Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.6197
Peer-review started: August 9, 2020
First decision: September 12, 2020
Revised: September 27, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 6, 2020
Processing time: 101 Days and 1.2 Hours
Polyostotic fibrous dysplasia (PFD) is an uncommon developmental bone disease in which normal bone and marrow are replaced by pseudotumoral tissue. The etiology of PFD is unclear, but it is generally thought to be caused by sporadic, post-zygotic mutations in the GNAS gene. Herein, we report the case of a young female with bone pain and lesions consistent with PFD, unique physical findings, and gene mutations.
A 27-year-old female presented with unbearable bone pain in her left foot for 4 years. Multiple bone lesions were detected by radiographic examinations, and a diagnosis of PFD was made after a biopsy of her left calcaneus with symptoms including pre-axial polydactyly on her left hand and severe ophthalmological problems such as high myopia, vitreous opacity, and choroidal atrophy. Her serum cortisol level was high, consistent with Cushing syndrome. Due to consanguineous marriage of her grandparents, boosted whole exome screening was performed to identify gene mutations. The results revealed mutations in HSPG2 and RIMS1, which may be contributing factors to her unique findings.
The unique findings in this patient with PFD may be related to mutations in the HSPG2 and RIMS1 genes.
Core Tip: Polyostotic fibrous dysplasia is an uncommon developmental bone disease. It mostly presents as progressive fibrous dysplasia with decreased skeletal strength and increased bone pain. Herein, we report the case of a 27-year-old female suffering multiple-sites bone pain on the left ischium, fibula, talus, and calcaneus with extreme high serum cortisol level, which might explain her Cushing syndrome. Preaxial polydactyly on her left hand and severe ophthalmological problems were also found in this patient. Boosted whole exome screening revealed unique gene mutations in HSPG2 and RIMS1 that may contribute to her symptoms.
- Citation: Lin T, Li XY, Zou CY, Liu WW, Lin JF, Zhang XX, Zhao SQ, Xie XB, Huang G, Yin JQ, Shen JN. Discontinuous polyostotic fibrous dysplasia with multiple systemic disorders and unique genetic mutations: A case report. World J Clin Cases 2020; 8(23): 6197-6205
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/6197.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.6197
Fibrous dysplasia (FD) is a genetic neoplastic bone disorder where normal bone and marrow are replaced by osteofibrous connective tissue, leading to bone pain, deformity, and fractures[1]. While the condition is benign, surgery is necessary to alleviate pain and repair or stabilize the affected bones. FD accounts for approximately 7% of all benign tumor-like bone lesions[2]. There are three defined subtypes of FD: (1) Monostotic; (2) Polyostotic fibrous dysplasia (PFD); and (3) McCune Albright syndrome (MAS). Among these, monostotic is the most common (70%), while PFD and MAS are relatively rare[3].
Bisphosphonates are the primary medical treatment for FD. Although a 1994 study showed that intravenous pamidronate (60 mg per day over 3 d, every 6 mo) can result in the refilling of bone lesions and cortical thickening of some patients with FD, its effectiveness in controlling disease progression remains uncertain[4]. Drugs such as denosumab have been reported to be effective in reducing bone pain and slowing the lesion growth rate in patients with receptor activator of nuclear factor kappa-B ligand expression, however, its use is debatable when the side effects are considered in light of the therapeutic effects[1]. Other treatments include surgery, physical rehabilitation, and long-term conservative management and close monitoring[5]. As such, to develop better treatments for FD, a better understanding of the genetic and molecular mechanisms of FD is needed.
FD is generally thought to be caused by sporadic, post-zygotic mutations in the GNAS gene, located on chromosome 20q13.3, which result in the activation of the signaling transduction pathway that generates cyclic adenosine monophosphate[6].
Herein, we report the case of a 27-year-old Chinese woman diagnosed as PFD with multiple discontinuous lesions, Cushing syndrome, and ophthalmological disorders. She was unresponsive to bisphosphonate treatment, and gene sequencing revealed mutations in the HSPG2 and RIMS1 genes.
The patient complained of increasing pain in her left foot and difficulty walking over the past 4 years.
Over a 4-year period, she experienced increasing pain in her left foot and difficulty walking. Radiographic examinations at local hospitals suggested a diagnosis of PFD, which was confirmed by a biopsy of her left calcaneus.
The patient had a history of frequent bone fractures since birth. She also presented with typical symptoms of Cushing syndrome and ophthalmological disorders including high myopia, vitreous opacity, and choroid atrophy. Hypothalamic amenorrhea and irregular menstruation were also present.
The patient’s maternal grandparents were consanguineous (cousins).
The patient experienced pain on palpation of her left foot. No obvious swelling was observed, and the foot was neurovascularly intact. Signs of Cushing syndrome included abdominal obesity with thin arms and legs, acne, a round face, and a fat lump between the shoulders (Figure 1A-C)[7]. Her teeth were noted to be poorly developed (Figure 1D), and she had thumb duplication on her left hand (Figure 1E and F).
Her serum calcium and phosphate levels were normal as well as the levels of hormones that regulate calcium metabolism, including parathyroid hormone, 25-hydroxy vitamin D, and osteocalcin. Her serum cortisol level was 1492.00 nmol/L, which was extremely high (reference range of 147.30-609.30 nmol/L), and her triglyceride and uric acid levels were also elevated (Table 1).
| Parameter | Unit | Reference | Result |
| Parathyroid hormone | pg/mL | 15.3-68.3 | 23.7 |
| 25-OH-VitD | nmol/L | 47.7-144 | 121.40 |
| Osteocalcin | ng/mL | Male, 9.80-26.40; Female, 7.70-21.70 | 7.05 |
| Calcium | mmol/L | 2.08-2.80 | 2.61 |
| Serum phosphate | mmol/L | 1.00-1.94 | 1.61 |
| Cortisol, 8:00-10:00 | nmol/L | 147.3-609.3 | 1492.00 |
| Triglyceride | mmol/L | < 1.70 | 1.88 |
| Uric acid | μmol/L | 150-360 | 689 |
| Bone specific alkaline phosphatase | μg/L | 11.4-24.0 | 15.86 |
| Hematocrit | % | Male, 41-53; Female, 36-46 | 47.80 |
| Eosinophils | % | 1-3 | 8.10 |
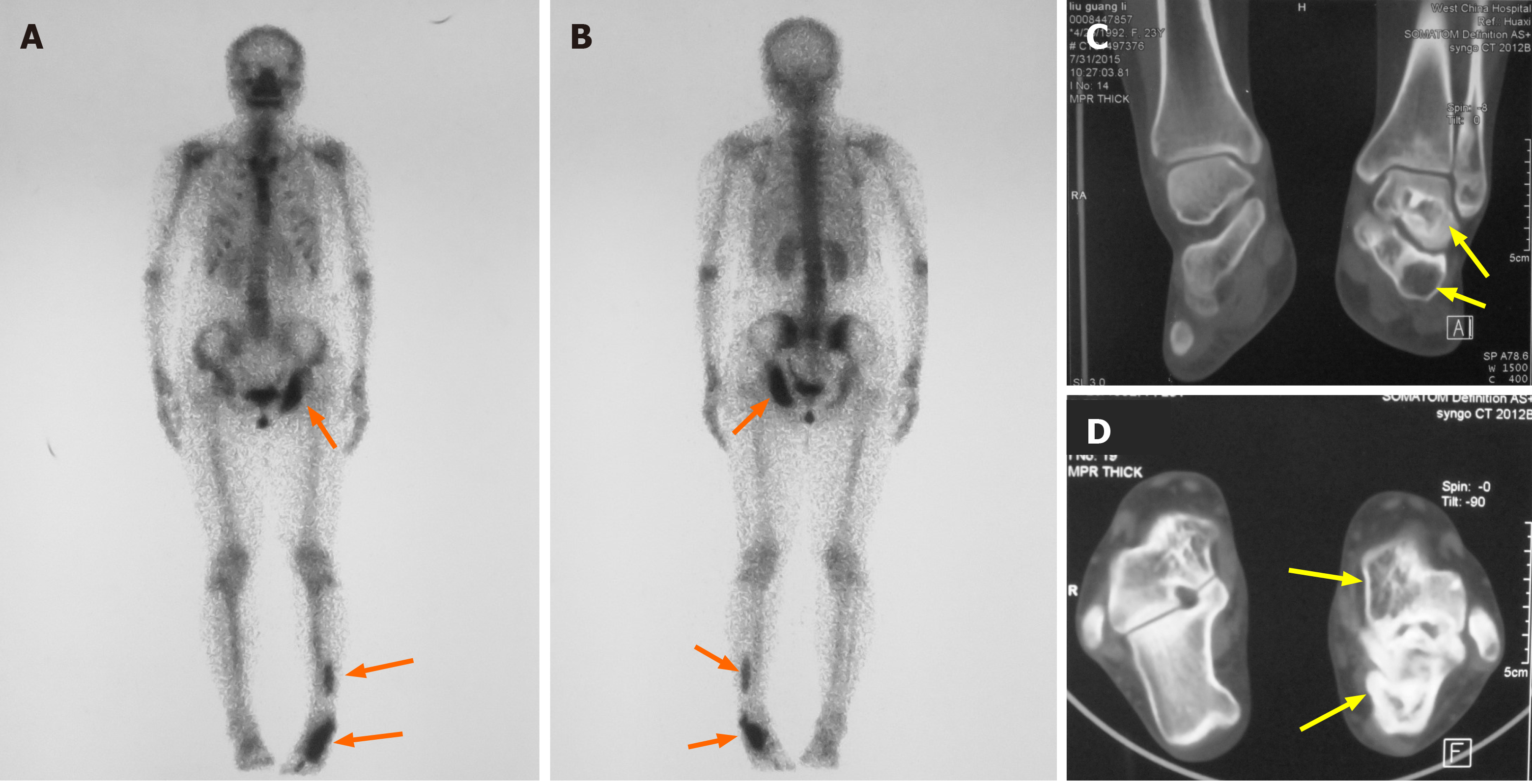
Skeletal scintigraphy showed multiple bone lesions on her left ischium, distal fibula, calcaneus, and talus (Figure 2A and B). Computed tomography (CT) revealed well-circumscribed bone lucencies and ground-glass opacities in the calcaneus and talus (Figure 2C and D). No evidence of pituitary or adrenal lesions was identified on brain computed tomography.
Based on the patient’s clinical symptoms, imaging studies, and biopsy results (Figure 3), a diagnosis of PFD was made after consultations with musculoskeletal oncologists, radiologists, and pathologists. Her other symptoms and signs were considered likely to be caused by her unique genetic mutations.
In 2016, the patient began intravenous zoledronic acid every 6 mo and received four doses, followed by sodium pamidronate every 3 mo and received three doses. She was then continued on oral alendronate weekly.
After evaluating the risk and expenses of surgery, the patient chose to continue bisphosphonate treatment with regular monitoring of disease progression. Over a 2-year period, the size of the lesions did not become markedly larger. However, dual-energy X-ray absorptiometry indicated a new lower bone mass in the proximal femur and the distal fibula as compared to an examination 2 years prior, suggesting possible disease progression. The treatments, however, did not alleviate the pain in her left foot, indicating resistance to the anti-bone resorption treatments. Nonsteroidal anti-inflammatory drugs were prescribed for her left foot bone pain.
Herein, we presented the case of a young woman with PFD combined with multiple systemic disorders and resistance to bisphosphonate treatment. PFD was confirmed by biopsy of her left calcaneus. However, her additional symptoms were markedly different from most patients with PFD.
We reviewed 6 cases of PFD identified in our search of the literature[2,8-12], and the details of the 6 cases along with the details of our case are summarized in Table 2. In patients with PFD, when lesions involve the orbital region the primary findings are facial asymmetry, orbital dystopia, and orbital proptosis[13], none of which were identified in our patient. Therefore, it is likely that the ophthalmological disorders of our patient are not related to her PFD, but rather to other genetic defects.
| Ref. | Year | Sex | Age | Location | Appendix | Treatment | Outcome |
| Sagmeister et al[12] | 2016 | F | 27 | Throughout the skeleton. Transverse fracture of the distal right femur | Continuous lesions, extensive bone expansion, cyst formation, cortical loss | Skin traction for 8 wk. Intensive physiotherapy for the fracture | Recovered well, returning to baseline 3 mo later |
| Wu et al[2] | 2014 | F | 38 | Sternum, thoracic spine, ribs, right femur, and tibia | Multiple lytic, expansile lesions, continuous pathologic fractures in the thoracic spine | Surgical therapy. Diphosphate therapy with Vit D and calcium | Completely recovered. Able to participate in daily life and work 2 yr later |
| Kodama et al[10] | 2012 | F | 8 | Right pelvis, bilateral femurs, and fibula | Discontinuous lesions | Thigh coxa valga osteotomy and plate fixation. Diphosphate therapy | No complaints of severe pain in lower extremity. Low bone turn-over rate |
| Aras et al[9] | 2012 | M | 48 | Cranium, left hemithorax, bilateral upper, lower extremities, and pelvic bones | Continuous lesions, bladder cancer | No treatment reported | No outcome reported |
| Boston et al[8] | 1994 | M | 3.3 | Proximal left femur and proximal left humerus | Albright-McCune syndrome, no café-au-lait pigmentation, Cushing syndrome | Bilateral adrenalectomy at 7-yr-old with steroid replacement | Cushing syndrome removed. Still with prepubertal and elevated liver enzyme |
| Lourenço et al[11] | 2015 | F | 17 d | Multiple lesions with fracture in left ulna | Multiple organs involved, Café-au-lait pigmentation, mosaic GNAS gene mutation | Metyrapone therapy for Cushing syndrome | Cushing syndrome recovered. Death due to respiratory infection |
| The current case | F | 27 | Left ischium, left distal fibula, calcaneus, and talus | Discontinuous lesions, intractable bone pain, Cushing syndrome | Diphosphate therapy | Still severe pain. Difficulty participating in daily life and job |
Some of the patients with PFD/MAS in our review did have multiple bone lesions and endocrine disorders (e.g., precocious puberty, hypercortisolism, and hyperthyroidism) and skin pigmentation (“café-au-lait” spots). Hypercortisolism was the rarest symptom associated with PFD/MAS and is reported to occur exclusively in newborns[14]. However, our patient had an extremely high serum cortisol level, a finding not reported in the cases we reviewed.
PFD is difficult to treat or cure because of multiple advanced bone lesions and genetic defects of the osteoprogenitors. Studies have shown that radiographic findings and bone pain are improved in approximately 50% patients treated with bisphosphonates[15-17]. However, neither oral nor intravenous bisphosphonates were effective in our patient. Curettage is indicated to alleviate bone pain and improve limb function; however, our patient declined any surgical treatment.
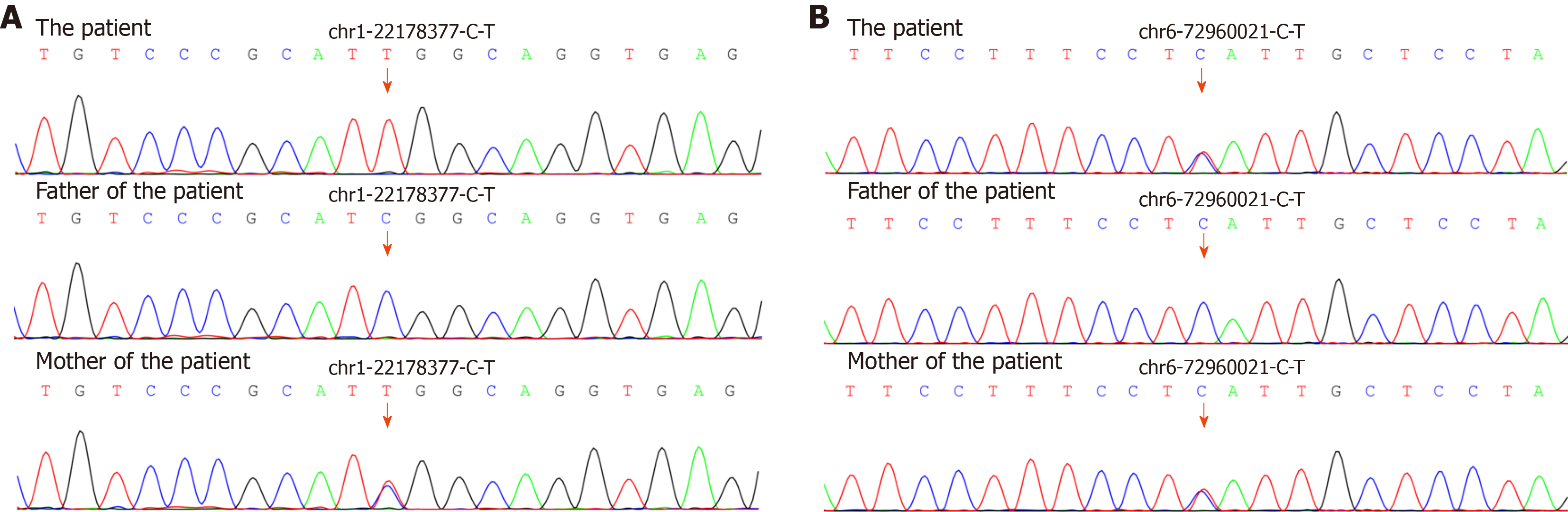
Since multiple gene mutations were identified by boosted whole exome screening in our patient, we hypothesize that her unique symptoms are likely due to the gene mutations (Table 3). Notably, the missense and non-sense point mutations in RIMS1 and HSPG2 are likely responsible for her unique symptoms (Figure 4), because mutations in these two genes are reported to be associated with bone and ocular diseases, respectively.
| Gene | Function of the protein coded | Mutation | Source | Associated disease |
| RIMS1 | A RAS gene superfamily member that regulates synaptic vesicle exocytosis | Point mutation Thr1047His | Maternal, heterozygous | Cone-rod dystrophy type 7 |
| HSPG2 | Perlecan that is found in the extracellular matrix | Point mutation Asp2305Asn | Maternal, homozygous | S-J syndrome type 1 |
| APC | Negative regulator of β-catenin/Wnt pathway | Point mutation Lys1586Met | Maternal, heterozygous | Colorectal cancer associated with FAP |
| BGN | A member of the SLRP family | Point mutation p.Asp168Glu | Maternal, heterozygous | SPD X-linked MLS |
| BMPR1B | Transmembrane serine/threonine kinases involving TGF-β pathway | Point mutation Met301Val | Maternal, heterozygous | Pulmonary arterial hypertension |
| CC2D2A | Play a critical role in cilia formation | Point mutation Gly317Arg | Maternal, heterozygous | Meckel syndrome type 6. Joubert syndrome type 9 |
| CDH23 | Cadherin superfamily involved in stereocilia organization and hair bundle formation | Point mutation Asp168Glu | Spontaneous, heterozygous | Breast cancer |
| CHD7 | Protein that contains several helicase family domains | Point mutation Asp1486Gly | Spontaneous, heterozygous | CHARGE syndrome |
| FLNA | An actin-binding protein that crosslinks actin filaments and links actin filaments to membrane glycoproteins | Point mutation Asp1314Asn | Maternal, heterozygous | Several syndromes including PNH, OPDS, FMD and so on |
| CILK1 | Eukaryotic protein kinases | Point mutation Val215Met | Maternal, homozygous | ECD |
Analysis of the genetic sequencing results of the patient and her parents indicated that the patient’s mutations were mostly heterozygous and similar to those of her mother. This indicates that the mutated genes came from her mother, whose parents had a consanguineous marriage. Surprisingly, the HSPG2 mutation was homozygous, which is likely due to uniparental disomy[18] [a single chromosome from her mother was duplicated leading to the homozygous mutation in HSPG2 (Figure 5)].
HSPG2 is an essential, highly conserved gene widely expressed throughout the development of cartilage and the formation and calcification of skeletal bone[19]. Mutations in HSPG2 can lead to two autosomal recessive inheritance skeletal disorders; Schwartz-Jampel syndrome (Online Mendelian Inheritance in Man 255800) and dys-segmental dysplasia, Silverman-Handmaker type (Online Mendelian Inheritance in Man 224410)[20]. Dys-segmental dysplasia, Silverman-Handmaker type can result in severe cartilage matrix anomalies, and even neonatal death, whereas patients with Schwartz-Jampel syndrome may have chondrodysplasias, myotonic myopathy, and facial and ocular abnormalities. Heterozygous mutations in RIMS1 mainly cause cone-rod dystrophy, which is characterized by reduced photophobia, central vision, and reduced color vision[21].
We did not identify mutations in GNAS in any of the blood samples we tested, although mutations in GNAS have been reported to be associated with PFD. It is generally thought that mutations in GNAS occur early in embryogenesis, and cells with defects are distributed in different tissues of the body as a result of embryonic cell migration[22]. To determine if GNAS mutations are present in our patient, bone lesion tissue samples require further sequencing analysis or molecular testing.
Herein, we presented the case of a patient with PFD with the unique findings of pre-axial polydactyly, Cushing syndrome, ophthalmological abnormalities, and resistance to bisphosphonate treatment. Gene sequencing revealed mutations in HSPG2 and RIMS1, which may be responsible for her unique findings.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ruiz MA S-Editor: Huang P L-Editor: Filipodia P-Editor: Xing YX
| 1. | Florez H, Peris P, Guañabens N. Fibrous dysplasia. Clinical review and therapeutic management. Med Clin (Barc). 2016;147:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Wu FL, Liu ZJ, Liu XG, Yang SM, Jiang L, Wei F, Yu M. Polyostotic fibrous dysplasia involving the thoracic spine with myelopathy: case report and review of the literature. Spine J. 2014;14:e11-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Basaran R, Kaksi M, Gur E, Efendioglu M, Balkuv E, Sav A. Monostotic fibrous dysplasia involving occipital bone: a case report and review of literature. Pan Afr Med J. 2014;19:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Liens D, Delmas PD, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. Lancet. 1994;343:953-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 138] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Boyce AM, Turner A, Watts L, Forestier-Zhang L, Underhill A, Pinedo-Villanueva R, Monsell F, Tessaris D, Burren C, Masi L, Hamdy N, Brandi ML, Chapurlat R, Collins MT, Javaid MK. Improving patient outcomes in fibrous dysplasia/McCune-Albright syndrome: an international multidisciplinary workshop to inform an international partnership. Arch Osteoporos. 2017;12:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1002] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 7. | Nieman LK, Ilias I. Evaluation and treatment of Cushing's syndrome. Am J Med. 2005;118:1340-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Boston BA, Mandel S, LaFranchi S, Bliziotes M. Activating mutation in the stimulatory guanine nucleotide-binding protein in an infant with Cushing's syndrome and nodular adrenal hyperplasia. J Clin Endocrinol Metab. 1994;79:890-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Aras M, Ones T, Dane F, Nosheri O, Inanir S, Erdil TY, Turoglu HT. False Positive FDG PET/CT Resulting from Fibrous Dysplasia of the Bone in the Work-Up of a Patient with Bladder Cancer: Case Report and Review of the Literature. Iran J Radiol. 2012;10:41-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Kodama Y, Ogose A, Oguri Y, Ubaidus S, Iizuka T, Takagi R. Alveolar bone grafting in association with polyostotic fibrous dysplasia and bisphosphonate-induced abnormal bone turnover in a bilateral cleft lip and palate patient: a case report. J Oral Maxillofac Surg. 2012;70:e500-e508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Lourenço R, Dias P, Gouveia R, Sousa AB, Oliveira G. Neonatal McCune-Albright syndrome with systemic involvement: a case report. J Med Case Rep. 2015;9:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Sagmeister ML, Miller G, Lewis TL. Polyostotic fibrous dysplasia: a rare cause of pathological fractures in young patients. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Choi JW, Lee SW, Koh KS. Correction of proptosis and zygomaticomaxillary asymmetry using orbital wall decompression and zygoma reduction in craniofacial fibrous dysplasia. J Craniofac Surg. 2009;20:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Brown RJ, Kelly MH, Collins MT. Cushing syndrome in the McCune-Albright syndrome. J Clin Endocrinol Metab. 2010;95:1508-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Chapurlat RD. Medical therapy in adults with fibrous dysplasia of bone. J Bone Miner Res. 2006;21 Suppl 2:P114-P119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Chapurlat RD, Delmas PD, Liens D, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. J Bone Miner Res. 1997;12:1746-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Chapurlat RD, Hugueny P, Delmas PD, Meunier PJ. Treatment of fibrous dysplasia of bone with intravenous pamidronate: long-term effectiveness and evaluation of predictors of response to treatment. Bone. 2004;35:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Yamazawa K, Ogata T, Ferguson-Smith AC. Uniparental disomy and human disease: an overview. Am J Med Genet C Semin Med Genet. 2010;154C:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Lowe DA, Lepori-Bui N, Fomin PV, Sloofman LG, Zhou X, Farach-Carson MC, Wang L, Kirn-Safran CB. Deficiency in perlecan/HSPG2 during bone development enhances osteogenesis and decreases quality of adult bone in mice. Calcif Tissue Int. 2014;95:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Martinez JR, Dhawan A, Farach-Carson MC. Modular Proteoglycan Perlecan/HSPG2: Mutations, Phenotypes, and Functions. Genes (Basel). 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Robson AG, Michaelides M, Luong VA, Holder GE, Bird AC, Webster AR, Moore AT, Fitzke FW. Functional correlates of fundus autofluorescence abnormalities in patients with RPGR or RIMS1 mutations causing cone or cone rod dystrophy. Br J Ophthalmol. 2008;92:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Robinson C, Collins MT, Boyce AM. Fibrous Dysplasia/McCune-Albright Syndrome: Clinical and Translational Perspectives. Curr Osteoporos Rep. 2016;14:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |