Published online Dec 26, 2020. doi: 10.12998/wjcc.v8.i24.6315
Peer-review started: September 8, 2020
First decision: September 24, 2020
Revised: October 8, 2020
Accepted: November 2, 2020
Article in press: November 2, 2020
Published online: December 26, 2020
Processing time: 102 Days and 6.3 Hours
In recent years, neoadjuvant chemoradiotherapy (NCRT) combined with surgery has been gradually applied in patients with locally advanced thoracic esophageal cancer, but its effectiveness and safety remains unclear. In this clinical trial, we prospectively investigated the efficacy and safety of NCRT plus surgery in the treatment of thoracic esophageal squamous cell carcinoma (TESCC).
To investigate the efficacy and safety of NCRT combined with surgery in the treatment of potentially resectable TESCC.
Thirty patients with advanced TESCC hospitalized in our hospital from July 2016 to June 2019 were prospectively studied. All patients received NCRT, which included intensity modulated conformal radiotherapy (40-44 Gy/20-22f, 2 Gy/f) and chemotherapy (paclitaxel 150-175 mg/m2d1, 22 + lobaplatin 25-30 mg/m2d2, 23 for two cycles). Surgery was performed after radiotherapy and chemotherapy. The effectiveness and safety of these treatments were observed.
Among these 30 patients, complete response was achieved in two cases (6.7%) and partial response in 26 cases (86.7%), yielding an objective response rate of 100%. All patients underwent radical surgery successfully. The R0 resection rate was 100%, and the pathologic complete response rate was 33.3%. The incidence of grade III- IV granulocytopenia was 10% during the NCRT, and anastomotic leakage occurred in one patient after surgery.
For patients with potentially resectable TESCC, NCRT can effectively reduce the tumor size, increase R0 resection rate, and achieve obvious pathological degradation, with mild adverse reactions. Thus, it is worthy of wider clinical application.
Core Tip: Esophageal cancer is one of the most common malignant tumors of the digestive system worldwide, with an estimated 572000 new cases and 509000 deaths annually. Treatments for locally advanced esophageal cancer include surgery and radical chemoradiotherapy. Although surgery is one of the mainstream treatments for esophageal cancer, the efficacy of surgery alone is poor. In recent years, neoadjuvant chemoradiotherapy combined with surgery has been gradually applied in patients with locally advanced thoracic esophageal cancer, but its effectiveness and safety remain unclear. In this clinical trial, we prospectively investigated the efficacy and safety of neoadjuvant chemoradiotherapy plus surgery in the treatment of thoracic esophageal squamous cell carcinoma.
- Citation: Yan MH, Hou XB, Cai BN, Qu BL, Dai XK, Liu F. Neoadjuvant chemoradiotherapy plus surgery in the treatment of potentially resectable thoracic esophageal squamous cell carcinoma. World J Clin Cases 2020; 8(24): 6315-6321
- URL: https://www.wjgnet.com/2307-8960/full/v8/i24/6315.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i24.6315
Esophageal cancer is one of the most common malignant tumors of the digestive system worldwide, with an estimated 572000 new cases and 509000 deaths annually[1,2]. Treatments for locally advanced esophageal cancer include surgery and radical chemoradiotherapy. Although surgery is one of the mainstream treatments for esophageal cancer, the efficacy of surgery alone is poor[3]. In recent years, neoadjuvant chemoradiotherapy (NCRT) combined with surgery has been gradually applied in patients with locally advanced thoracic esophageal cancer, but its effectiveness and safety remains unclear. In this clinical trial, we prospectively investigated the efficacy and safety of NCRT plus surgery in the treatment of thoracic esophageal squamous cell carcinoma (TESCC).
Patients with pathologically confirmed TESCC who were admitted to our center from July 2016 to July 2019 were prospectively enrolled. The inclusion criteria were as follows: (1) Aged 18-70 years; (2) With an Eastern Cooperative Oncology Group performance status score of 2 or lower; (3) With normal results in routine blood test, liver and kidney function tests, and cardiopulmonary examinations; (4) With at least one measurable lesion (≥ 1 cm in diameter on positron emission tomography-computed tomography (CT) or ≥ 2 cm on other imaging modalities); (5) With stage II-III esophageal cancer, which had not been treated with other anti-tumor drugs other than the study drug within the past 4 wk; the patient could receive specialized anti-tumor treatment; and (6) With good tolerance and compliance to radiotherapy and chemotherapy. The exclusion criteria included: (1) Pregnant, lactating, and fertile women who had not taken contraceptive measures; (2) Patients with severe acute infection, purulent/chronic infection, or protracted wound healing; (3) Patients with esophageal perforation (existing or possible tracheoesophageal fistula), who had shown obvious symptoms and multiple distant metastases; and/or (4) Patients with poor tolerance to radiotherapy and chemotherapy due to coagulation dysfunction, mental disorders, poor cardiopulmonary function, poor liver and kidney function, and other conditions. The general data of these patients are shown in Table 1.
| Patient characteristics | n (%) |
| Gender | |
| Males | 29 (96.7%) |
| Female | 1 (3.3%) |
| Median age (range), yr | 55 (48-70) |
| Clinical stage | |
| I | 0 |
| II | 3 (10%) |
| III | 27 (90%) |
| IV | 0 |
| Tumor location | |
| Upper chest | 0 |
| Middle chest | 4 (21.0%) |
| Lower chest | 26 (86.7%) |
All the patients underwent the intensity-modulated radiation therapy (40-44 Gy/20 - 22f, 2 Gy/f). Two cycles of chemotherapy was administered on day 1 and day 21 of radiotherapy: Paclitaxel injection 150–175 mg/m2 intravenous continuous medication drips (ivgtt) on day 1 and day 22 + lobaplatin 25-30 mg/m2 ivgtt on day 1 and day 22. Radical surgical resection was performed within 6-8 wk after the completion of the concurrent radiotherapy and chemotherapy. After surgery, adjuvant chemotherapy regimen (paclitaxel injection 150–175 mg/m2 ivgtt on day 1 + lobaplatin 25-30 mg/m2 ivgtt on day 1; two cycles) was adopted depending on the patient's pathological conditions.
The efficacy evaluation was based on the version 1.1 Response Evaluation Criteria in Solid Tumors, which included complete response (CR), partial response (PR), stable disease, and progression disease; the objective remission rate (ORR) was calculated using the following formula: ORR = (CR + PR)/ total cases × 100%. Chest CT, magnetic resonance imaging, and positron emission tomography-CT (if necessary) were performed 1 mo after NCRT, 1 mo after surgery, and 1 mo after adjuvant chemotherapy. Gastroscopy was performed 3 mo after treatment for efficacy evaluation. Adverse reactions were evaluated according to the criteria of the National Cancer Institute Common Toxicity for Adverse Events version 3.0.
The primary outcome measures included progression-free survival (PFS), overall survival (OS), ORR after NCRT, pathologic complete response after surgery, and R0 resection rate after surgery; the secondary outcome measures included adverse reactions after NCRT and postoperative complications. All patients were followed up via telephone or outpatient visits.
All data were processed and analyzed using the Statistic Package for Social Science software package version 19.0 (Armonk, NY, United States). The count data are expressed in percentages and rates.
A total of 30 patients with locally advanced TESCC were enrolled in this study. Among them 4 patients did not undergo surgery (2 were clinically evaluated as CR and 2 refused surgery), and 26 received surgical treatment following NCRT (1 patient received one cycle of postoperative adjuvant chemotherapy and 11 patients received two cycles of postoperative adjuvant chemotherapy).
Preoperative therapeutic responses included CR in 2 cases (6.7%), PR in 26 cases (86.7%), and stable disease in 2 cases (6.7%); the ORR was 100%. All patients underwent laparoscopic radical surgery successfully. R0 resection was achieved in all patients (100%), and 10 patients (33.3%) achieved pathological CR (pCR) after surgery. After surgery, there were 10, 6, 1, 9, and 0 patients at stages 0, I, II, III, and IV, respectively (Table 2).
| Stage | Preoperative grade, n (%) | Postoperative grade, n (%) |
| 0 | 0 | 10 (52.6) |
| I | 0 | 6 (36.8) |
| II | 3 (10) | 1 (53.3) |
| III | 16 (90) | 9 (30) |
| IV | 0 | 0 |
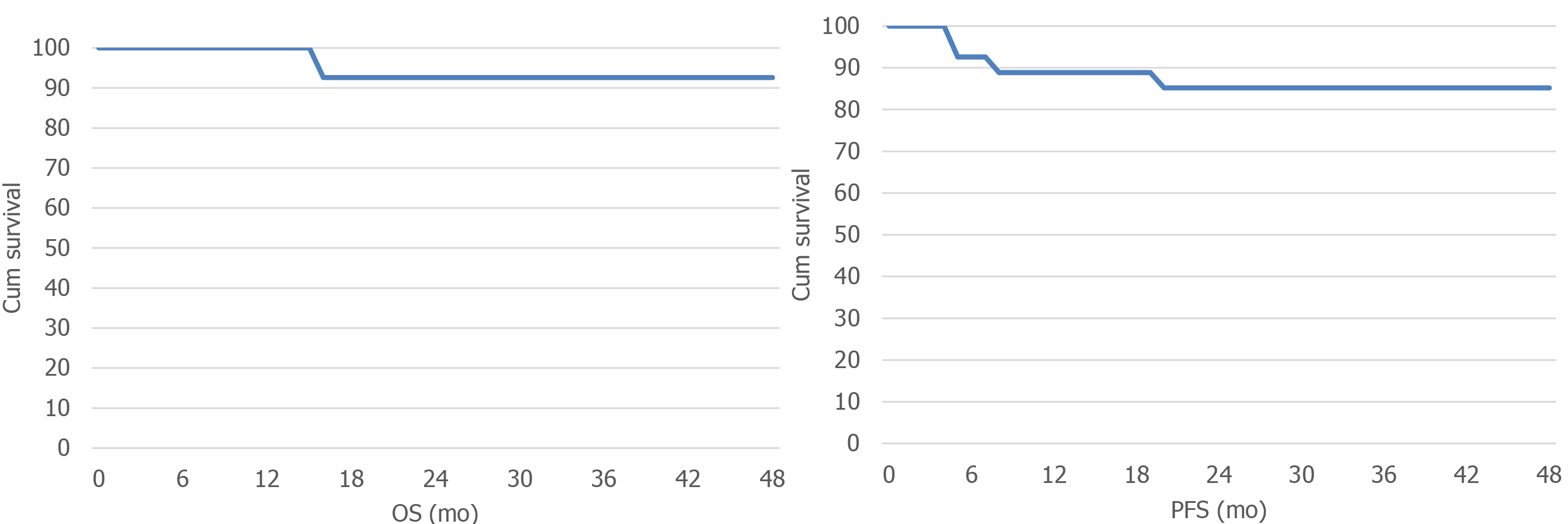
All patients were followed up till November 2019. Thirty patients were followed up for 5-40 mo, with a median follow-up period of 24 mo. The median PFS was 30 mo, and the median OS was 32 mo (Figure 1). As of the deadline of the follow-up, there were 5 cases of disease progression, including 2 cases of supraclavicular lymph node metastasis, 1 case of mediastinal lymph node metastasis, and 2 cases of distant metastasis (lung and liver).
The main adverse event of NCRT was granulocytopenia (grade IV in 2 cases, grade III in 1 case), which was improved after granulocyte colony-stimulating factor treatment. No side effects such as obvious radiation pneumonitis were seen. One patient developed anastomotic leak after surgery, which was improved after nutritional support and anti-infection treatments.
Surgery is the first treatment of choice for esophageal cancer. In China, preoperative NCRT is an important strategy for increasing the chances of surgical treatment[4]. The findings of CROSS study[5] made NCRT a hot research topic in esophageal cancer, and NCRT has become the standard treatment for esophageal adenocarcinoma. The study showed that preoperative NCRT significantly prolonged the OS and disease free survival of ESCC patients. A meta-analysis by Deng et al[6] included five clinical studies (including 709 patients) comparing preoperative NCRT vs neoadjuvant chemotherapy for advanced esophageal cancer. It was found that both NCRT and neoadjuvant chemotherapy improved the postoperative pathological complete remission rate, R0 resection rate, and 3-year survival rate in esophageal cancer patients. Further stratified analysis revealed that the 3-year survival rates of patients receiving NCRT and those receiving neoadjuvant chemotherapy were 56.8% and 42.8%, respectively. There was no significant survival benefit in patients with esophageal adenocarcinoma treated with NCRT, suggesting that NCRT could increase the survival rate of locally advanced ESCC. In our study, a total of 30 patients with locally advanced thoracic esophageal cancer were prospectively enrolled and treated with NCRT, and 26 patients successfully underwent surgical resection.
Lobaplatin is a new-generation platinum anti-tumor drug. It has also been proved to have significant therapeutic efficacy in the treatment of advanced esophageal cancer, with controllable side effects[7-9]. However, the application of lobaplatin in the NCRT for esophageal cancer has not been reported. In the present study, all patients were treated with paclitaxel combined with lobaplatin as the preoperative neoadjuvant chemotherapy regimen for potentially resectable TESCC. The same regimen was also applied in the postoperative adjuvant chemotherapy. No severe chemotherapy-induced nausea or vomiting was noted. The incidence of grade III-IV granulocytopenia was 10% during the NCRT, and no severe thrombocytopenia occurred.
There are only a few reports on the optimal radiation dose in concurrent NCRT for esophageal cancer. In 2018, Ji et al[10] retrospectively analyzed the clinic pathological characteristics of 8881 patients treated with NCRT for esophageal cancer. According to the different doses of radiotherapy, these patients were divided into high-dose group (n = 6248, 50.4 Gy), moderate-dose group (n = 2194, 45 Gy), and low-dose group (n = 439, 41.4 Gy). The median OS was 40.7 mo, 37.2 mo, and 52.6 mo and the 5-year survival rates were 40.2%, 38.7%, and 48.3%, respectively. Therefore, a radiation dose of 41.4 Gy could reduce perioperative mortality and improve survival in patients with locally advanced esophageal cancer. In the present study, the radiation dose in the NCRT was 40-44 Gy/20-22f. Initially, seven patients with esophageal cancer were treated with a radiation dose of 40 Gy/20f. Subsequently, all the remaining patients received radiotherapy at a dose of 44 Gy/22f, resulting in a postoperative pCR rate of 33.3%. Only one patient experienced postoperative anastomotic leakage, and the remaining patients had no serious radiotherapy-related side effects.
In 2018, Yang et al[11] reported the findings of a multicenter phase III clinical trial comparing the safety of NCRT plus surgery vs surgery alone in patients with resectable ESCC from 2007 to 2014; 451 patients were randomly allocated to NCRT plus surgery (group CRT; n = 224) and surgery alone (group S; n = 227). In group CRT, patients were treated with vinorelbine and cisplatin for two cycles, with a total concurrent radiation dose of 40.0 Gy administered in 20 fractions, and the pCR rate was 43.2%. The R0 resection rates were 98.4%and 91.2%, respectively, in group CRT and group S, with a median OS of 100.1 mo and 66.5 mo. Grade III or IV leukocytopenia (48.9%) and neutropenia (45.7%) were the most common adverse events in group CRT, and the postoperative complications showed no significant difference between these two groups. It was suggested that preoperative NCRT plus surgery can achieve pathological downgrading, increase the resection rate, and prolong the survival in patients with locally advanced ESCC, with controllable adverse effects. Similarly, the postoperative R0 resection rate reached 100% and the pCR rate was 33.3% in our study, and the pathological downgrading effect was obvious after NCRT.
In summary, for patients with potentially resectable TESCC, NCRT with paclitaxel combined with lobaplatin plus surgery can effectively reduce the tumor size, increase R0 resection rate, and obviously lower the pathological grading without increasing surgical complications. Paclitaxel combined with lobaplatin has mild toxicities and is worthy of wider clinical application. Our study was limited by its short follow-up period, and patient survival and tumor recurrence/metastasis need to be further investigated. In addition, the optimal dose and fractionation schedule of the neoadjuvant radiotherapy for ESCC deserve further clinical research.
In recent years, neoadjuvant chemoradiotherapy (NCRT) combined with surgery has been gradually applied in patients with locally advanced thoracic esophageal cancer, but its effectiveness and safety remains unclear.
In this clinical trial, we prospectively investigated the efficacy and safety of NCRT plus surgery in the treatment of thoracic esophageal squamous cell carcinoma (TESCC).
To investigate the efficacy and safety of NCRT combined with surgery in the treatment of potentially resectable TESCC.
Thirty patients with advanced TESCC hospitalized in our hospital from July 2016 to June 2019 were prospectively studied. All patients received NCRT, which included intensity modulated conformal radiotherapy and chemotherapy. Surgery was performed after radiotherapy and chemotherapy. The effectiveness and safety of these treatments were observed.
Among these 30 patients, complete response was achieved in 2 cases (6.7%) and partial response in 26 cases (86.7%), yielding an objective response rate of 100%. All patients underwent radical surgery successfully. The R0 resection rate was 100%, and the pathologic complete response rate was 33.3%. The incidence of grade III-IV granulocytopenia was 10% during the NCRT, and anastomotic leakage occurred in one patient after surgery.
In summary, for patients with potentially resectable TESCC, NCRT with paclitaxel combined with lobaplatin plus surgery can effectively reduce the tumor size, increase R0 resection rate, and obviously lower the pathological grading without increasing surgical complications. Paclitaxel combined with lobaplatin has mild toxicities and is worthy of wider clinical application.
Our study was limited by its short follow-up period, and patient survival and tumor recurrence/metastasis need to be further investigated. In addition, the optimal dose and fractionation schedule of the neoadjuvant radiotherapy for ESCC deserve further clinical research.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hashash JG, Kim ES S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13170] [Article Influence: 1881.4] [Reference Citation Analysis (4)] |
| 3. | Gockel I, Niebisch S, Ahlbrand CJ, Hoffmann C, Möhler M, Düber C, Lang H, Heid F. Risk and Complication Management in Esophageal Cancer Surgery: A Review of the Literature. Thorac Cardiovasc Surg. 2016;64:596-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Weijs TJ, Goense L, van Rossum PSN, Meijer GJ, van Lier AL, Wessels FJ, Braat MN, Lips IM, Ruurda JP, Cuesta MA, van Hillegersberg R, Bleys RL. The peri-esophageal connective tissue layers and related compartments: visualization by histology and magnetic resonance imaging. J Anat. 2017;230:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1829] [Article Influence: 182.9] [Reference Citation Analysis (0)] |
| 6. | Deng HY, Wang WP, Wang YC, Hu WP, Ni PZ, Lin YD, Chen LQ. Neoadjuvant chemoradiotherapy or chemotherapy? Eur J Cardiothorac Surg. 2017;51:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Jia XJ, Huang JZ. Clinical Study on Lobaplatin Combined with 5-Fu and Concurrent Radiotherapy in Treating Patients with Inoperable Esophageal Cancer. Asian Pac J Cancer Prev. 2015;16:6595-6597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Chen MQ, Chen C, Lu HJ, Xu BH. The efficacy and toxicities of combined lobaplatin with paclitaxel as a first-line chemotherapy for advanced esophageal squamous cell carcinoma. J Thorac Dis. 2015;7:1749-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 9. | Chen J, Su T, Lin Y, Wang B, Li J, Pan J, Chen C. Intensity-modulated radiotherapy combined with paclitaxel and platinum treatment regimens in locally advanced esophageal squamous cell carcinoma. Clin Transl Oncol. 2018;20:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Ji KSY, Thomas SM, Roman SA, Czito B, Anderson KL Jr, Frakes J, Adam MA, Sosa JA, Robinson TJ. Low- vs. High-Dose Neoadjuvant Radiation in Trimodality Treatment of Locally Advanced Esophageal Cancer. J Gastrointest Surg. 2019;23:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Yang H, Li T, Lordick F, D'Journo XB, Cerfolio RJ, Korst RJ, Novoa NM, Swanson SJ, Brunelli A, Ismail M, Fernando HC, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J; AME Thoracic Surgery Collaborative Group. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol. 2018;36:2796-2803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 696] [Article Influence: 99.4] [Reference Citation Analysis (0)] |