Published online Dec 26, 2020. doi: 10.12998/wjcc.v8.i24.6358
Peer-review started: August 26, 2020
First decision: September 24, 2020
Revised: September 28, 2020
Accepted: October 13, 2020
Article in press: October 13, 2020
Published online: December 26, 2020
Processing time: 114 Days and 16.9 Hours
In the last decade, confocal laser endomicroscopy (CLE) has emerged as a new endoscopic imaging modality for real-time in vivo histological examination at the microscopic level. CLE has been shown to be useful for distinguishing benign and malignant lesions and has been widely used in many digestive diseases. In our study, we used CLE for the first time to examine the morphology of cholesterol polyps as well as the different parts of normal gallbladder mucosa.
A 57-year-old woman was diagnosed by ultrasound with a polyp of 21 mm in the gallbladder wall. She consented to polyp removal by laparoscopic choledo-choscopy. During laparoscopic cholecystectomy combined with choledochoscopic polyp resection, CLE was used to observe the morphology of the polyp surface cells. The appearance of the mucosa and microvessels in various parts of the gallbladder were also observed under CLE. Through comparison between postoperative pathology and intraoperative CLE diagnosis, the reliability of intraoperative CLE diagnosis was confirmed. CLE is a reliable method to examine living cell pathology during cholecystectomy. Based on our practice, CLE should be prioritized in the diagnosis of gallbladder polyps.
Compared with traditional histological examination, CLE has several advantages. We believe that CLE has great potential in this field.
Core Tip: Confocal laser endomicroscopy (CLE) has shown to be useful in distinguishing benign and malignant lesions and has been widely used in many digestive diseases, but in gallbladder disease, it has not been that popular. In our study, we used CLE for the first time to examine the morphology of cholesterol polyps as well as the different parts of normal gallbladder mucosa. Based on our practice, CLE should be prioritized in the diagnosis of gallbladder polyps. We believe that CLE has great potential in this field.
- Citation: Tang BF, Dang T, Wang QH, Chang ZH, Han WJ. Confocal laser endomicroscopy distinguishing benign and malignant gallbladder polyps during choledochoscopic gallbladder-preserving polypectomy: A case report. World J Clin Cases 2020; 8(24): 6358-6363
- URL: https://www.wjgnet.com/2307-8960/full/v8/i24/6358.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i24.6358
Confocal laser endomicroscopy (CLE) is an emerging endoscopic imaging technique. It is not simply an imitation of histopathology, and it offers a unique microscopic view of the epithelial physiology and pathology in the natural state[1], with a magnification of 500-1000. Because of its virtual histological imaging and real-time detection, it can be used in endoscopic investigation of living cells. For this reason, CLE is often known as optical biopsy.
Gallbladder polyps are commonly seen in clinical practice. To prevent misdiagnosis of gallbladder cancer, cholecystectomy is often performed for polyps > 1 cm. With the new technological development in laparoscopic gallbladder preservation, the idea of retaining the gallbladder has become more accepted[2]. However, since positive diagnosis of gallbladder cancer through imaging techniques is low, most gallbladder cancers are discovered by chance during pathological analysis after cholecystectomy. To obtain an accurate pathological picture with cholecystectomy is difficult. Consequently, the risk of malignant transformation is high.
Probe-based CLE (pCLE) could get through the working channels of various standard endoscopes[3]. Therefore, we used laparoscopy combined with choledochoscopy to place the pCLE in the gallbladder cavity to obtain diagnosis of gallbladder polyps, to observe the morphology and structure of normal gallbladder mucosal cells, and to evaluate the risk of gallbladder malignancy without damaging the gallbladder.
On October 8, 2019, a 57-year-old woman presented to The Second Affiliated Hospital of Baotou Medical College (Baotou, China). Ultrasound examination revealed a polyp in her gallbladder. She did not complain of abdominal pain.
The patient had had a gallbladder polyp identified 3 years prior to this visit. She did not complain of abdominal pain.
The patient had had a gallbladder polyp identified by ultrasound examination 3 years ago.
She had no personal or family history.
When the patient presented to our hospital, her temperature was 36.6 °C, heart rate 80 bpm, respiratory rate 16 breaths/min, blood pressure 130/90 mmHg, and oxygen saturation in room air 98%.
Blood routine examination and blood biochemical indicators were all in normal ranges.
The patient was diagnosed by ultrasound with a polyp of 21 mm in the gallbladder wall.
The patient was diagnosed with a gallbladder polyp.
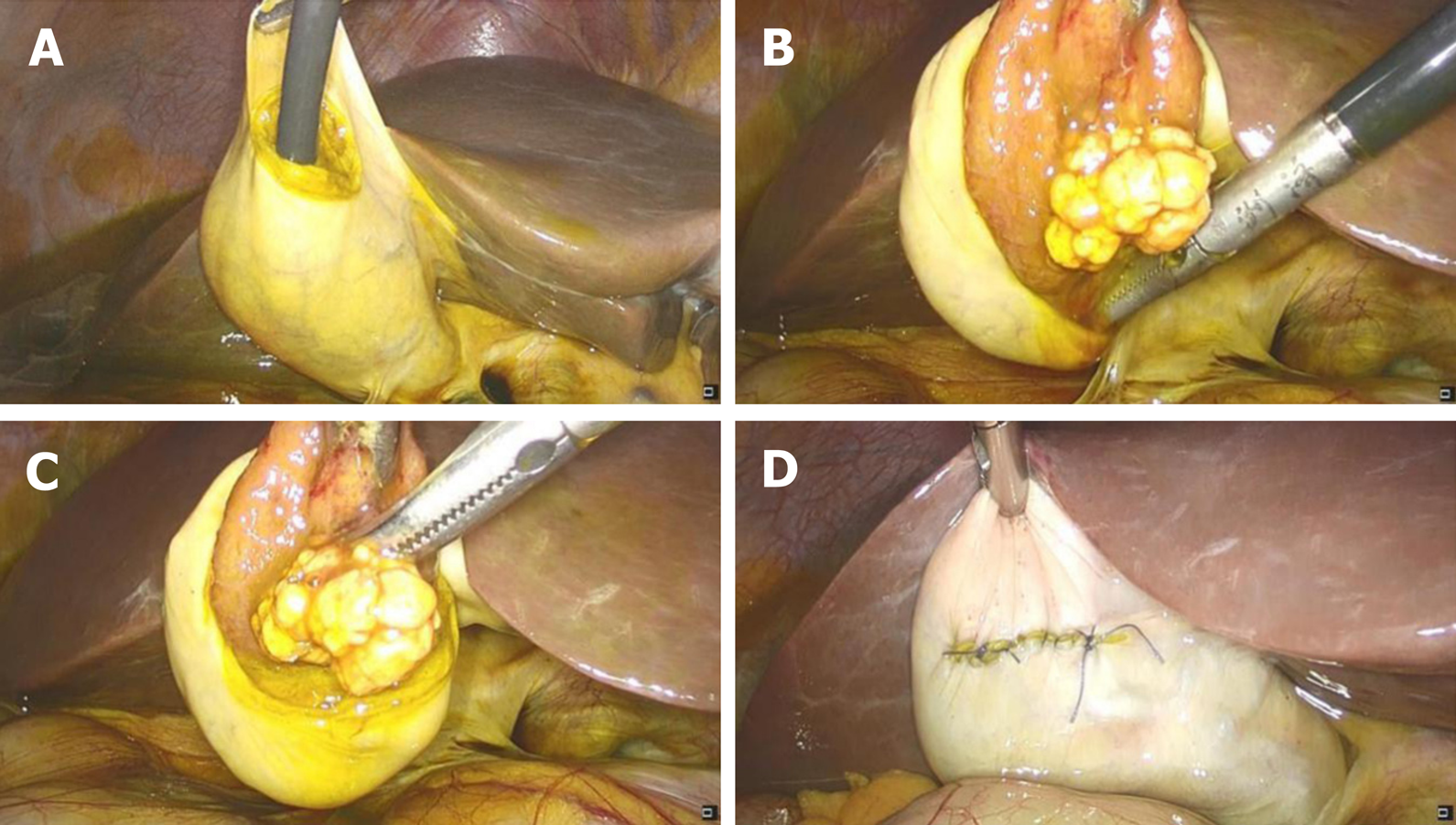
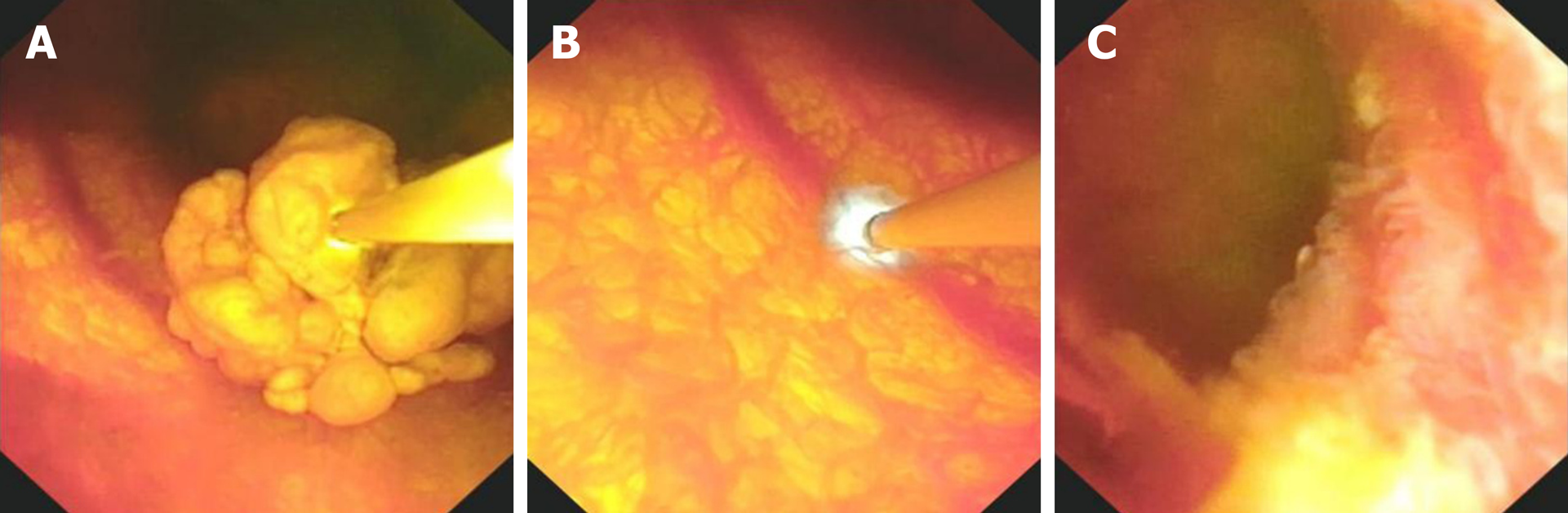
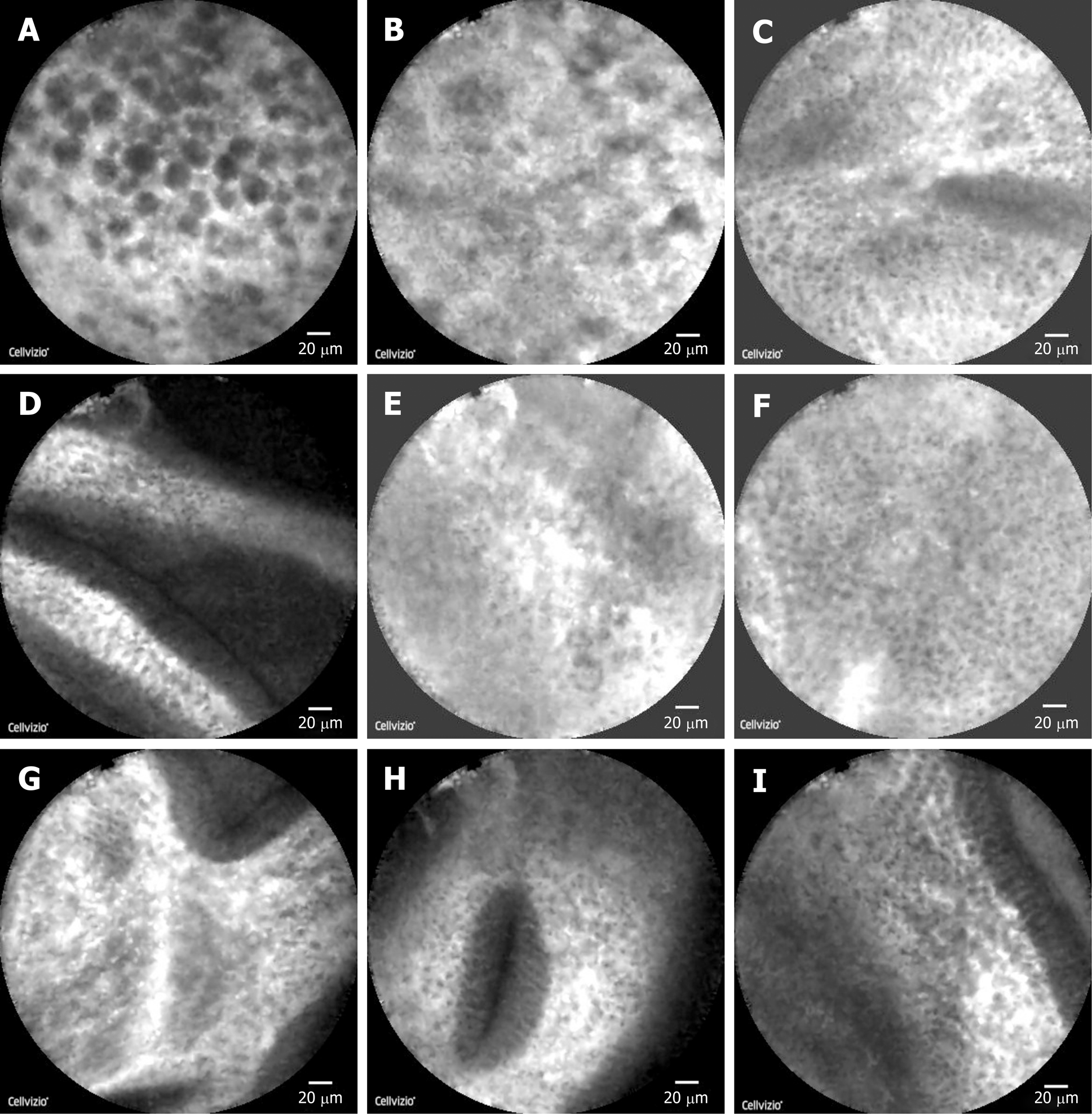
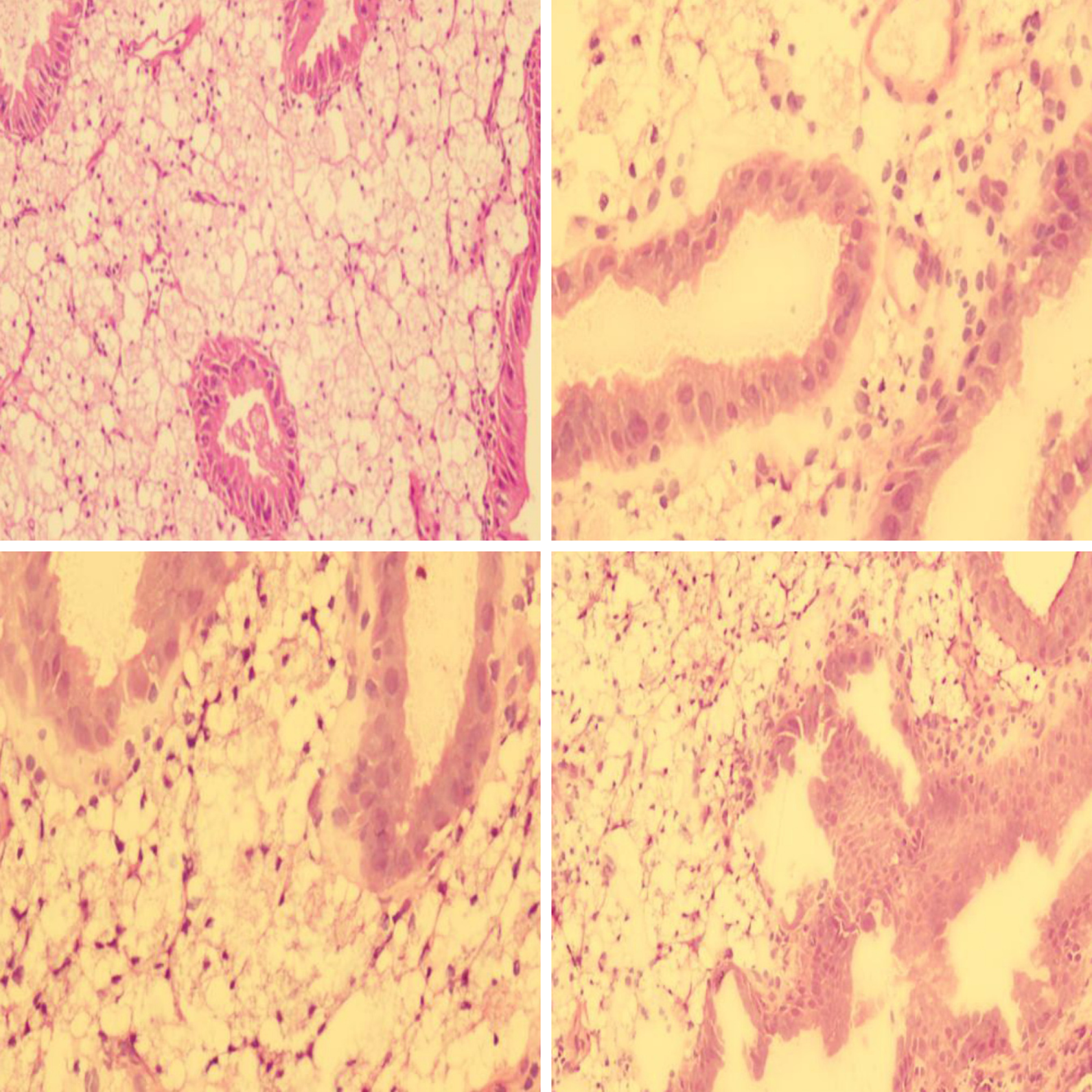
The patient consented to polyp removal by laparoscopic choledochoscopy. During the operation, the gallbladder was cut open under laparoscopy and the choledochoscope was placed in the gallbladder cavity (Figure 1A). Choledochoscopy revealed a single yellow polyp of 20 mm × 20 mm with a nodular surface on the gallbladder body (Figure 1B). Thirty seconds after intravenous injection of sodium fluorescein (10%, 2 mL, Alcon Laboratories, Cardinal Health Manufacturing Services B.V. Inc., Dublin, Ireland), pCLE (Cholangioflex; Mauna Kea Technologies, Paris, France) was placed through the choledochoscopic biopsy pore to examine the polyp surface and normal gallbladder wall (Figure 2). CLE revealed the structures of mucosal cells and microvessels in different parts of the gallbladder. The polyp cells were in a close arrangement, with round shape and black color, with a maximum diameter of about 20 μm (Figure 3A). The polyps had a few microvessels of maximum diameter around 10 μm (Figure 3B). Cholesterol polyps were considered. The remaining normal gallbladder mucosal cells were round, with nuclei located in the center of the cell, about 5-10 μm in diameter (Figure 3C). Microvessels ran in a straight line, with a diameter of 15-20 μm (Figure 3D). The gallbladder fundus mucosa contained loose cells and fewer microvessels (Figure 3E). The cells in the gallbladder body were in tighter arrangement, and microvessels were abundant (Figure 3F and G). An oval glandular structure was seen in the neck of the gallbladder, with a size of about 25 μm × 120 μm, that was black in the center with highlighted brush/dot contour at the edges (Figure 3H). The basal cells were dense, with a diameter of about 5-10 μm (Figure 3I). Combined with pCLE examination, the patient was considered to have a benign polyp surrounded by normal gallbladder mucosa. The patient underwent laparoscopic and choledochoscopic electrocoagulation resection of the polyp (Figure 1C). The gallbladder was sutured under laparoscopy (Figure 1D). Postoperative pathology confirmed the diagnosis made by intraoperative pCLE (Figure 4).
A follow-up was conducted 3 mo after the operation, and no obvious discomfort was found.
CLE has been shown to be useful for distinguishing benign and malignant lesions and has been widely used in many digestive diseases, including biliary strictures, Barrett’s esophagus, and inflammatory bowel diseases[4]. Only one case was reported in gallbladder disease[5], where CLE was used in the gallbladder cavity after endosonographic drainage. In our study, we used CLE for the first time to examine the morphology of cholesterol polyps as well as the different parts of normal gallbladder mucosa. The gallbladder mucosal structure seen using CLE was consistent with the pathological observation[6,7]. A few black strip-like structures were seen on the mucosa of the gallbladder neck, similar to the bile duct observed with CLE in other studies[8], which might be fibrin structures. In this case, we did not see gallbladder adenocarcinoma cells as in Teoh’s report[5]. Therefore, although polyp cells seen under pCLE were black, which was similar to malignant cells, we believed that they were caused by cholesterol deposition rather than lack of absorbance of the fluorescent dye. This was confirmed by postoperative pathology.
Compared with traditional histological examination, CLE has the following advantages. Firstly, in the case of gallbladder preservation operation, general pathological investigation is impossible. Therefore, preoperative computed tomography and magnetic resonance imaging are usually used to judge benign and malignant gallbladder lesions, but the accuracy is poor[9]. Especially for cystic mucosal tumors, misdiagnosis of early cancer and other lesions happens often. This reduces the reliability of gallbladder preservation. In contrast, CLE has shown great value in the distinction of benign and malignant lesions in other parts of the digestive tract. Our work is the first application of CLE in the gallbladder. It provided reliable pathological data without the destruction of gallbladder tissues. Secondly, based on our study, CLE is capable of providing accurate diagnosis of the whole gallbladder mucosa and obtaining pathological information during the operation. In addition, with CLE, we can monitor the morphology of living cells in the gallbladder wall and dynamic blood flow. In these areas, operative pathological biopsy diagnosis is incomparable. Lastly, CLE has a learning curve, which is easy to be controlled by doctors[4]. With a short learning curve, doctors can be trained to reach a reliable level of CLE diagnosis.
Based on our practice, CLE technology should be prioritized in the diagnosis of gallbladder polyps. We believe that CLE has great potential in this field.
We are grateful for the understanding and cooperation of the patient.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nakada K, Treepongkaruna S S-Editor: Chen XF L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Goetz M, Malek NP, Kiesslich R. Microscopic imaging in endoscopy: endomicroscopy and endocytoscopy. Nat Rev Gastroenterol Hepatol. 2014;11:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 2. | Zha Y, Zhou ZZ, Chen XR, Gan P, Tan J. Gallbladder-preserving cholelithotomy in laparoscopic and flexible choledochoscopic era: a report of 316 cases. Surg Laparosc Endosc Percutan Tech. 2013;23:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Hoffman A, Manner H, Rey JW, Kiesslich R. A guide to multimodal endoscopy imaging for gastrointestinal malignancy - an early indicator. Nat Rev Gastroenterol Hepatol. 2017;14:421-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | East JE, Vleugels JL, Roelandt P, Bhandari P, Bisschops R, Dekker E, Hassan C, Horgan G, Kiesslich R, Longcroft-Wheaton G, Wilson A, Dumonceau JM. Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy. 2016;48:1029-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 5. | Teoh AY, Chan AW, Chiu PW, Lau JY. In vivo appearances of gallbladder carcinoma under magnifying endoscopy and probe-based confocal laser endomicroscopy after endosonographic gallbladder drainage. Endoscopy. 2014;46 Suppl 1 UCTN:E13-E14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Frierson HF Jr. The gross anatomy and histology of the gallbladder, extrahepatic bile ducts, Vaterian system, and minor papilla. Am J Surg Pathol. 1989;13:146-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 55] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Yamamoto M, Nakajo S, Tahara E. Histological classification of epithelial polypoid lesions of the gallbladder. Acta Pathol Jpn. 1988;38:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Wen J, Ji JM, Gong B. Cholangioscopy and probe-based confocal laser endomicroscopy for real-time diagnosis of intraductal papillary mucinous neoplasm of the bile duct. Dig Endosc. 2019;31:595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Sandrasegaran K, Menias CO. Imaging and Screening of Cancer of the Gallbladder and Bile Ducts. Radiol Clin North Am. 2017;55:1211-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |