Published online Dec 26, 2020. doi: 10.12998/wjcc.v8.i24.6296
Peer-review started: August 26, 2020
First decision: September 13, 2020
Revised: September 15, 2020
Accepted: September 25, 2020
Article in press: September 25, 2020
Published online: December 26, 2020
Processing time: 114 Days and 17.2 Hours
Endoscopic submucosal dissection (ESD) has been advocated by digestive endoscopists because of its comparable therapeutic effect to surgery, reduced trauma, faster recovery, and fewer complications. However, ESD for lesions of the duodenum is more challenging than those occurring at other levels of the gastrointestinal tract due to the thin intestinal wall of the duodenum, narrow intestinal space, rich peripheral blood flow, proximity to vital organs, and high risks of critical adverse events including intraoperative and delayed bleeding and perforation. Because of the low prevalence of the disease and the high risks of severe adverse events, successful ESD for lesions of the duodenum has rarely been reported in recent years.
To investigate the efficacy and safety of ESD in the treatment of duodenal space-occupying lesions.
Clinical data of 24 cases of duodenal lesions treated by ESD at the Digestive Endoscopy Center of the Affiliated Hospital of Qingdao University from January 2016 to December 2019 were retrospectively analyzed.
All of the 24 cases from 23 patients underwent ESD treatment for duodenal space-occupying lesions under general anesthesia, including 15 male and 8 female patients, with a mean age of 58.5 (32.0-74.0) years. There were 12 lesions (50%) in the duodenal bulb, 9 (37.5%) in the descending part, and 3 (12.5%) in the ball-descending junction. The mean diameter of the lesion was 12.75 (range, 11-22) mm. Thirteen lesions originated from the mucosa, of which 4 were low-grade intraepithelial neoplasia, 3 were hyperplastic polyps, 2 were chronic mucositis, 2 were adenomatous hyperplasia, 1 was high-grade intraepithelial neoplasia, and 1 was tubular adenoma. Eleven lesions were in the submucosa, including 5 neuroendocrine neoplasms, 2 cases of ectopic pancreas, 1 stromal tumor, 1 leiomyoma, 1 submucosal duodenal adenoma, and 1 case of submucosal lymph follicular hyperplasia. The intraoperative perforation rate was 20.8% (5/24), including 4 submucosal protuberant lesions and 1 depressed lesion. The mean length of hospital stay was 5.7 (range, 3-10) d, and the average follow-up time was 25.8 (range, 3.0–50.0) mo. No residual disease or recurrence was found in all patients, and no complications, such as infection and stenosis, were found during the follow-up period.
ESD is safe and effective in the treatment of duodenal lesions; however, the endoscopists should pay more attention to the preoperative preparation, intraoperative skills, and postoperative treatment.
Core Tip: Endoscopic submucosal dissection (ESD) has been advocated by digestive endoscopists because of its comparable therapeutic effect to surgery, reduced trauma, faster recovery, and fewer complications. ESD for lesions of the duodenum is challenging due to the thin intestinal wall of the duodenum, narrow intestinal space, rich peripheral blood flow, and proximity to vital organs, such as the common bile duct and pancreas. Therefore, the duodenal lesion biopsy before ESD should be small and parallel to the fold to avoid scarring and hindering subsequent dissection. Duodenal ESD should be performed quickly to shorten the operation time and reduce delayed perforation and bleeding caused by electrocoagulation syndrome and intestinal wall edema, especially when the lesions are on the medial side of the descending segment of the duodenum because of proximity to huge arteries, the common bile duct, and pancreas tissues. The wounds after ESD should be sutured with metal clips to avoid late perforation.
- Citation: Li XY, Ji KY, Qu YH, Zheng JJ, Guo YJ, Zhang CP, Zhang KP. Application of endoscopic submucosal dissection in duodenal space-occupying lesions. World J Clin Cases 2020; 8(24): 6296-6305
- URL: https://www.wjgnet.com/2307-8960/full/v8/i24/6296.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i24.6296
Currently, with the technological development of digestive endoscopy and the increasing awareness of timely gastrointestinal tumor screening, more patients with early gastrointestinal tumors and precancerous lesions have been promptly detected and treated by minimally invasive endoscopy. Endoscopic submucosal dissection (ESD) has been advocated by digestive endoscopists because of its comparable therapeutic effect to surgery, reduced trauma, faster recovery, and fewer com-plications[1,2]. The incidence of duodenal space-occupying lesions is low[3], however, due to special anatomical characteristics, such as the abundant blood supply to the duodenum, thin intestinal wall, and small intestinal cavity space, the incidence of complications such as perforation and bleeding is high[4], and ESD treatment is challenging, though this has rarely been reported in recent years. Twenty-three patients (24 sites) with duodenal space-occupying lesions were treated by ESD in the Digestive Endoscopy Center of the Affiliated Hospital of Qingdao University from January 2016 to December 2019. In the present study, the clinical data of these cases, including therapeutic effect and postoperative follow-up, were analyzed to summarize the safety and clinical experience of ESD in the treatment of duodenal space-occupying lesions.
Patients with duodenal space-occupying lesions admitted to the Digestive Endoscopy Center of the Affiliated Hospital of Qingdao University were included in the study from January 2016 to December 2019. Patients with duodenal papillary lesions were excluded. A total of 23 patients were included, including 16 male and 7 female patients, with a mean age of 58.5 (range, 32-74) years. Among them, 15 cases had dull epigastric pain, abdominal distension, belching, and other atypical symptoms usually seen in the upper gastrointestinal tract disorders; 1 had black stools; and 7 did not complain of any obvious discomfort. Twenty-four lesions were noted in 23 patients, including 12 (50%) in the duodenal bulb, 9 (37.5%) in the descending part, and 3 (12.5%) in the ball-descending junction. The mean diameter of the lesions was 12.75 (range, 11-22) mm.
Gastroscope (Olympus GIF-H290, GIF-Q260J), duodenoscope (Olympus TJF-260V), ultrasonic endoscope (Olympus GF-UE260), ultrasonic small probe (UM-DP20-25R, 20MHZ), high-frequency electrotome (ERBE VIO-200D), flush knife, dual knife, transparent cap, injection needle, snare, hemostats, and harmonic clamp were used.
Conventional white light endoscopy revealed 11 cases of duodenal submucosal protuberant lesions, and so ultrasound endoscopy was performed. Using the degassed water filling method, an ultrasound endoscope was employed to scan at a distance of 2.0-3.0 cm from the lesion, and the location, source, boundary, size, echo, and blood supply of the lesion were recorded.
The patient's general health, underlying diseases, and lesion location, size, depth of invasion, and preoperative pathology were assessed. All patients who had surgical indications, with no surgical contraindications, were informed of endoscopic and alternative treatment options, and all signed the informed consent form. Antiplatelet and anticoagulant medications were discontinued 1 wk prior to surgery. Routine preoperative blood tests, coagulation function tests, ultrasound, computed tomography, and other auxiliary examinations were completed. Diet was prohibited for 8 h preoperatively.
Twenty-three patients underwent ESD for duodenal space-occupying lesions under general anesthesia with endotracheal intubation. Surgical procedures conducted were as follows: (1) Marker: A flush knife or dual knife was used to coagulate an area 5 mm around the lesion; (2) Submucosal injection: Submucosal injection with normal saline containing methylene blue and sodium hyaluronate was administered; (3) Mucosal layer incision; (4) Lesion dissection: The lesion was gradually dissected along the submucosa with a flush knife or dual knife. Owing to the thin wall of the duodenum, adequate submucosal injection was required to avoid injury of the muscle layer. Small submucosal vessels, especially the muscularis communicating branches, should be coagulated in advance to cut off the vessels to avoid blurred vision owing to blood in the area as well as shrinking or even vascular stump perforation after electro-coagulation. In cases of difficult dissection, after incising the whole peripheral mucosa and dissecting to a certain extent, the remaining lesions were removed with a snare (Hybrid ESD); and (5) Wound treatment: The exposed vessels on the wound surface were coagulated using hemostatic forceps. The exposed wound surface was mainly closed with a harmonious clamp or the Boston Scientific hemostatic clip. If further closure was needed, a nylon rope purse-string suture was used.
Oral intake was not permitted post-surgery. Moreover, acid suppression, gastrointestinal decompression, antibiotics to prevent infection, and water and electrolyte supplementation were employed. A liquid diet was started after exsufflation, although the fasting time was prolonged in those patients with intraoperative perforation and large wounds. Gastroscopy was performed 3 mo after the surgery to observe wound healing and detect the presence or absence of residual lesions, and endoscopy was performed every 6 mo or 1 year to review the status based on the results of the initial reexamination and postoperative pathology.
Continuous variables are expressed as the median [interquartile range (IQR)] and were compared using Student’s t test or the Mann-Whitney U test. Stata software version 22 (Stata Corp., College Station, TX, United States) was used for all statistical analyses.
A total of 24 lesions in 23 patients were removed using the ESD method: 12 (50%) duodenal bulb lesions, including 7 in the anterior wall, 3 in the greater curvature, 1 in the posterior wall, and 1 in the lesser curvature; 3 (12.5%) descending junction lesions; and 9 (37.5%) descending lesions, including 3 in the proximal segment and 6 in the distal segment (papilla side, 2 cases; opposite side of the papilla, 4 cases). The mean lesion diameter was 12.75 (range, 11-22) mm. Thirteen lesions were originating from the mucosa, of which 4 were low-grade intraepithelial neoplasia, 3 were hyperplastic polyps, 2 were chronic mucositis, 2 were adenomatous hyperplasia, 1 was high-grade intraepithelial neoplasia, and 1 was tubular adenoma. Eleven lesions were in the submucosa, including 5 neuroendocrine neoplasms, 2 cases of ectopic pancreas, 1 stromal tumor, 1 leiomyoma, 1 submucosal duodenal adenoma, and 1 case of submucosal lymphofollicular hyperplasia. The demographics and treatment strategies for the 23 patients are shown in Table 1.
| Serial number | Gender | Age(yr) | Lesion location | Diameter (mm) | Pathological hierarchy | Pathological result | En bloc resection/R0 resection | Complication | Length of hospital stay (d) | Follow-up time (mo) |
| 1 | Male | 46 | Descending distal segment | 11 | Mucosa | Low-grade intraepithelial neoplasia | Yes | 5 | 21 | |
| 2 | Male | 41 | Anterior wall of the bulb | 12 | Submucosa | Gastrointestinal stromal tumors | Yes | Perforation | 7 | 13 |
| 3 | Male | 63 | Superior wall of the bulb | 11 | Submucosa | Neuroendocrine tumor | Yes | 3 | 9 | |
| 4 | Female | 65 | Anterior wall of the bulb | 11 | Submucosa | Neuroendocrine tumor | Yes | 5 | 39 | |
| 5 | Female | 70 | Anterior wall of the bulb | 12 | Mucosa | Adenomatous hyperplasia | Yes | Perforation | 5 | 36 |
| 6 | Male | 69 | Proximal descending segment | 15 | Mucosa | Tubular adenoma | Yes | Perforation | 7 | 24 |
| 7 | Male | 74 | Descending distal segment | 11 | Mucosa | Low-grade intraepithelial neoplasia | Yes | 8 | 14 | |
| 8 | Female | 45 | Descending distal segment | 16 | Mucosa | Low-grade intraepithelial neoplasia | Yes | 5 | 24 | |
| 9 | Male | 49 | Anterior wall of the bulb | 15 | Submucosa | Neuroendocrine tumor | Yes | 7 | 29 | |
| 10 | Female | 70 | Anterior wall of the bulb | 11 | Mucosa | Adenomatous hyperplasia | Yes | 3 | 46 | |
| 11 | Male | 71 | Superior angle of the bulb | 12 | Mucosa | Hyperplastic polypModerate chronic inflammation | Yes | 7 | 45 | |
| ProximalDescending segment | 15 | Mucosa | ||||||||
| 12 | Female | 71 | Superior angle of the bulb | 11 12 | Submucosa | Ectopic pancreas | Yes | Perforation | 7 | 39 |
| 13 | Male | 32 | Ball-descending junction | 11 12 | Submucosa | Ectopic pancreas | Yes | 6 | 6 | |
| 14 | Male | 69 | Descending distal segment | 11 | Mucosa | Low-grade intraepithelial neoplasia | Yes | 4 | 45 | |
| 15 | Male | 73 | Anterior wall of the bulb | 13 | Mucosa | Hyperplastic polyp | Yes | 6 | 27 | |
| 16 | Male | 54 | Anterior wall of the bulb | 12 | Submucosa | Neuroendocrine tumor | Yes | 5 | 11 | |
| 17 | Female | 47 | Descending distal segment | 11 | Mucosa | Glandular hyperplasia with polypoid changes | Yes | 4 | 34 | |
| 18 | Male | 48 | Proximal descending segment | 20 22 | Submucosa | Leiomyoma | Yes | 10 | 11 | |
| 19 | Male | 59 | Posteriorwall of the bulb | 11 | Submucosa | Submucosal duodenal adenoma | Yes | 6 | 8 | |
| 20 | Male | 63 | Descending distal segment | 12 | Mucosa | Low- and high-grade intraepithelial neoplasia | Yes | 5 | 3 | |
| 21 | Female | 66 | Anterior wall of the bulb | 12 | Submucosa | Neuroendocrine tumor | Yes | Perforation | 7 | 50 |
| 22 | Female | 40 | Ball-descending junction | 15 | Submucosa | Submucosal lymphofollicular hyperplasia | Yes | 5 | 34 | |
| 23 | Male | 60 | Ball-descending junction | 11 | Mucosa | Chronic inflammation of the mucosal tissue | Yes | 5 | 26 |
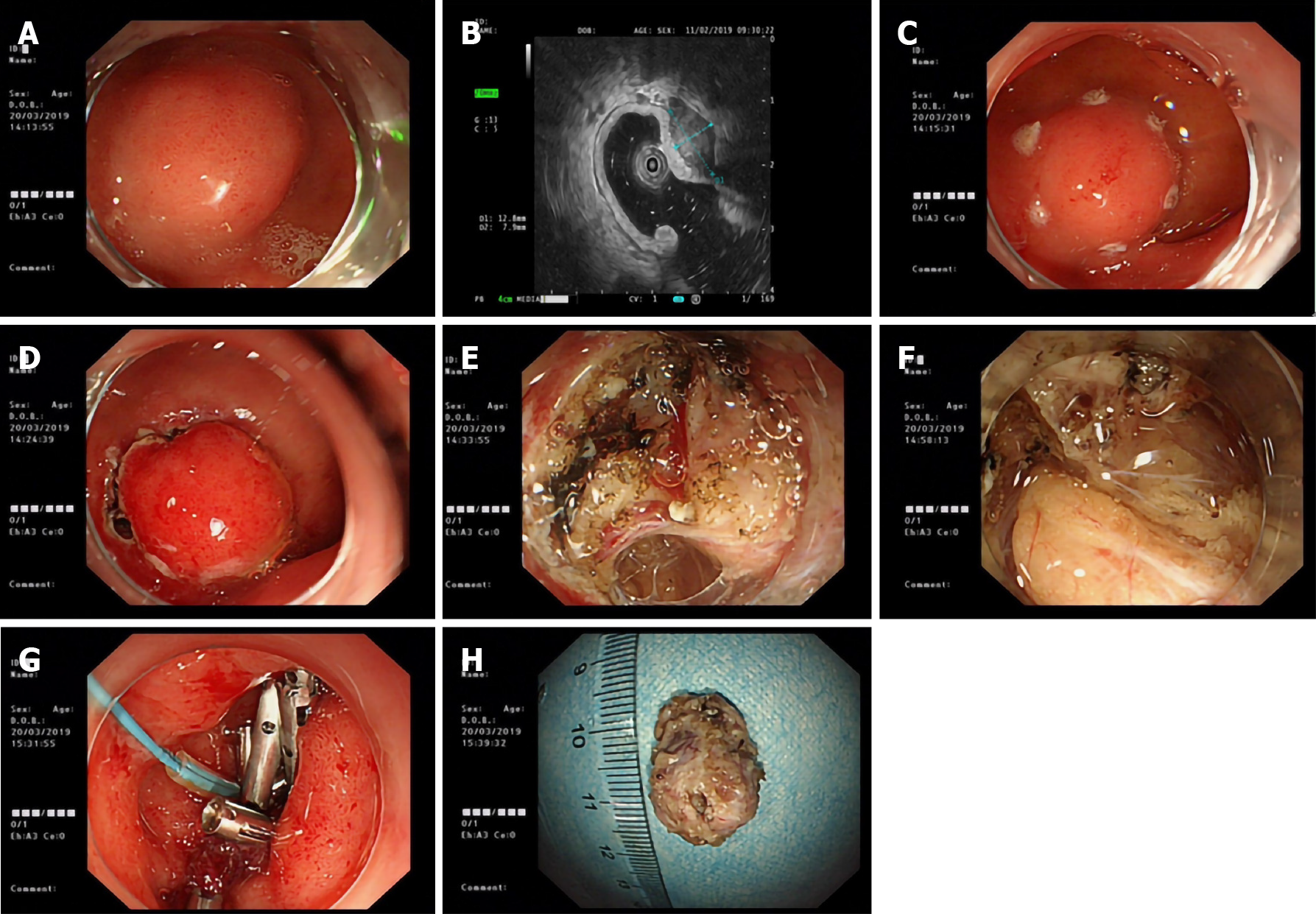
Surgery time was 30-96 min. The mean postoperative hospital stay was 5.7 (range, 3-10) d. There were 5 (5/24, 20.8%) cases of intraoperative perforation, 4 of which were in those with submucosal protuberant lesions (1 case each of ectopic pancreas, stromal tumor, and neuroendocrine tumor located in the submucosa; 1 case of tubular adenoma located in the mucosa), and 1 in a patient with a depressed lesion. The submucosal lesion shown in Figure 1 was located on the anterior wall of the greater curvature of the duodenal bulb, with a diameter of approximately 12 mm, and so a diagnosis of a duodenal submucosal mass (bulb stromal tumor was likely) was made. During ESD, the white tumor was in the muscularis propria, and a perforation of approximately 5 mm in size was observed after stripping the lower tumor. The perforation was sutured with nylon suture and a metal clip following the straight-line method, and the wound was clamped with the Boston Scientific hemostatic clip and metal clip.
Furthermore, a gastric tube was indwelled under endoscopic monitoring. Postoperative pathology was consistent with gastrointestinal stromal tumor (size, 2 cm × 1 cm × 1 cm).
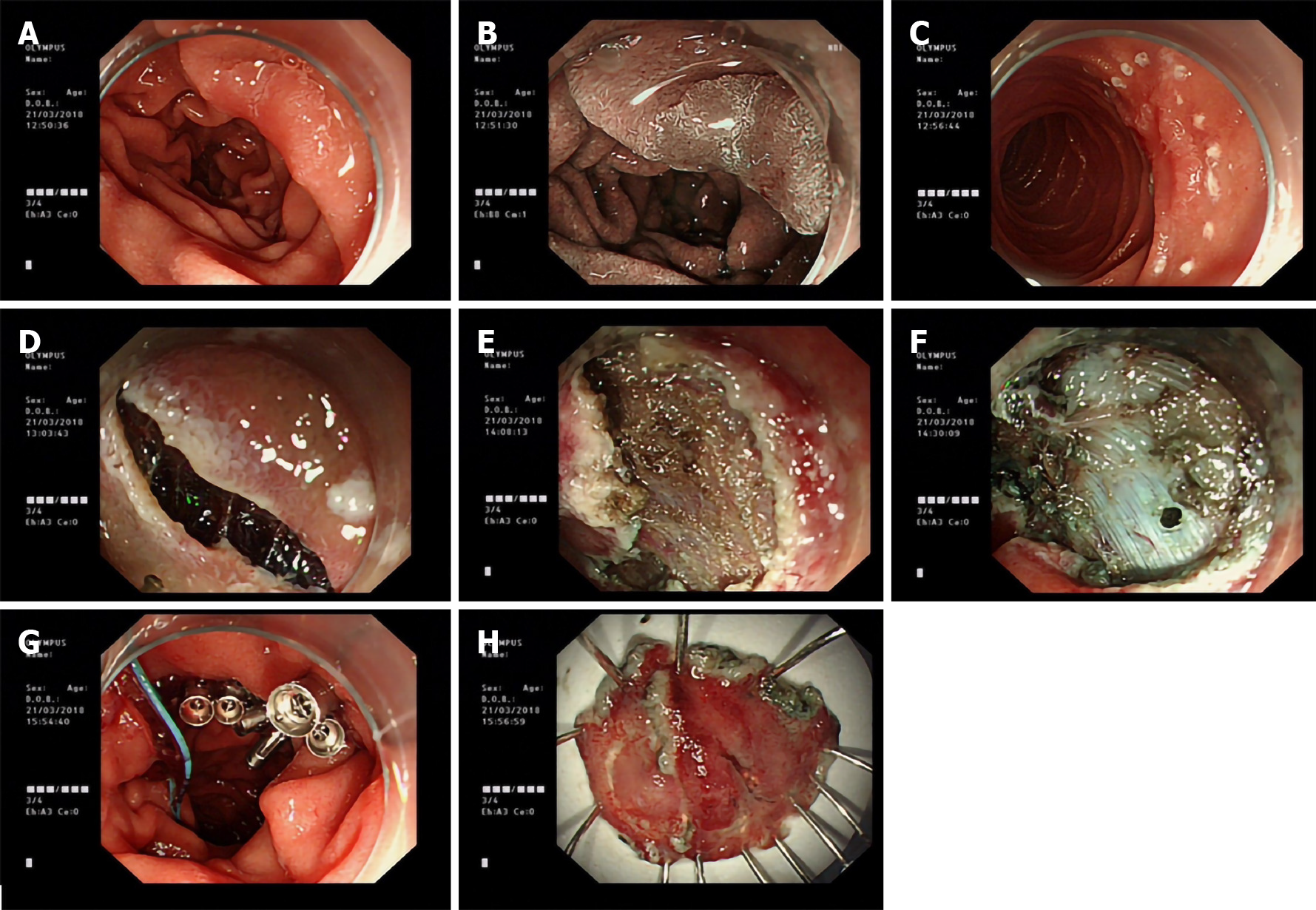
As shown in Figure 2, for the depressed lesion in the descending segment of the duodenum, after marking the boundary with a flush knife and administering the submucosal injection, the mucosal layer was incised along the whole circumference and dissected along the submucosa. Because it was contralateral to the duodenal papilla, the visual field of endoscopy was not clearly exposed; this led to intraoperative perforation, and the remaining lesion was snared after perforation. Later, the wound was closed with nylon suture, harmonious clip, or Boston Scientific hemostatic clip. A nasogastric tube was then indwelled, and postoperative gastrointestinal decompression was performed. After adequate intake of a restricted diet and provision of medical symptomatic treatment, good recovery was noted. No patient in the study had delayed postoperative hemorrhage or perforation.
The mean follow-up time was 25.8 (range, 3.0-50.0) mo. All patients showed no residual or recurrent disease, and all had good quality of life. There were no complications, such as infection and stenosis.
The incidence of duodenal space-occupying lesions is low, with most being benign lesions and having nonspecific early clinical symptoms and signs. Of the 23 patients in this group, 15 showed atypical symptoms of upper gastrointestinal disorders, such as dull epigastric pain, abdominal distension, and belching; 1 had melena; and the remaining 7 had no obvious symptoms. Therefore, the diagnosis of duodenal space-occupying lesions was mainly based on endoscopic and histopathological examinations[5,6]. The pathological types observed were as follows: 5 cases of high and low-grade intraepithelial neoplasia, 5 neuroendocrine tumors, 4 adenomas, 3 hyperplastic polyps, 2 cases of ectopic pancreas, 2 cases of chronic inflammation of the mucosal tissue, 1 stromal tumor, 1 leiomyoma, and 1 submucosal lymph follicular hyperplasia. This is like case reports detailed in earlier studies[7-9]. The bulb was the most common lesion location (50%).
The duodenum is in the retroperitoneum, surrounded by abundant blood vessels, adjacent to the pancreas and common bile duct[10]. Surgery (Whipple's operation, pylorus-preserving pancreaticoduodenectomy, etc.) for the treatment of duodenal lesions is invasive, and serious complications, such as pancreatic fistula, intestinal fistula, and retroperitoneal infection, can easily occur after surgery[11]. Endoscopic treatment of duodenal lesions has the advantages of less trauma and higher safety. For smaller lesions, EMR is simple and convenient[12,13]. However, for larger, irregularly shaped lesions, the complete resection rate with EMR is low, and the resection depth is shallow, thus leading to limitations. ESD can completely remove the lesion, which is conducive to the evaluation of postoperative pathology. Backes et al[14] reported that ESD can aid in complete removal of the lesion and that it has significant advantages over EMR in long-term prognosis.
ESD for duodenal lesions is associated with a higher incidence of bleeding and perforation than those occurring at other levels of the gastrointestinal tract[15-17]. This is because the duodenum is located in the retroperitoneum, especially on the papillary side, adjacent to the abdominal large vessels, common bile duct, pancreas, and other vital organs, the duodenal bowel cavity has a large curvature, thin intestinal wall, and rich peripheral blood flow, and the bile and pancreatic juice are strongly corrosive[18,19]. The occurrence of duodenal ESD complications is mainly affected by the following factors of the lesions: Size, location, depth of invasion, and positional relationship with the surrounding vascular organs[20-22].
According to previous reports, the incidence of duodenal ESD perforation is 21%-35.7%[23]. The perforation rate in our study was 20.8 %, which was consistent with the data reported in the literature. Among the 5 cases of perforation, 4 were submucosal protuberant lesions and 1 was a depressed lesion. Perforation occurs in 80% of submucosal protuberant lesions, and this is due to the thin duodenal intestinal wall and deep infiltration of the lesion. For the submucosal lesions, such as GISTs, which are extensively adhesive to muscularis propria or serosa, we usually actively perform full-thickness resection or choose laparoscopic endoscopic cooperative surgery in order to ensure complete resection of lesions.
For the lesions originating from the mucosal layer, an important reason for intraoperative perforation is that the submucosal layer is severely adherent and difficult to dissect. Therefore, when taking the biopsy for the first time when duodenal lesions are detected, it should be noted that the biopsy tissue sample should be small and parallel to the folds[24]. If the biopsy is taken across the folds, a post-biopsy scar is easily formed, causing the submucosal layer to be adherent to the muscular layer, which makes the dissection layers unclear. It will be difficult to enter the submucosal layer, and be easy to damage the muscle layer, resulting in extended operation time and intraoperative perforation. This is our operation experience, and more scientific research data will be collected in future research. We also suggest that the duodenal ESD should be performed quickly to shorten the operation time and reduce delayed perforation or bleeding caused by electrocoagulation syndrome and intestinal wall edema[25,26].
The incidence of delayed postoperative perforation is also high due to the continuous intestinal lumen dilatation, erosion of the wound by digestive juices, and electrocoagulation syndrome during surgery. Therefore, the wound after duodenal ESD should be closed with metal clips, even there were no perforation when just finishing ESD[27,28]. Of the 23 patients in this group, 13 were treated with prophylactic metal clips to close the wound, and 4 were treated with prophylactic metal clips combined with nylon suture to close the wound. No delayed perforation occurred in these patients. Japanese scholars advocated laparoscopic endoscopic cooperative surgery for duodenal lesions[29,30]; that is, after ESD, the part of the lesion missing the mucosa and submucosa layers is sutured from the abdominal cavity with the help of laparoscopy to avoid delayed postoperative perforation[31].
ESD is safe and effective for the treatment of duodenal lesions and is characterized with less trauma and rapid recovery compared with surgery. However, ESD is challenging to perform due to the thin intestinal wall of the duodenum, narrow intestinal space, rich peripheral blood flow, and proximity to vital organs, such as the common bile duct and pancreas. Therefore, the duodenal lesion biopsy should be done parallel to the fold and should be small to avoid scarring and prevent from subsequent dissection. Duodenal ESD should be performed quickly to shorten the operation time and reduce delayed perforation and bleeding caused by electrocoagulation syndrome and intestinal wall edema, especially when the lesions are on the medial side of the descending segment of the duodenum because of proximity to huge arteries, the common bile duct, and pancreas tissues. The wounds after ESD should be sutured with metal clips to avoid delayed postoperative perforation.
Endoscopic submucosal dissection (ESD) for lesions of the duodenum is more challenge than those occurring at other levels of the gastrointestinal tract due to the thin intestinal wall of the duodenum, narrow intestinal space, rich peripheral blood flow, proximity to vital organs, and high risks of critical adverse events including intraoperative and delayed bleeding and perforation. Because of the low prevalence of the disease and the high risks of severe adverse events, successful ESD for lesions of the duodenum has rarely been reported in recent years.
To investigate the efficacy and safety of ESD in the treatment of duodenal space-occupying lesions.
Based on the research background, we analyzed the clinical data of 24 cases of duodenal lesions treated by ESD and investigated the effectiveness of ESD in these cases.
This study analyzed the clinical data of 24 cases of duodenal lesions treated by ESD at the Digestive Endoscopy Center of the Affiliated Hospital of Qingdao University from January 2016 to December 2019 and investigated the complications and hands-on experiences.
Bleeding and perforation were the main adverse events. The intraoperative perforation rate was 20.8% (5/24), including 4 submucosal protuberant lesions and 1 depressed lesion. No residual disease or recurrence was found in all patients, and no complications, such as infection and stenosis, were found during a median follow-up period of 25.8 mo. No patient died due to tumor recurrence.
ESD is safe and effective in the treatment of duodenal lesions; however, the endoscopists should pay more attention to the preoperative preparation, intraoperative skills, and postoperative treatment.
For duodenal lesions, ESD is safe and effective.
We thank all the authors for helping with the writing and publication of this article.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hirasawa K S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Kakushima N, Kanemoto H, Tanaka M, Takizawa K, Ono H. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;20:12501-12508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Nonaka S, Oda I, Tada K, Mori G, Sato Y, Abe S, Suzuki H, Yoshinaga S, Nakajima T, Matsuda T, Taniguchi H, Saito Y, Maetani I. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Yahagi N, Kato M, Ochiai Y, Maehata T, Sasaki M, Kiguchi Y, Akimoto T, Nakayama A, Fujimoto A, Goto O, Uraoka T. Outcomes of endoscopic resection for superficial duodenal epithelial neoplasia. Gastrointest Endosc. 2018;88:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Chen WC, Wallace MB. Endoscopic management of mucosal lesions in the gastrointestinal tract. Expert Rev Gastroenterol Hepatol. 2016;10:481-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Goda K, Kikuchi D, Yamamoto Y, Takimoto K, Kakushima N, Morita Y, Doyama H, Gotoda T, Maehata Y, Abe N. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: Multicenter case series. Dig Endosc. 2014;26 Suppl 2:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Matsumoto S, Yoshida Y. Selection of appropriate endoscopic therapies for duodenal tumors: an open-label study, single-center experience. World J Gastroenterol. 2014;20:8624-8630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Honda T, Yamamoto H, Osawa H, Yoshizawa M, Nakano H, Sunada K, Hanatsuka K, Sugano K. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Ichikawa T, Kudo M, Matsui S, Okada M, Kitano M. Endoscopic ultrasonography with three miniature probes of different frequency is an accurate diagnostic tool for endoscopic submucosal dissection. Hepatogastroenterology. 2007;54:325-328. [PubMed] |
| 9. | Park SM, Ham JH, Kim BW, Kim JS, Kim CW, Kim JI, Lim CH, Oh JH. Feasibility of endoscopic resection for sessile nonampullary duodenal tumors: a multicenter retrospective study. Gastroenterol Res Pract. 2015;2015:692492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Klein A, Nayyar D, Bahin FF, Qi Z, Lee E, Williams SJ, Byth K, Bourke MJ. Endoscopic mucosal resection of large and giant lateral spreading lesions of the duodenum: success, adverse events, and long-term outcomes. Gastrointest Endosc. 2016;84:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Cloyd JM, Kastenberg ZJ, Visser BC, Poultsides GA, Norton JA. Postoperative serum amylase predicts pancreatic fistula formation following pancreaticoduodenectomy. J Gastrointest Surg. 2014;18:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Tomizawa Y, Ginsberg GG. Clinical outcome of EMR of sporadic, nonampullary, duodenal adenomas: a 10-year retrospective. Gastrointest Endosc. 2018;87:1270-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Jamil LH, Kashani A, Peter N, Lo SK. Safety and efficacy of cap-assisted EMR for sporadic nonampullary duodenal adenomas. Gastrointest Endosc. 2017;86:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Backes Y, Moons LM, van Bergeijk JD, Berk L, Ter Borg F, Ter Borg PC, Elias SG, Geesing JM, Groen JN, Hadithi M, Hardwick JC, Kerkhof M, Mangen MJ, Straathof JW, Schröder R, Schwartz MP, Spanier BW, de Vos Tot Nederveen Cappel WH, Wolfhagen FH, Koch AD. Endoscopic mucosal resection (EMR) versus endoscopic submucosal dissection (ESD) for resection of large distal non-pedunculated colorectal adenomas (MATILDA-trial): rationale and design of a multicenter randomized clinical trial. BMC Gastroenterol. 2016;16:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Tashima T, Ohata K, Sakai E, Misumi Y, Takita M, Minato Y, Matsuyama Y, Muramoto T, Satodate H, Horiuchi H, Matsuhashi N, Nonaka K, Ryozawa S. Efficacy of an over-the-scope clip for preventing adverse events after duodenal endoscopic submucosal dissection: a prospective interventional study. Endoscopy. 2018;50:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Dohi O, Yoshida N, Naito Y, Yoshida T, Ishida T, Azuma Y, Kitae H, Matsumura S, Takayama S, Ogita K, Mizuno N, Nakano T, Majima A, Hirose R, Inoue K, Kamada K, Uchiyama K, Takagi T, Ishikawa T, Konishi H, Morinaga Y, Kishimoto M, Itoh Y. Efficacy and safety of endoscopic submucosal dissection using a scissors-type knife with prophylactic over-the-scope clip closure for superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2019;:. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Kim GH, Kim JI, Jeon SW, Moon JS, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Lee YC; Korean College of Helicobacter and Upper Gastrointestinal Research. Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J Gastroenterol Hepatol. 2014;29:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Inoue T, Uedo N, Yamashina T, Yamamoto S, Hanaoka N, Takeuchi Y, Higashino K, Ishihara R, Iishi H, Tatsuta M, Takahashi H, Eguchi H, Ohigashi H. Delayed perforation: a hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc. 2014;26:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Hoteya S, Furuhata T, Takahito T, Fukuma Y, Suzuki Y, Kikuchi D, Mitani T, Matsui A, Yamashita S, Nomura K, Kuribayashi Y, Iizuka T, Kaise M. Endoscopic Submucosal Dissection and Endoscopic Mucosal Resection for Non-Ampullary Superficial Duodenal Tumor. Digestion. 2017;95:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Yamamoto Y, Yoshizawa N, Tomida H, Fujisaki J, Igarashi M. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc. 2014;26 Suppl 2:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Ohata K, Murakami M, Yamazaki K, Nonaka K, Misumi N, Tashima T, Minato Y, Shozushima M, Mitsui T, Matsuhashi N, Fu K. Feasibility of endoscopy-assisted laparoscopic full-thickness resection for superficial duodenal neoplasms. ScientificWorldJournal. 2014;2014:239627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kakushima N, Kanemoto H, Sasaki K, Kawata N, Tanaka M, Takizawa K, Imai K, Hotta K, Matsubayashi H, Ono H. Endoscopic and biopsy diagnoses of superficial, nonampullary, duodenal adenocarcinomas. World J Gastroenterol. 2015;21:5560-5567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Kakushima N, Ono H, Takao T, Kanemoto H, Sasaki K. Method and timing of resection of superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014;26 Suppl 2:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Miura Y, Shinozaki S, Hayashi Y, Sakamoto H, Lefor AK, Yamamoto H. Duodenal endoscopic submucosal dissection is feasible using the pocket-creation method. Endoscopy. 2017;49:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Ichikawa D, Komatsu S, Dohi O, Naito Y, Kosuga T, Kamada K, Okamoto K, Itoh Y, Otsuji E. Laparoscopic and endoscopic co-operative surgery for non-ampullary duodenal tumors. World J Gastroenterol. 2016;22:10424-10431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Andrisani G, Di Matteo FM. Endoscopic full-thickness resection of duodenal lesions (with video). Surg Endosc. 2020;34:1876-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Hiki N, Nunobe S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications. Ann Gastroenterol Surg. 2019;3:239-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 30. | Otowa Y, Kanaji S, Morita Y, Suzuki S, Yamamoto M, Matsuda Y, Matsuda T, Oshikiri T, Nakamura T, Kawara F, Tanaka S, Ishida T, Toyonaga T, Azuma T, Kakeji Y. Safe management of laparoscopic endoscopic cooperative surgery for superficial non-ampullary duodenal epithelial tumors. Endosc Int Open. 2017;5:E1153-E1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Yanagimoto Y, Omori T, Jeong-Ho M, Shinno N, Yamamoto K, Takeuchi Y, Higashino K, Uedo N, Sugimura K, Matsunaga T, Miyata H, Ushigome H, Takahashi Y, Nishimura J, Yasui M, Asukai K, Yamada D, Tomokuni A, Wada H, Takahashi H, Ohue M, Yano M, Sakon M. Feasibility and Safety of a Novel Laparoscopic and Endoscopic Cooperative Surgery Technique for Superficial Duodenal Tumor Resection: How I Do It. J Gastrointest Surg. 2019;23:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |