Published online Dec 26, 2020. doi: 10.12998/wjcc.v8.i24.6252
Peer-review started: August 31, 2020
First decision: September 24, 2020
Revised: October 1, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 26, 2020
Processing time: 110 Days and 5.5 Hours
Understanding a virus shedding patterns in body fluids/secretions is important to determine the samples to be used for diagnosis and to formulate infection control measures.
To investigate the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shedding patterns and its risk factors.
All laboratory-confirmed coronavirus disease 2019 patients with complete medical records admitted to the Shenzhen Third People’s Hospital from January 28, 2020 to March 8, 2020 were included. Among 145 patients (54.5% males; median age, 46.1 years), three (2.1%) died. The bronco-alveolar lavage fluid (BALF) had the highest virus load compared with the other samples. The viral load peaked at admission (3.3 × 108 copies) and sharply decreased 10 d after admission.
The viral load was associated with prolonged intensive care unit (ICU) duration. Patients in the ICU had significantly longer shedding time compared to those in the wards (P < 0.0001). Age > 60 years [hazard ratio (HR) = 0.6; 95% confidence interval (CI): 0.4-0.9] was an independent risk factor for SARS-CoV-2 shedding, while chloroquine (HR = 22.8; 95%CI: 2.3-224.6) was a protective factor.
BALF had the highest SARS-CoV-2 load. Elderly patients had higher virus loads, which was associated with a prolonged ICU stay. Chloroquine was associated with shorter shedding duration and increased the chance of viral negativity.
Core Tip: Severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2) can be found in various samples, including eye discharge. The virus load increased sharply at admission and dropped dramatically thereafter. Later admission was associated with longer virus shedding, higher ICU admission and lower survival probability. As for clinical treatment, we found that chloroquine could potentially increase the chance of viral shedding.
- Citation: Chen PF, Yu XX, Liu YP, Ren D, Shen M, Huang BS, Gao JL, Huang ZY, Wu M, Wang WY, Chen L, Shi X, Wang ZQ, Liu YX, Liu L, Liu Y. Virus load and virus shedding of SARS-CoV-2 and their impact on patient outcomes. World J Clin Cases 2020; 8(24): 6252-6263
- URL: https://www.wjgnet.com/2307-8960/full/v8/i24/6252.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i24.6252
The coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus that is closely related to SARS-CoV, which caused an outbreak in 2003[1]. The outbreak of COVID-19 was first reported in December 2019 in Wuhan, China[2,3]. Such outbreak can cause emotional distress and anxiety[4], which can occur even in people not at high risk of getting sick, in the face of a virus that the common people are unfamiliar with[4]. The common signs of COVID-19 include fever, cough, and shortness of breath[5]. While a significant proportion of patients develop neurological manifestations, especially olfactory and gustatory dysfunction[6,7]. There is no specific treatment, but supportive care is necessary in severe and critical cases[5,8]. Acute respiratory distress syndrome and sepsis were reported in 100% of the patients with confirmed COVID-19 who died[9].
The factors dictating the severity of illness and outcome among patients with COVID-19 are still not well defined. Observational studies reported that the median duration of viral shedding was 20.0 d (IQR: 17.0-24.0) among survivors[10,11]. In some other coronavirus respiratory illnesses, the higher virus load and longer shedding duration were related to a worse outcome[12-14]. The longest observed duration of viral shedding in survivors was 37 d[11]. The viral shedding duration among patients with COVID-19 has been reported to be associated with age and comorbidities[2,9]. Prolonged Middle East respiratory syndrome (MERS) viral shedding in the respiratory tract was associated with severe outcomes in patients with MERS[15,16]. In addition, studies suggested that patients with MERS-CoV requiring intensive care unit (ICU) admission had a higher viral load than the patients not requiring ICU admission[17]. Nevertheless, whether the virus load and shedding duration of SARS-CoV-2 are associated with the severity of illness is still unknown.
Data on viral load and shedding in the respiratory tract are limited, and risk factors for viral shedding have yet to be fully clarified. COVID-19 has proven to be highly infectious and is transmitted person-to-person[5]. Respiratory droplets were suspected to be the main route of transmission for SARS-CoV[12] and SARS-CoV-2[5]. Aerosols and fomite are possible transmission routes since SARS-CoV-2 survives for 0.8-6.8 h on different surfaces[18]. It is of great epidemic significance to understand the virus shedding patterns in different body fluids and secretions to determine which samples are the most suitable for diagnosis, and how to formulate appropriate infection control measures and isolation duration. Most importantly, virus shedding could be used as a reliable marker to scrutinize the effectiveness of antiviral drugs.
This study aimed to examine the distribution of COVID-19 virus in the tissues of the patients and the shedding pattern of COVID-19 virus in the respiratory secretions and to investigate potential factors associated with viral load and shedding and patient outcomes.
A cycle threshold value (Ct value) < 37 was defined as a positive test, and a Ct value of ≥ 40 was defined as a negative test. A medium load, defined as a Ct value ≥ 37 but < 40, required retesting. By regression analysis of the standard curves provided by the manufacture (Shanghai Jienuo Co., Ltd., Shanghai, China) (Supplementary Figure 1), the following equations of the standard curve (plot of Ct values against the log of the standard sample amount) were determined for SARS-CoV-2 (R² = 0.995): Ct = -3.39 × log(copies) + 40.52.
The following data were extracted from the medical records: Demographics (age, sex, body mass index, history of Hubei contact, and smoking), symptoms (at admission and in the ICU), comorbidities (hypertension, diabetes, heart diseases, and chronic obstructive pulmonary disease), laboratory findings at admission (temperature, white blood cells, platelets, lymphocytes, interleukin (IL)-6, serum creatinine, respiratory tract viral load, and PaO2/FiO2), severity scores [Acute Physiology And Chronic Health Evaluation II (APACHEII), Sequential Organ Failure Assessment (SOFA), and Glasgow Coma Scale (GCS)], therapy, outcomes (viral shedding, ICU stay, hospital stay, death, and discharge).
Continuous variables are presented as mean values with 95% confidence intervals (CI). The means for continuous variables were compared using the independent t test when the data were normally distributed (Kolmogorov-Smirnov test); otherwise, the Mann-Whitney U-test was used. Data with non-Gaussian distribution from repeated measures were compared using the generalized linear mixed model. The proportions for categorical variables were compared using the χ-square test, while the Fisher’s exact test was used when the data were limited. Multivariable regression analysis or time-dependent Cox regression was conducted to assess the relative influence of virus shedding on ICU admission and hospital duration. P values ≤ 0.05 were considered statistically significant. All analyses were performed using R (http://www.R-project.org) and EmpowerStats software (www.empowerstats.com, X&Y solutions, Inc. Boston, MA, United States).
All consecutive patients with confirmed COVID-19 admitted to the Shenzhen Third People’s Hospital from January 28, 2020 to March 8, 2020, were screened for eligibility. The Shenzhen Third People’s Hospital is the only designated infectious disease hospital responsible for the treatments of COVID-19 in Shenzhen, China. This retrospective study was approved by the Institutional Review Board of Shenzhen Third People’s Hospital. Informed written consent was obtained from all subjects prior to the study.
All patients with COVID-19 included in this study were diagnosed according to World Health Organization interim guidance[19]. The hospitalized patients with more than one positive nucleic acid test for SARS-CoV-2 virus at least one day apart and with complete medical records were included in the analysis. To investigate the possible transmission capacity, the specimens were obtained from a variety of sources, including sputum, nasopharyngeal swabs, blood, endotracheal aspirate, saliva, and eye discharges.
The study initially screened 1461 patients, and 111 were excluded for missing virological records (Figure 1). Among those 1350 patients with COVID-19, 7404 virological tests were performed. Of 6959 (94.0%) nasopharyngeal swab specimens, 41.6% were positive for COVID-19. Among 144 (1.9%) blood samples, 14.6% showed positive results. Among 213 (2.9%) bronchoalveolar lavage fluid (BALF) specimens, 56.3% were positive. Among 47 (0.6%) saliva samples, 29.8% were positive. Among 38 (0.5%) eye discharge samples, 15.8% were positive. In addition, two cerebrospinal fluid (CSF, 0.03%) samples were available, and both were negative. One (0.01%) anus swab sample was negative for COVID-19 (Supplementary Table 1).
The patients with incomplete medical records were excluded, and 145 patients with full inpatient records were included (Figure 1). Among them, 79 (54.28%) were males, and the mean age was 46.1 (95%CI: 42.6-49.9) years. Of these patients, 107 (73.8%) were admitted to the isolation wards, and 38 (26.2%) were admitted and transferred to the ICU. The median duration from the first symptoms to hospital admission was 4.6 d (95%CI: 4.1-5.1 d) (Table 1). Hypertension [26 (17.9%)], cardiovascular disease [18 (12.4%)], and diabetes [11 (7.6%)] were the most common comorbidities. During the first 3 d after admission, 142 were treated with interferon, 16 (11.0%) received no antiretrovirals (ARV), while 84 (57.9%) received one ARV, 38 (26.2%) received two ARVs, and seven (4.8%) received three ARVs.
| All | Viral shedding (d) | P value | |||
| < 14 | ≥ 14 | ||||
| Total | 145 | 53 (37%) | 92 (63%) | ||
| Demographics | Age, yr | 46.1 (42.6-49.9) | 39.6 (34.2-45.9) | 50.3 (46.1-54.9) | 0.004 |
| Sex, male, n (%) | 79 (54.5%) | 25 (47.2%) | 54 (58.7%) | 0.18 | |
| BMI (kg/m2) | 23.6 (22.9-24.2) | 22.9 (21.9-24.1) | 23.9 (23.2-24.7) | 0.131 | |
| History of Hubei contact | 129 (89.0%) | 50 (94.3%) | 79 (85.9%) | 0.117 | |
| Smoking | 3 (2.1%) | 0 | 3 (3.3%) | 0.184 | |
| Symptom to admission (d) | 4.6 (4.1-5.1) | 4.4 (3.6-5.3) | 4.7 (4.1-5.3) | 0.553 | |
| Symptom to ICU (d) | 12.0 (10.8-13.4) | 10.5 (8.7-12.7) | 12.4 (10.9-14.2) | 0.236 | |
| Comorbidities | Hypertension | 26 (17.9%) | 5 (9.4%) | 21 (22.8%) | 0.043 |
| Diabetes | 11 (7.6%) | 4 (7.6%) | 7 (7.6%) | 0.989 | |
| Heart disease | 18 (12.4%) | 3 (5.7%) | 15 (16.3%) | 0.061 | |
| COPD | 4 (2.8%) | 1 (1.9%) | 3 (3.3%) | 0.627 | |
| Laboratory findings on admission | Temperature (°C) | 37.4 (37.3-37.5) | 37.3 (37.0-37.5) | 37.5 (37.3-37.6) | 0.256 |
| WBC (× 109/L) | 4.84 (4.58-5.12) | 4.89 (4.46-5.36) | 4.82 (4.49-5.16) | 0.784 | |
| PLT (× 1012/L) | 175.51 (166.47-185.05) | 201.84 (185.82-219.25) | 161.75 (151.91-172.23) | < 0.001 | |
| LYMPH (× 109/L) | 1.20 (1.10-1.29) | 1.21 (1.06-1.37) | 1.19 (1.07-1.31) | 0.839 | |
| IL-6 (ug/L) | 18.24 (14.93-22.29) | 12.55 (8.58-18.36) | 21.72 (17.34-27.20) | 0.012 | |
| CR (mg/L) | 67.47 (64.12-71.00) | 62.03 (57.87-66.50) | 70.87 (66.21-75.86) | 0.013 | |
| Viral load in respiratory tract [log (copies)] | 3.69 (3.06-5.07) | 3.36 (2.49-4.83) | 4.22 (3.10-5.12) | 0.192 | |
| PAO2/FIO2 | 0.897 | ||||
| < 100 | 1 (0.7%) | 0 | 1 (1.1%) | ||
| ≥ 100, < 200 | 8 (5.9%) | 3 (6.4%) | 5 (5.7%) | ||
| ≥ 200, < 300 | 19 (14.1%) | 7 (14.9%) | 12 (13.6%) | ||
| ≥ 300 | 107 (79.3%) | 37 (78.7%) | 70 (79.6%) | ||
| Severity score | APACHEII | 4.2 (3.7-4.7) | 3.4 (2.8-4.1) | 4.8 (4.2-5.5) | 0.003 |
| SOFA | 1.7 (1.5-1.9) | 1.4 (1.2-1.7) | 1.9 (1.6-2.2) | 0.027 | |
| GCS | 14.8 (14.5-15.2) | 15.0 (15.0-15.0) | 14.7 (14.2-15.3) | 0.45 | |
| Symptoms | Fever | 123 (84.8%) | 41 (77.4%) | 82 (89.1%) | 0.057 |
| Sore muscles | 32 (22.1%) | 13 (24.5%) | 19 (20.7%) | 0.588 | |
| Cough | 84 (57.9%) | 31 (58.5%) | 53 (57.6%) | 0.917 | |
| ARV in the first 3 d | 0.008 | ||||
| None | 16 (11.0%) 10 (18.9%) | ||||
| Kaletra | 40 (27.6%) | 8 (15.1%) | 32 (34.8%) | ||
| Kaletra & Other ARV | 40 (27.6%) | 19 (35.9%) | 21 (22.8%) | ||
| Other ARV but Kaletra | 49 (33.8%) | 16 (30.2%) | 33 (35.9%) | ||
| ARV in the first 3 d | 0.014 | ||||
| 0 | 16 (11.0%) | 10 (18.9%) | 6 (6.5%) | ||
| 1 | 84 (57.9%) | 22 (41.5%) | 62 (67.4%) | ||
| 2 | 38 (26.2%) | 18 (34.0%) | 20 (21.7%) | ||
| 3 | 7 (4.8%) | 3 (5.7%) | 4 (4.4%) | ||
| Globulin | 61 (42.1%) | 12 (22.6%) | 49 (53.3%) | < 0.001 | |
| Steroid | 57 (39.3%) | 12 (22.6%) | 45 (48.9%) | 0.002 | |
| Thymosin alpha-1 | 59 (40.7%) | 13 (24.5%) | 46 (50.0%) | 0.003 | |
| Antibiotics | 50 (34.5%) | 15 (28.3%) | 35 (38.0%) | 0.235 | |
| Other treatments | 11 (7.6%) | 0 | 11 (12.0%) | 0.009 | |
| HFO | 18 (12.4%) | 7 (13.2%) | 11 (12.0%) | 0.826 | |
| MV | 16 (11.0%) | 1 (1.9%) | 15 (16.3%) | 0.008 | |
| NMV | 46 (31.7%) | 11 (20.8%) | 35 (38.0%) | 0.031 | |
| CRRT | 4 (2.8%) | 0 | 4 (4.4%) | 0.124 | |
| Outcome | Negative | 108 (74.5%) | 53 (100%) | 55 (59.8%) | < 0.001 |
| Viral shedding (d) | 17.3 (15.8-18.9) | 12.1 (11.0-13.3) | 24.4 (22.6-26.3) | < 0.001 | |
| Length of ICU stay (d) | 13.6 (9.4-19.8) | 7.0 (3.7-13.1) | 16.3 (10.7-24.9) | 0.067 | |
| Length of hospital stay (d) | 25 (23-28) | 21 (18-24) | 28 (25-32) | 0.002 | |
| Death | 3 (2.1%) | 0 | 3 (3.3%) | 0.184 | |
| Discharge | 57 (39.3%) | 29 (54.7%) | 28 (30.4%) | 0.004 | |
In 92 (63.5%) patients, viral negativization took longer than 2 wk. These patients were older (50.3 vs 39.6 years) and were more likely to have hypertension and heart diseases compared with patients who achieved viral negativization in less than 2 wk (Table 1). In addition, they had lower platelet and higher IL-6 and serum creatinine levels. Their conditions were more severe, with higher SOFA and APACHEII scores.
The mean viral load at admission for the 145 patients was 1.16 × 104 copies/mL (26.7 ± 4.4 in Ct values), but it could reach 11.7 in Ct value (3.3 × 108 copies/mL). The mean viral load in the respiratory tract was 4.9 × 103 copies/mL. The median viral load in nasopharyngeal swab samples was 1.32 × 104 copies/mL (26.6 ± 4.4 in Ct value); from sputum samples, it was 2.9 × 102 copies/mL (32.2 in Ct value); from BALF it was 5.7 × 104 copies/mL (24.5 ± 3.6 in Ct value); and from blood samples, it was 4.2 × 103 copies/mL (28.2 ± 7.4 in Ct value) (Supplementary Figure 1). The BALF had the highest virus load compared with the other samples.
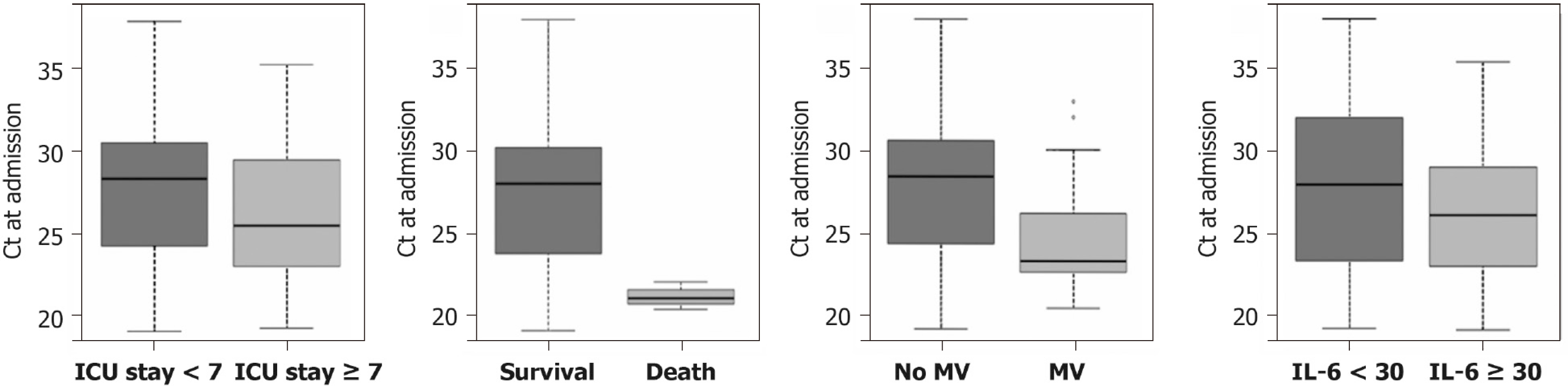
The patients in the ICU had higher virus loads than those in wards. The average Ct values were 2.0 × 104 copies/mL (25.9 ± 4.0 in Ct value) and 6.8 × 103 copies/mL (27.5 ± 4.7 in Ct value) for 38 patients in the ICU and for 107 patients in wards, respectively. The Ct values for patients with longer ICU stay (≥ 7 d) were significantly lower than those with shorter ICU stay (< 7 d, P = 0.02) (Figure 2A). Of those patients who survived, the Ct values were significantly higher compared with those who died (P < 0.0001) (Figure 2B). In addition, patients with mechanical ventilation had lower Ct values at admission compared to those without mechanical ventilation (P = 0.004) (Figure 2C). Moreover, patients with IL-6 > 30 µg/L had higher Ct values compared to those with IL-6 < 30 ug/L (P = 0.05) (Figure 2D). The average Ct values did not significantly differ in terms of comorbidities, sex, and Hubei contact (all P > 0.05).
The average duration of SARS-CoV-2 viral shedding was 17.3 d (95%CI: 15.8-18.9 d). For the ward patients, the average duration of SARS-CoV-2 viral shedding was 12.1 d (95%CI: 11.0-13.3 d), while for the ICU patients, it was 24.4 d (95%CI: 22.6-26.3 d) (P < 0.0001). Patients with lower first positive Ct values tended to have longer viral shedding time (P = 0.0176).
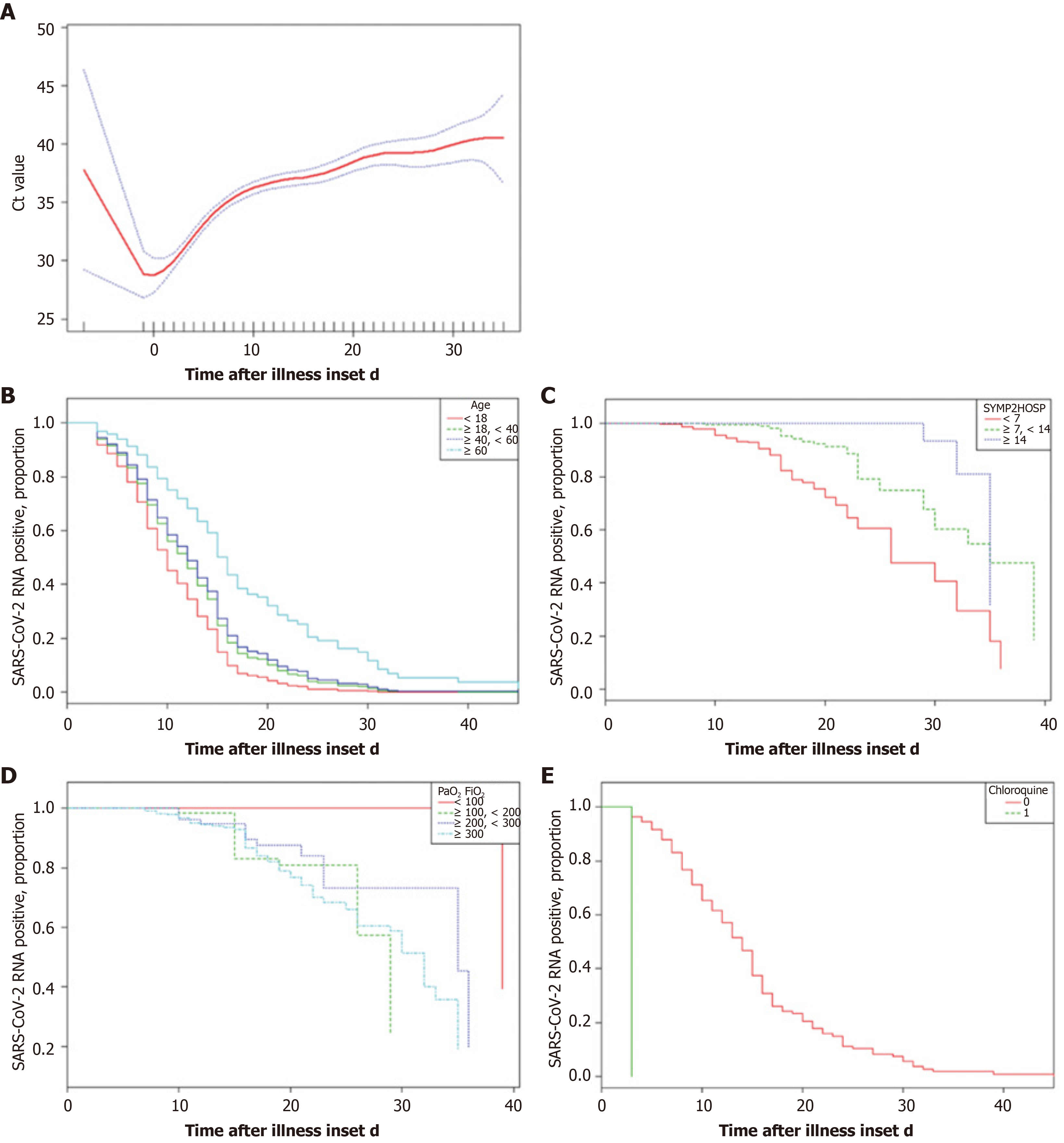
Smooth curves were fitted for Ct value and the illness onset to hospital admission (Figure 3A). Virus load peaked at admission and sharply decreased within 10 d after admission, after which it gradually dropped until reaching a low level or negativization. Moreover, the results also indicated the longer the time from symptom to hospital admission, the longer was the duration of viral shedding (Figure 3C). We further compared the proportion of patients who tested positive for SARS-CoV-2 RNA over time after admission, and significant differences were seen in the duration of viral shedding for the elderly population (Figure 3B), patients with PaO2/FiO2 < 100 (Figure 3D), and chloroquine prescribed within 3 d after admission (Figure 3E).
All available data from 145 patients were incorporated in a time-dependent Cox proportional hazards model. Chloroquine was positively correlated with virus shedding, increasing the possibility of viral shedding. Age > 60 years was negatively correlated with viral shedding and could potentially decrease the chance of viral shedding and was thus associated with longer shedding duration. Age > 60 years [hazard ratio (HR) = 0.6; 95%CI: 0.4-0.9] was an independent risk factor for SARS-CoV-2 shedding, while chloroquine (HR = 22.8; 95%CI: 2.3-224.6) was a protective factor (Table 2); immunoglobulin, antibiotics, steroid, PaO2/FiO2 ratio, and the neutrophil-lymphocyte ratio were not independently associated with virus shedding (Table 2). Viral shedding was associated with longer ICU duration, but not with hospital stay and in-hospital mortality (Table 3).
| Variables | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex, male | 1.1 (0.7, 1.6) | 0.757 | 0.8 (0.5, 1.3) | 0.422 |
| Age (yr) | ||||
| < 60 | Reference | |||
| ≥ 60 | 0.5 (0.4, 0.8) | 0.002 | 0.6 (0.4, 1.0) | 0.030 |
| Diabetes | 0.9 (0.5, 1.8) | 0.870 | 1.4 (0.7, 2.7) | 0.370 |
| IVIG | 0.5 (0.3, 0.7) | < 0.001 | 0.6 (0.3, 1.2) | 0.182 |
| Antibiotics | 0.5 (0.4, 0.8) | 0.002 | 0.7 (0.4, 1.3) | 0.273 |
| Steroids | 0.5 (0.3, 0.7) | <0.001 | 0.8 (0.4, 1.7) | 0.588 |
| PAO2/FIO2 | ||||
| < 300 | Reference | |||
| ≥ 300 | 1.3 (0.8, 2.2) | 0.247 | 1.0 (0.5, 1.7) | 0.898 |
| Chloroquine | 26.8 (3.0, 239.3) | 0.003 | 19.0 (1.9, 186.2) | 0.012 |
| NLR | 1.0 (0.9, 1.0) | 0.386 | 1.0 (1.0, 1.1) | 0.632 |
| Non-adjusted | Model I | Model II | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| ICU duration | 0.52 (0.90-0.96) | 0.02 | 0.49 (0.42-0.94) | 0.03 | 0.48 (-0.01-0.97) | 0.05 |
| Hospital stay | 0.15 (-0.01,0.32) | 0.07 | 0.11 (-0.05-0.26) | 0.18 | 0.11 (-0.05-0.26) | 0.18 |
| In-hospital mortality | 1.07 (0.98, 1.17) | 0.15 | 0.06 (-0.04-0.16) | 0.23 | 0.06 (-0.04-0.16) | 0.23 |
In this study, we analyzed the virus nucleic acid content of SARS-CoV-2 from different samples, described the features of virus load and virus shedding, identified the risk factors for prolonged SARS-CoV-2 RNA shedding and evaluated the impact of prolonged SARS-CoV-2 RNA shedding on patients’ outcomes, including ICU duration and in-hospital mortality.
SARS-CoV-2 was detected in samples from eye discharge, blood, saliva, nose swab, and BALF. Although the SARS-CoV-2 virus is mainly distributed in the respiratory system, it can also be found in the digestive tract, blood, and eye discharge, which may contribute to the high contagious capacity of the virus[20]. In the present study, SARS-CoV-2 was detected in the nasopharyngeal swab, blood, BALF, saliva, and eye discharge. It was not found in the CSF and anus swab, but these two sample types were too few to draw a conclusion.
The shedding pattern of SARS-CoV-2 RNA demonstrated that the virus load increased sharply before admission and dropped dramatically thereafter. Based on the samples collected from 145 patients, the mean virus load at admission was 1.16 × 104 copies/mL, which is basically similar to SARS[12], instead of 1000 times higher, as reported[21], and the highest viral load was 3.3 × 108 copies/mL according to the previous research[21]. Moreover, the present study suggested that the viral load peaked at admission, decreased right after the start of treatment, decreased sharply within 10 d after admission, and dropped gradually until reaching a quite low level or negativization. In addition, the mean duration of SARS-CoV-2 viral shedding was much longer in ICU patients compared to those in the wards (21.8 vs. 14.8 d). The shedding duration of SARS-CoV-2 RNA was reported to be considerably longer than that of MERS and SARS[15,22]. Moreover, the duration of virus shedding was influenced by the time between the onset of illness and admission, where a later admission was associated with longer virus shedding, higher ICU admission, and lower survival probability. Those data suggest that the early detection and clinical intervention would help shorten the viral shedding duration, lower the risk of ICU admission, and increase the survival probability.
This study suggested that senior age was an independent risk factor for virus shedding. Previous research suggested that advanced age is associated with prolonged illness and poor outcomes in patients hospitalized with COVID-19[23-25]. The present study further demonstrated that the elderly had higher initial viral loads in contrast to younger patients. A higher viral load may reflect a disability of the immune system to contain viral proliferation, thus contributing to the prolonged viral shedding period. Another independent risk factor was the use of chloroquine within 3 d after admission. The interim conclusions from the published literature is that there is no current evidence of use of chloroquine for treatment of COVID-19. Chloroquine should be restricted to clinical trials with strict vigilance and follow-up[26]. The results in the present study indicated that chloroquine could greatly increase the probability of viral negativity by 19.0 times while shortening the virus shedding duration. This finding is consistent with previous publications[5,27]. Nevertheless, due to the limitation of observational studies, randomized clinical trials are necessary before any definitive conclusions on the effectiveness of chloroquine can be reached.
The effectiveness of several clinical treatments was also examined. Contrary to the intuitive experience, the present study suggests that immunomodulation, such as thymosin and intravenous immunoglobulin (IVIG), had no impact on the pattern of virus shedding. This effect has been explored in other researches, and the studies for IVIG were inconclusive due to potential confounding effects of patient comorbidities, stage of illness, or effect of other treatments[28,29]. Our findings suggest that immunomodulation treatment might not reduce the shedding duration after adjusting for the confounding factors. Similar findings were observed about the effect of antivirus treatment. Kaletra, a combination of lopinavir and ritonavir for human immunodeficiency virus treatment, has shown efficacy in a case report in China[25], but after adjusting for the confounding factors, it was not superior to ribavirin plus interferon. It is also true for the other antiviral drugs. Thus, any wide administration of this antiviral treatment (AVT) should be carefully evaluated by randomized clinical trials.
This study has some limitations. First, due to the retrospective study design, not all comorbidities were well documented in all patients. Therefore, their role might be underestimated in identifying the risk factors for virus shedding. Second, the patients received AVT empirically, which greatly varied in the selection of antiviral drugs and duration, thus making it extremely challenging to evaluate the role of antiviral drugs. Third, the estimated duration of viral shedding was limited by the frequency of respiratory specimen collection, lack of quantitative viral RNA detection, and a relatively low positive rate of SARS-CoV-2 RNA detection in throat swabs. Fourth, by excluding patients still in hospital as of March 8, 2020, and the low mortality in our group, the impact of virus shedding on mortality might be underestimated. Finally, the interpretation of our findings is limited by the sample size. Nevertheless, by including all adult patients in the designated hospitals in Shenzhen admitted for COVID-19, we believe that our study population is representative of the cases diagnosed and treated in a large city outside Wuhan.
The present study is the first to examine the patterns of SARS-CoV-2 virus shedding and its risk factors. The results strongly suggest that SARS-CoV-2 was identified in a wide range of samples, including the respiratory tract, blood, and eye discharge. In addition, the data suggested that earlier hospitalization might help reduce the virus-shedding period and ICU stay. Regarding clinical therapy, the analysis did not confirm the effectiveness of immunomodulation or AVT, but chloroquine could have the potential to shorten the duration and increase the possibility of shedding, although with limited power due to the small sample size. The findings of this retrospective study have important implications for the clinical treatment and policy making with regard to reducing the damage of the current pandemic caused by SARS-CoV-2.
The outbreak of coronavirus disease 2019 (COVID-19) cause emotional distress and anxiety worldwide. However, the factors for the severity of illness and its outcomes still remain unclear.
The goal of this study was to characterize the viral shedding patterns and risk factors in hospitalized patients with COVID-19.
The study aimed to identify the characteristics of viral load and shedding, the risk factors affecting the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus clearance and to evaluate the effect of prolonged viral shedding on the outcome of the patients.
This was a retrospective study on all laboratory-confirmed COVID-19 patients with complete medical records admitted to the Shenzhen Third People’s Hospital from January 1, 2020 to March 8, 2020. A total of 7404 virological tests in 1350 patients were analyzed to identify the pattern of virus load in different samples. Furthermore, 145 patients with full inpatient records were statistically analyzed to reveal the risk factors associated with the viral shedding and ICU admission by multivariate cox regression.
SARS-CoV-2 virus was identified in a wide range of samples, including eye discharge. Earlier hospitalization might help reduce the virus-shedding period and intensive care unit (ICU) stay. Chloroquine is associated with the shortened shedding duration.
Among various samples the SARS-CoV-2 virus, bronchoalveolar lavage fluid had the highest SARS-CoV-2 load. Elderly patients had higher virus loads, which was associated with a prolonged ICU stay. Chloroquine was associated with a shorter shedding duration and increased the chance of viral negativity.
The findings about the virus shedding patterns and its risk factors suggested that early hospitalization has the potential to reduce the virus shedding time and the ICU stay. Also, the confirmation of effectiveness of immunomodulation and chloroquine might help in clinical treatment and policy making.
We sincerely thank Mr. Wang CY from Shanghai Jienuo Company, for providing the standard curve (plot of Ct value against the log of the standard sample amount).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Laassri M, Montemurro N, Singh S S-Editor: Huang P L-Editor: MedE-Ma JY P-Editor: Xing YX
| 1. | Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5202] [Cited by in RCA: 4631] [Article Influence: 926.2] [Reference Citation Analysis (0)] |
| 2. | Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1670] [Cited by in RCA: 1769] [Article Influence: 353.8] [Reference Citation Analysis (0)] |
| 3. | Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2274] [Cited by in RCA: 1961] [Article Influence: 392.2] [Reference Citation Analysis (0)] |
| 4. | Montemurro N. The emotional impact of COVID-19: From medical staff to common people. Brain Behav Immun. 2020;87:23-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 5. | Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1597] [Cited by in RCA: 1601] [Article Influence: 320.2] [Reference Citation Analysis (0)] |
| 6. | Di Carlo DT, Montemurro N, Petrella G, Siciliano G, Ceravolo R, Perrini P. Exploring the clinical association between neurological symptoms and COVID-19 pandemic outbreak: a systematic review of current literature. J Neurol. 2020;1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Özdağ Acarli AN, Samanci B, Ekizoğlu E, Çakar A, Şirin NG, Gündüz T, Parman Y, Baykan B. Coronavirus Disease 2019 (COVID-19) From the Point of View of Neurologists: Observation of Neurological Findings and Symptoms During the Combat Against a Pandemic. Noro Psikiyatr Ars. 2020;57:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Montemurro N, Perrini P. Will COVID-19 change neurosurgical clinical practice? Br J Neurosurg. 2020;1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2548] [Article Influence: 509.6] [Reference Citation Analysis (2)] |
| 10. | He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, Mo X, Chen Y, Liao B, Chen W, Hu F, Zhang Q, Zhong M, Wu Y, Zhao L, Zhang F, Cowling BJ, Li F, Leung GM. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2588] [Cited by in RCA: 2857] [Article Influence: 571.4] [Reference Citation Analysis (0)] |
| 11. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18189] [Article Influence: 3637.8] [Reference Citation Analysis (0)] |
| 12. | Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY; HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767-1772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1737] [Cited by in RCA: 1786] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 13. | Chan KH, Poon LL, Cheng VC, Guan Y, Hung IF, Kong J, Yam LY, Seto WH, Yuen KY, Peiris JS. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis. 2004;10:294-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Oh MD, Park WB, Choe PG, Choi SJ, Kim JI, Chae J, Park SS, Kim EC, Oh HS, Kim EJ, Nam EY, Na SH, Kim DK, Lee SM, Song KH, Bang JH, Kim ES, Kim HB, Park SW, Kim NJ. Viral Load Kinetics of MERS Coronavirus Infection. N Engl J Med. 2016;375:1303-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 15. | Memish ZA, Assiri AM, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014;29:307-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Al-Dorzi HM, Aldawood AS, Khan R, Baharoon S, Alchin JD, Matroud AA, Al Johany SM, Balkhy HH, Arabi YM. The critical care response to a hospital outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infection: an observational study. Ann Intensive Care. 2016;6:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Feikin DR, Alraddadi B, Qutub M, Shabouni O, Curns A, Oboho IK, Tomczyk SM, Wolff B, Watson JT, Madani TA. Association of Higher MERS-CoV Virus Load with Severe Disease and Death, Saudi Arabia, 2014. Emerg Infect Dis. 2015;21:2029-2035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564-1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5894] [Cited by in RCA: 5665] [Article Influence: 1133.0] [Reference Citation Analysis (0)] |
| 19. | World Health Organization. Surveillance case definitions for human infection with novel coronavirus (nCoV). 2020; Available from: https://apps.who.int/iris/bitstream/handle/10665/330376/WHO-2019-nCoV-Surveillance-v2020.1-eng.pdf. |
| 20. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30098] [Article Influence: 6019.6] [Reference Citation Analysis (3)] |
| 21. | Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4682] [Cited by in RCA: 4809] [Article Influence: 961.8] [Reference Citation Analysis (0)] |
| 22. | Cheng PK, Wong DA, Tong LK, Ip SM, Lo AC, Lau CS, Yeung EY, Lim WW. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699-1700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 245] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14762] [Article Influence: 2952.4] [Reference Citation Analysis (0)] |
| 24. | Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4385] [Article Influence: 877.0] [Reference Citation Analysis (1)] |
| 25. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12970] [Article Influence: 2594.0] [Reference Citation Analysis (1)] |
| 26. | Gupta N, Agrawal S, Ish P. Chloroquine in COVID-19: the evidence. Monaldi Arch Chest Dis. 2020;90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Roberts GC. Origins of specificity in the binding of small molecules to dihydrofolate reductase. Ciba Found Symp. 1977;(60):89-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Ho JC, Wu AY, Lam B, Ooi GC, Khong PL, Ho PL, Chan-Yeung M, Zhong NS, Ko C, Lam WK, Tsang KW. Pentaglobin in steroid-resistant severe acute respiratory syndrome. Int J Tuberc Lung Dis. 2004;8:1173-1179. [PubMed] |
| 29. | Chen CY, Lee CH, Liu CY, Wang JH, Wang LM, Perng RP. Clinical features and outcomes of severe acute respiratory syndrome and predictive factors for acute respiratory distress syndrome. J Chin Med Assoc. 2005;68:4-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |