoma mostly occurs in adolescent females. It is characterized by the presence of cysts of varying sizes. Microscopically, cystic follicular structures of varying sizes and shapes are seen under the microscope, often accompanied by luteinization, rare nuclear grooves and Call-Exner bodies.
The only treatment for STT is surgical resection. If the frozen section indicates the diagnosis during surgery, the tumor can be simply removed. In 2016, Goebel et al[21] reported 6 cases of ovarian SST with obvious mitotic appearance and suggested that SST with a mitotic appearance > 4/10 HPF should be named mitotically active ovarian SST. One of the cases was a 24-year-old female with SST, who showed obvious necrosis, mitotic appearance of 5/10 HPE, and pelvic recurrence within 1 year after operation, but did not metastasize. Therefore, it is recommended that the number of mitotic figures be noted in the pathology report, indicating the possible risk of recurrence, and long-term follow-up is recommended.
SSTs are extremely rare in children and adolescents; therefore, when children or adolescents are encountered with virilizing manifestations, the possibility of SSTs should be considered, and at the same time as resection of the tumor, attention should be paid to the physical and mental health of these children. GnRH could be selected to inhibit the hypothalamic-pituitary-gonadal axis[15] and promote the normal development of children and adolescents.
This case may help in the detailed understanding of the rare clinical manifestations of SST of the ovary. SSTs are rare benign ovarian neoplasms that are usually diagnosed in young women, they can also occur in children and adolescents, which may cause precocious puberty, affecting the physical and mental health of these patients. The only treatment for STT is surgical resection. Therefore, gynecologists should be aware of this potential complication in adolescent girls with a mass in the ovary.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ullah M S-Editor: Huang P L-Editor: Webster JR P-Editor: Liu JH
| 1. | Chalvardjian A, Scully RE. Sclerosing stromal tumors of the ovary. Cancer. 1973;31:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Morowitz M, Huff D, von Allmen D. Epithelial ovarian tumors in children: a retrospective analysis. J Pediatr Surg. 2003;38:331-335; discussion 331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Schneider DT, Jänig U, Calaminus G, Göbel U, Harms D. Ovarian sex cord-stromal tumors--a clinicopathological study of 72 cases from the Kiel Pediatric Tumor Registry. Virchows Arch. 2003;443:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Gulati A, Kaushik R, Sharma J. Sclerosing stromal tumor of the ovary associated with benign endometrioid peritoneal implants. Indian J Pathol Microbiol. 2009;52:594-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Marelli G, Carinelli S, Mariani A, Frigerio L, Ferrari A. Sclerosing stromal tumor of the ovary. Report of eight cases and review of the literature. Eur J Obstet Gynecol Reprod Biol. 1998;76:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Lee CM, Lim S, Cho HY, Lee JS, Shin JW. Erratum to: Sclerosing Stromal Tumor of the Ovary in Postmenopausal Women: A Report of Two Cases. J Menopausal Med. 2015;21:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Damajanov I, Drobnjak P, Grizelj V, Longhino N. Sclerosing stromal tumor of the ovary: A hormonal and ultrastructural analysis. Obstet Gynecol. 1975;45:675-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Duke DS, Yoo EY, Newton C, Schwartz MZ. A rare cause of vaginal bleeding in a 7-month-old female infant. J Pediatr Surg. 2008;43:E1-E4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Grechi G, Clemente N, Tozzi A, Ciavattini A. Laparoscopic Treatment of Sclerosing Stromal Tumor of the Ovary in a Woman With Gorlin-Goltz Syndrome: A Case Report and Review of the Literature. J Minim Invasive Gynecol. 2015;22:892-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Ismail SM, Walker SM. Bilateral virilizing sclerosing stromal tumours of the ovary in a pregnant woman with Gorlin's syndrome: implications for pathogenesis of ovarian stromal neoplasms. Histopathology. 1990;17:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Zhai T, Cui M, Chen G, Zhang S, Li N. Sclerosing Stromal Ovarian Tumor Combined with Early Onset Severe Preeclampsia. A Case Report. J Reprod Med. 2015;60:249-253. [PubMed] |
| 12. | Huang SC, Chen HC, Chang KC, Chou CY. Ascites and elevated androgen level in a pregnant patient with an ovarian sclerosing stromal tumor. J Formos Med Assoc. 2003;102:124-126. [PubMed] |
| 13. | Cashell AW, Cohen ML. Masculinizing sclerosing stromal tumor of the ovary during pregnancy. Gynecol Oncol. 1991;43:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Özdemir Ö, Atalay C, Şen E, Özhamam E. Sclerosing stromal tumour of the ovary in a postmenopausal woman presenting with virilization. J Exp Ther Oncol. 2016;11:213-216. [PubMed] |
| 15. | Yen E, Deen M, Marshall I. Youngest reported patient presenting with an androgen producing sclerosing stromal ovarian tumor. J Pediatr Adolesc Gynecol. 2014;27:e121-e124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Park SM, Kim YN, Woo YJ, Choi HS, Lee JS, Heo SH, Kim CJ. A sclerosing stromal tumor of the ovary with masculinization in a premenarchal girl. Korean J Pediatr. 2011;54:224-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Kuscu E, Oktem M, Karahan H, Bilezikci B, Demirhan B. Sclerosing stromal tumor of the ovary: a case report. Eur J Gynaecol Oncol. 2003;24:442-444. [PubMed] |
| 18. | Boussaïd K, Meduri G, Maiza JC, Gennero I, Escourrou G, Bros A, Leguevaque P, Bennet A, Caron P. Virilizing sclerosing-stromal tumor of the ovary in a young woman with McCune Albright syndrome: clinical, pathological, and immunohistochemical studies. J Clin Endocrinol Metab. 2013;98:E314-E320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Terauchi F, Onodera T, Nagashima T, Kobayashi Y, Moritake T, Oharaseki T, Ogura H. Sclerosing stromal tumor of the ovary with elevated CA125. J Obstet Gynaecol Res. 2005;31:432-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Tian T, Zhu Q, Chen W, Wang S, Sui W, Wu J. CT findings of sclerosing stromal tumor of the ovary: A report of two cases and review of the literature. Oncol Lett. 2016;11:3817-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Goebel EA, McCluggage WG, Walsh JC. Mitotically Active Sclerosing Stromal Tumor of the Ovary: Report of a Case Series With Parallels to Mitotically Active Cellular Fibroma. Int J Gynecol Pathol. 2016;35:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |