Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.6110
Peer-review started: June 12, 2020
First decision: September 13, 2020
Revised: September 22, 2020
Accepted: October 13, 2020
Article in press: October 13, 2020
Published online: December 6, 2020
Processing time: 175 Days and 0.1 Hours
Grade II and III meningiomas [World Health Organization (WHO) classification] rarely have extracranial metastases via the blood circulation; however, we experienced a case with a metaplastic atypical meningioma and local de-differentiation that metastasized to the jugular vein, carotid artery and subclavian artery at the cervicothoracic junction. Such cases have seldom been reported before.
The patient was a 30-year-old man who developed right neck masses with dysphagia, labored breathing, dizziness, and occasional earaches. Eight months earlier the patient was diagnosed with a right parietal lobe neoplasm and hemorrhage at a local hospital due to the sudden onset of headaches and left limb weakness, and the post-operative pathology was a metaplastic atypical meningioma (WHO grade II) with local de-differentiation (WHO III). Magnetic resonance imaging revealed a calcified mass at the root of the neck on the right and a large cystic mass in the right parapharyngeal space. Head and neck angiography showed that the right common carotid artery was compressed and completely occluded, and the jugular vein was enveloped by the tumor and occluded. A balloon occlusion test showed no perfusion in the right common carotid artery. Tumor resection, carotid artery ligation, and subclavian artery reconstruction were performed. The tumor was a malignant meningioma. Post-operatively, the patient had Horner's syndrome and hoarseness.
This case highlights the importance of the link between a large cervical mass and a primary intracranial tumor. Malignant meningioma should not be considered merely as an intracranial metastasis spread through cerebrospinal fluid, it can also be transferred through the circulation to the parapharyngeal space and the cervical great vessels.
Core Tip: Malignant meningioma is considered to have a low incidence, and metastases to the cervical great vessels has been much less reported. We present herein, a rare case of primary intracranial malignant meningioma metastasizing to the parapharyngeal space, the common carotid artery and jugular vein in a young male patient. The pathology of the surgically resected tumor confirmed malignant meningioma. This case highlights the importance of the link between a large cervical mass and a primary intracranial tumor. Malignant meningioma should not be considered merely as intracranial metastasis spread through cerebrospinal fluid, it can also be transferred through the circulation to the parapharyngeal space and the cervical great vessels.
- Citation: Chen HY, Zhao F, Qin JY, Lin HM, Su JP. Malignant meningioma with jugular vein invasion and carotid artery extension: A case report and review of the literature. World J Clin Cases 2020; 8(23): 6110-6121
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/6110.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.6110
A meningioma is the most common intracranial tumor in adults, accounting for approximately 37% of all intracranial tumors[1]. Based on cell morphology and mitosis, meningiomas are classified pathologically as benign [(World Health Organization (WHO) grade I (approximately 90%)], atypical [WHO grade II, low-grade malignant (5%-7%)], and malignant [WHO grade III (3%-5%)][2]. Malignant meningiomas can be transmitted to intracranial sites, spinal canal, and extracranial sites via the cerebrospinal fluid or blood, but this is rare in clinical practice. Moreover, malignant tumors that metastasize to the cervical thoracic junction are closely related to the surrounding blood vessels and nerves; therefore, the operation is difficult and high risk, which may require the cooperation of multiple disciplines, such as neurosurgery, head and neck surgery, vascular surgery, cardiothoracic surgery, and orthopedics. Single disciplinary management is not conducive to complete resection of the tumor, and serious complications may occur, which is a clinical challenge.
Herein we describe the diagnosis and treatment of a young man with a malignant meningioma, jugular vein invasion, and carotid and subclavian artery extension. In addition, the literature was reviewed regarding the clinical features, histopathologic findings, and extracranial metastasis of malignant meningiomas, as well as carotid artery resection and reconstruction, which will provide important clinical information for the diagnosis and treatment of rare tumors in this anatomic region.
A 30-year-old male patient presented with a 5-mo history of right neck masses, complaining of dysphagia and labored breathing.
Five months ago, two thumb-sized masses grew in the upper and middle parts of the anterior right neck, then gradually enlarged, especially the tumor in the middle part, which enlarged rapidly. The patient disregarded the masses and did not seek evaluation and treatment. The patient subsequently developed dysphagia, labored breathing, dizziness, and occasional earaches. He was diagnosed with right neck masses in another hospital and underwent neck exploratory surgery, but the masses could not be removed due to severe bleeding. The two tumors continued to grow after surgery. The upper tumor increased in size over the short term and eventually developed into two adult fist-sized masses with associated symptoms.
The patient was diagnosed with a right parietal lobe neoplasm and hemorrhage at a local hospital due to the sudden onset of headaches and left limb weakness 8 mo ago. The patient underwent a craniotomy with hematoma removal and tumor resection, and the post-operative pathology was a metaplastic atypical meningioma (WHO grade II) with local de-differentiation (WHO grade III). Post-operatively, the patient had left limb weakness and hoarseness, but did not receive radiotherapy as recommended by the physician.
No family history of meningiomas or neck tumors.
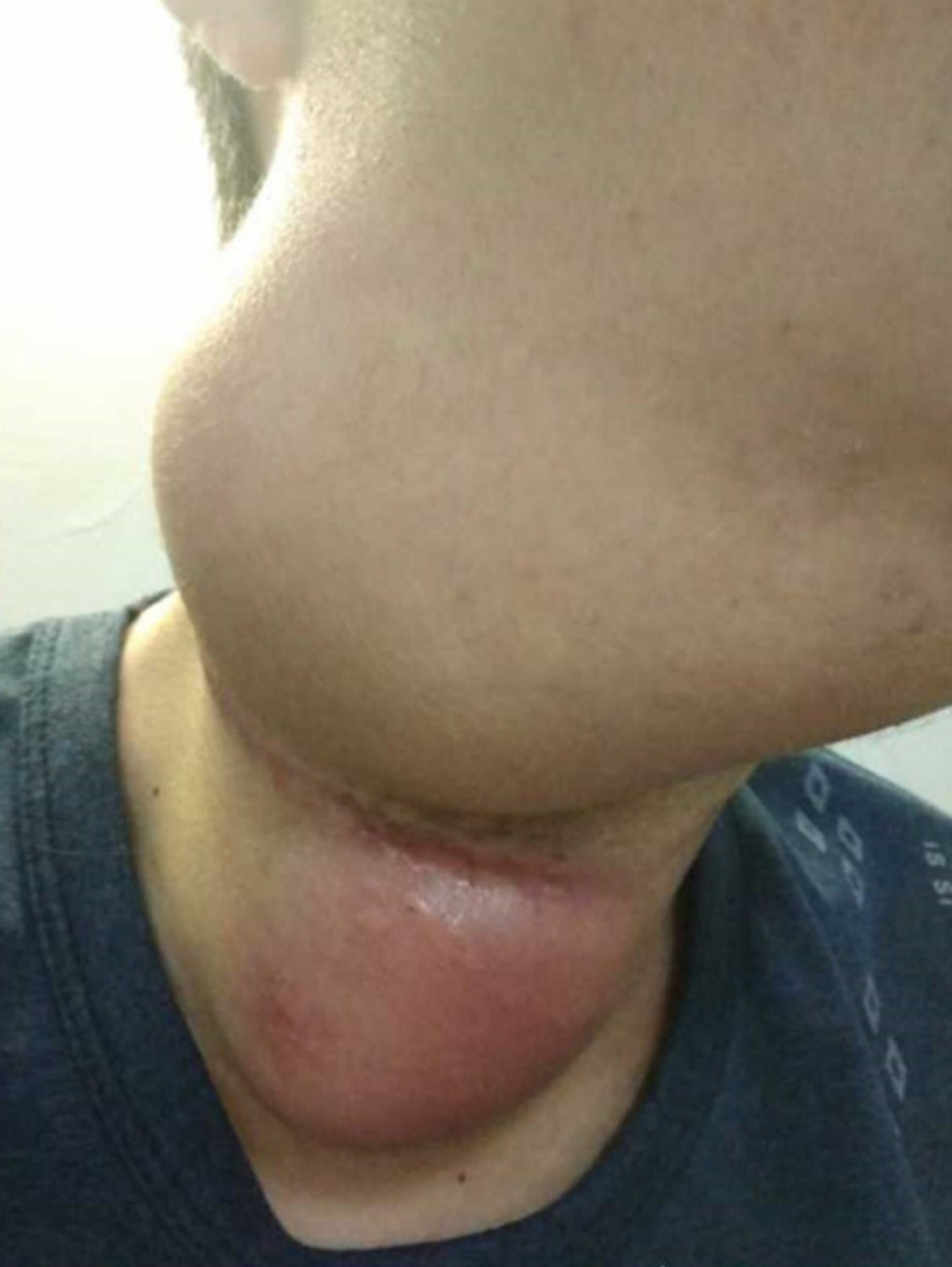
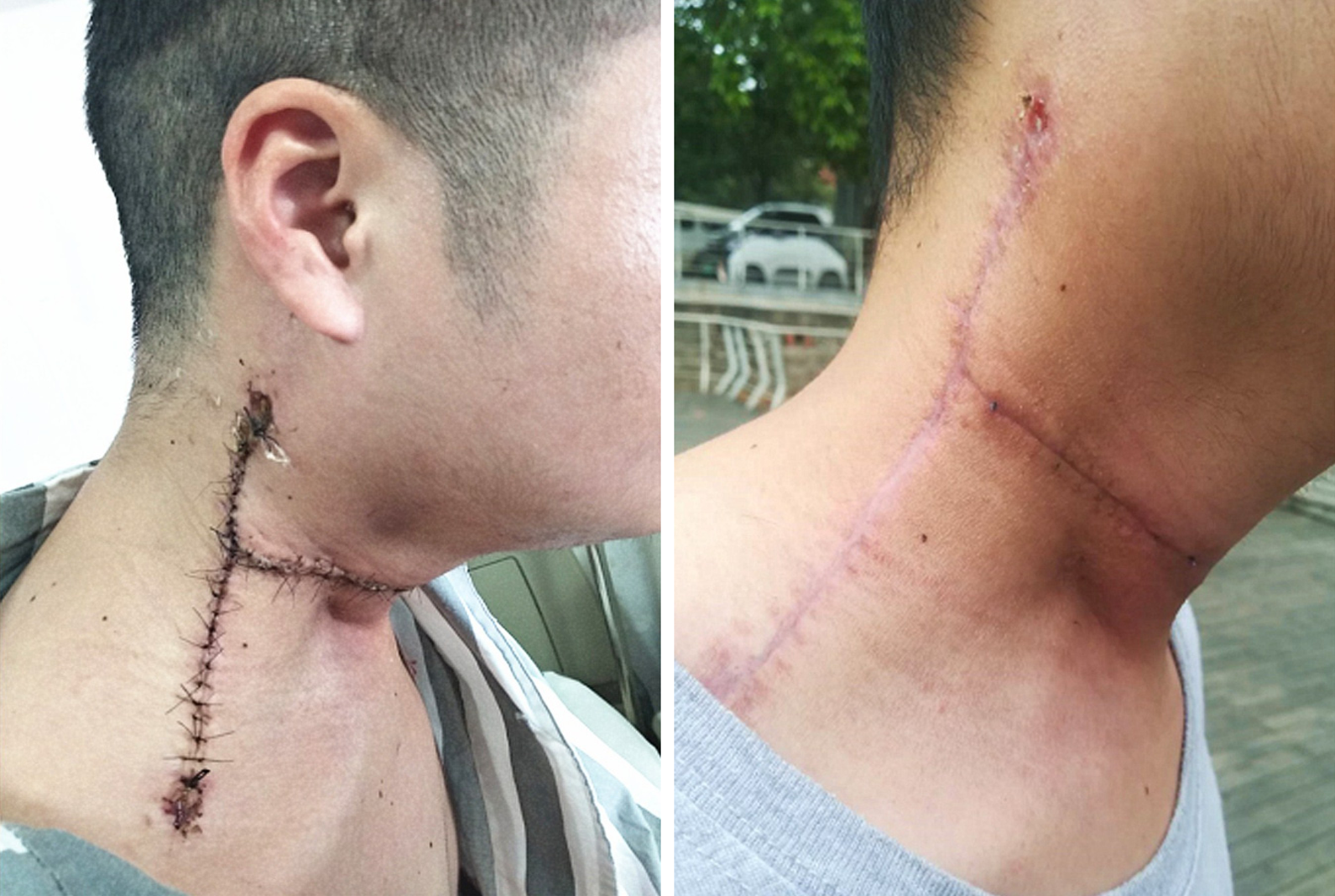
Two masses were identified in the upper and middle parts of the right neck, about 10 cm × 8 cm in the upper part and 8 cm × 5 cm in the middle part, and an 18.0-cm lateral surgical scar was present between the two masses (Figure 1). The trachea was deviated to the left and the right vocal cords were paralyzed. Grade 3 muscle strength was demonstrated in the left upper and lower limbs.
There were no obvious abnormalities in routine blood tests, coagulation function, liver and kidney function, blood electrolytes and other blood biochemical tests.
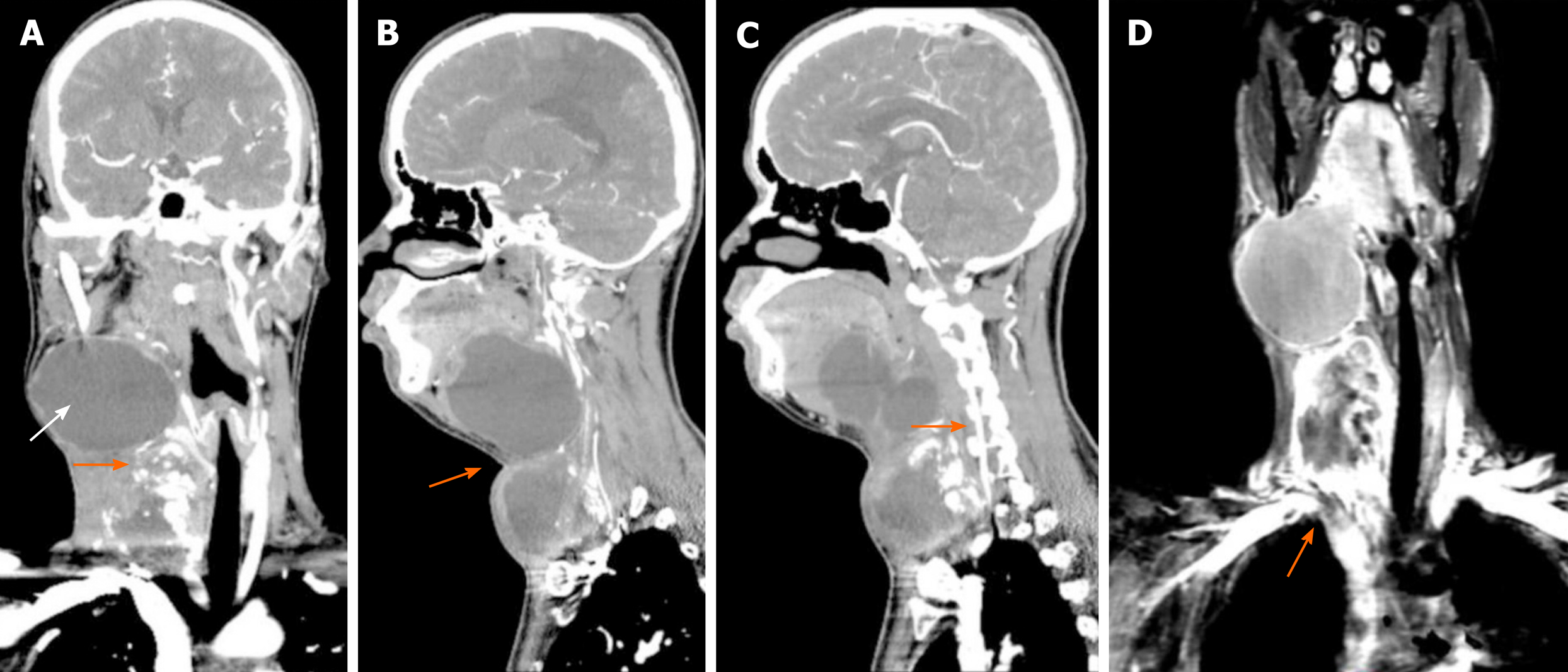
Computed tomography and magnetic resonance imaging (MRI) revealed a right side calcified mass and an unclear boundary at the root of the neck measuring 7.1 cm × 5.8 cm × 6.0 cm. Another large cystic mass was present in the right parapharyngeal space and measured 5.9 cm × 9.2 cm × 8.1 cm. The two masses reached the right sublingual space and extended downward to the thorax. The adjacent blood vessels were displaced and the trachea, oropharynx, and hypopharynx were deviated to the left (Figure 2). Head and neck angiography showed that the right common carotid artery was compressed and completely occluded and the diameter of the internal carotid artery was reduced. The jugular vein was enveloped by the tumor and occluded. A balloon occlusion test (BOT) showed no perfusion in the right common carotid artery. The anterior communicating artery was opened so that the left carotid artery could compensate for the contralateral cerebral circulation.
The final diagnosis of the presented case was metastatic cervical malignant meningioma with jugular vein invasion and carotid artery extension.
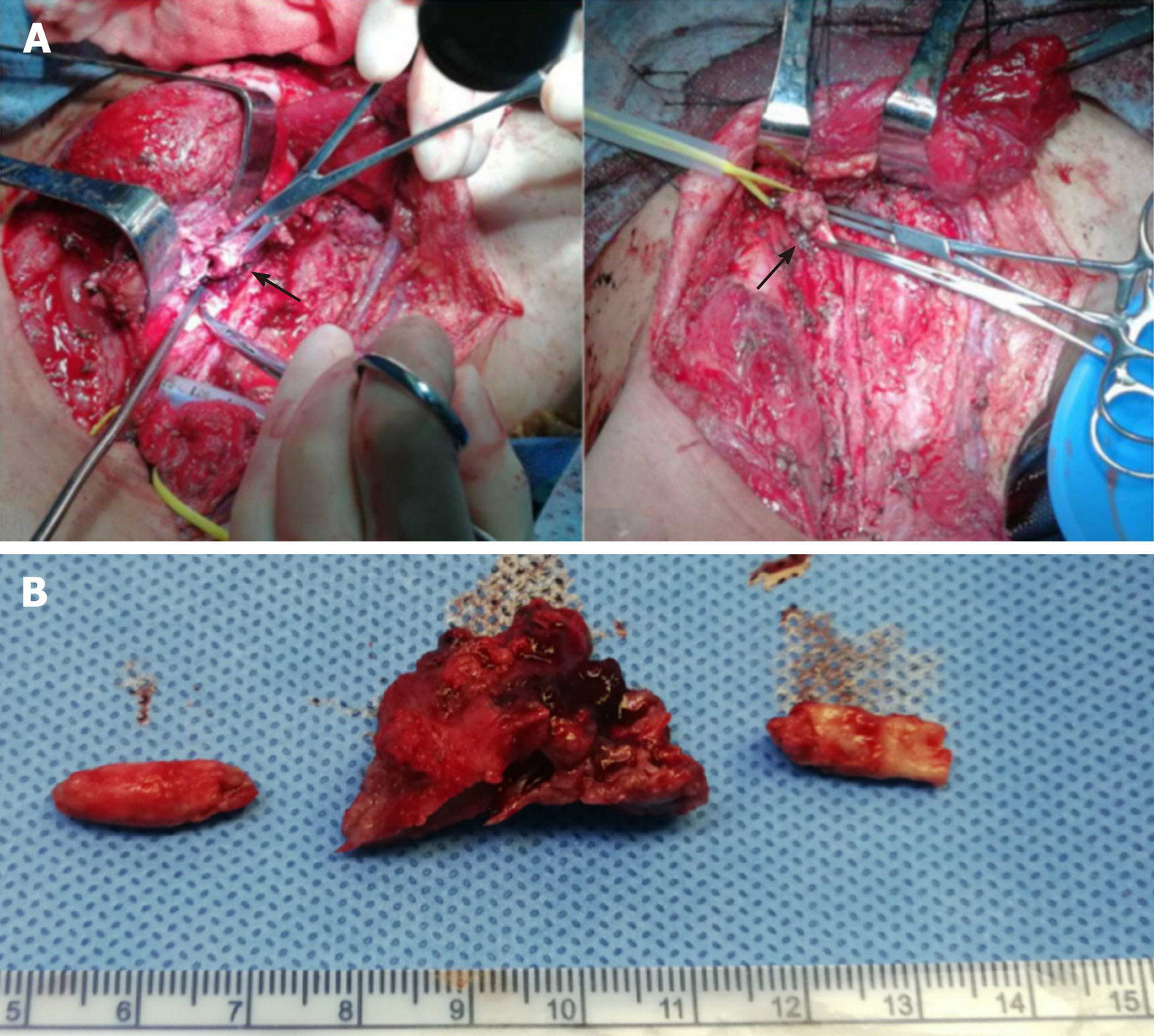
(1) The original surgical scar was removed and separated upward and downward. The right upper neck mass was removed and the skin flap was separated along the surface of the mass. The upper medial part of the mass was closely adhered to the submandibular gland, the lower part was adhered to the sub-cervical mass, and the lateral part was adhered to the sternocleidomastoid muscle. The sternocleidomastoid muscle was cut and separated upward and downward. The common carotid artery, jugular vein, vagus nerve, cervical sympathetic nerve, and recurrent laryngeal nerve were encased in the deep surface of the mass. The tumor was further separated deeply facing up to the floor of the mouth and completely removed. During the separation, the tumor leaked a dark red liquid, but there was no severe active bleeding; (2) the right sub-neck tumor was resected. The internal jugular vein, common carotid artery, vagus nerve, cervical sympathetic nerve, and recurrent laryngeal nerve were completely covered by the mass, which adhered tightly to the thyroid, trachea, and esophagus. There was a large amount of hard calcified tissue in the tumor and brown liquefied necrosis (Figure 3). The jugular vein and common carotid artery were calcified. A calcified body was embolized to the jugular vein and common carotid artery. The lumen of the jugular vein was destroyed and there was no blood flow. The jugular vein was transected and ligated at the level of the common carotid artery bifurcation. No embolism was noted in the ligated end. The reflux pressure of the right common carotid artery was > 70 cm H2O. The right common carotid artery was ligated 1 cm below the bifurcation and resected at the level of the common carotid artery; no embolism was noted. The vagus nerve was isolated and released. The thyroid, trachea, and esophagus were isolated along the inside of the mass and the cervical sympathetic nerve and recurrent laryngeal nerve, which were encased by the mass, were resected and ligated; (3) further exploration revealed that there was remnant tumor tissue in the cross between the right subclavian and common carotid arteries; the outer wall was infiltrated by the tumor. The end of the common carotid artery was cut at the bifurcation of the brachiocephalic trunk and the tumor tissue was removed until the normal vascular wall was visualized; (4) the right common carotid artery was removed and there was a 1 cm × 2 cm defect at the junction of the right subclavian artery and the brachiocephalic trunk. The 1 cm × 2 cm vein wall of the residual end of the jugular vein was secured and the defect was repaired by a continuous suture with 5-0 prolene; and (5) the sternocleidomastoid muscle was fashioned into a muscle flap covering and strengthening the vascular repair site. A drainage tube was placed, the incision was sutured, and a compression bandage was placed. The operation lasted approximately 7 h.
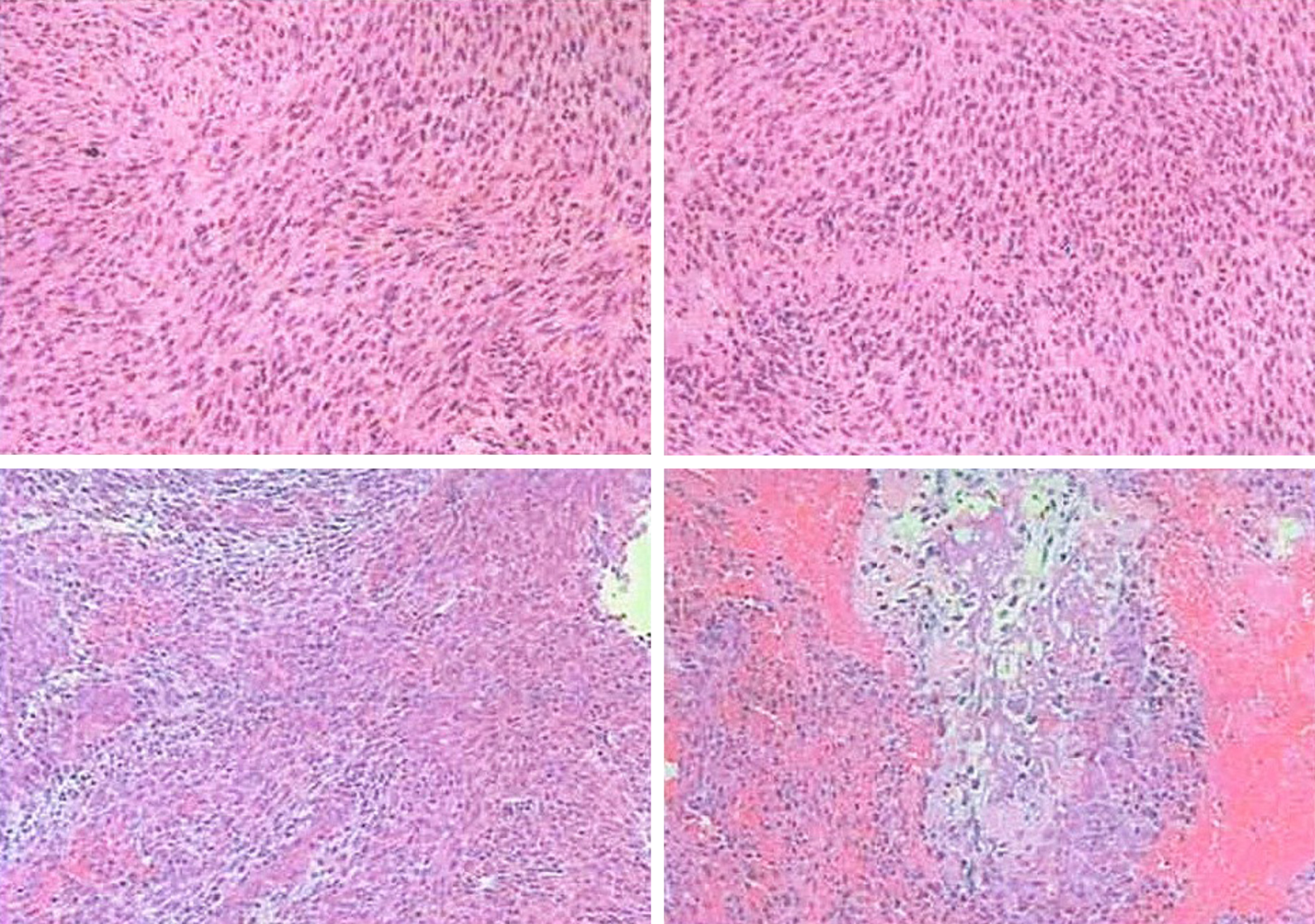
Post-operatively, the incision healed normally and the patient had unobstructed breathing, a good appetite, no headache, and no episodes of emesis. The left limb muscle strength was unchanged from that pre-operatively. The right limb muscle strength was grade 5. The head was slightly deviated to the right, the right eye was sunken, and the pupil was small; together, the findings were consistent with Horner syndrome. Microscopic pathology (Figure 4) showed the following: (1) A spindle cell malignant tumor with local osteogenesis; and (2) vimentin (+), CK (-), EMA (+), CK5 / 6 (-), P40 (-), CD34 (-), S-100 (-), SMA (+), TTF-1 (-), calponin (small focus) (+), desmin (-), PR (-), D2-40 (-), E-CAD (-), and Ki-67 [+ (50%)]. In situ hybridization showed EBERs (-). The following diagnoses were considered: (1) Osteosarcoma; (2) Malignant meningiomas with heterogeneous differentiation; (3) Tumor tissue noted in the common carotid artery; (4) Tumor tissue noted in the brachiocephalic trunk; and (5) One tracheoesophageal lymph node without metastasis (Figure 4). The consultation at The First Affiliated Hospital of Sun Yat-sen University (Guangzhou City, China) showed that the tumor cells were positive for vimentin, CD99, INI-1, and EMA; desmin was slightly positive in some of the cells; Ki67 expression varied between 10% and 30%; and was negative for S-100, PR, GFAP, CD34, Syn, NSE, MyoD1, and myogenin. Based on the immunohistochemistry results, a malignant meningioma was diagnosed. The patient was followed for 9 mo. Hoarseness was apparent, Horner's syndrome was unchanged, the appearance of the neck had improved, and no signs of tumor were noted (Figure 5). The right upper limb was normal, muscle strength was grade 5, and the left upper limb muscle strength was grade 3 (unchanged since the pre-operative assessment). A repeat computed tomography (CT) scan showed that compared with the pre-operative CT, the tumor had been resected, pulmonary nodules were present and the larger nodule was 0.7 cm × 0.6 cm with clear enhancement.
The annual incidence of malignant meningiomas is approximately 0.17/100000 per year, including three subtypes (anaplastic, rhabdoid, and papillary). Anaplastic meningioma is the most common malignant meningioma, accounting for approximately 1.0%-2.8%. Malignant meningiomas grow rapidly, and have high invasion, recurrence, and mortality rates. Malignant meningiomas can damage the arachnoid membrane, infiltrate into the brain tissue and the full-thickness of the dura mater and destroy the skull[3]. A study[3] reported that the 2- and 5-year survival rates for malignant meningiomas were 70% and 28% after total resection, 44% and 0% after subtotal resection, 100% and 57% after total resection plus radical radiotherapy, and 87% and 0% after subtotal resection plus radical radiotherapy, respectively. The median survival time of recurrences was 18 mo after re-operation and 34.1 mo after re-operation plus radiotherapy. Meningioma cells can detach in the cerebrospinal fluid and cause ventricular metastasis or dissemination of the subarachnoid space, or enter the spinal canal and cause extracranial implantation in the cervical, thoracic, lumbar, sacral, and cauda equina. A study has shown that 8 patients with meningiomas developed cerebral spinal cord metastases after multiple surgeries, accounting for 4% (8/200) of meningiomas at the same time[4].
Extracranial metastases of meningiomas via the blood are rare. A medical checkup of the brain by MRI scanning in a 59-year-old male revealed asymptomatic intracranial tumors in the right occipital area, which invaded the right transverse and sigmoid sinuses. The upper portion of the tumor was removed by temporoccipital craniotomy. To avoid the risk of damage to the Labbe vein, the intravenous sinus tumor was not removed. The patient received postoperative radiotherapy for the residual tumor in the intravenous sinus. Despite radiation, the residual tumor developed caudally and eventually extended to the right internal jugular vein. The average regeneration rate of the extracranial mass was 3.6 mm/month. The patient underwent surgery for recurrent tumors located in the transverse sinus, sigmoid sinus, jugular bulb, and internal jugular vein. The pathological characteristics of both operations were the same: WHO grade I meningioma[5].
A 14-year-old girl had headaches, dizziness, and neck pain for 6 mo, accompanied by abnormal hearing and smell. CT and MRI examinations revealed that a tumor of the right posterior temporal and occipital lobes encroached upon the transverse sinus, sigmoid sinus, and jugular bulb. The internal jugular vein was blocked to the C2 Level. The intracranial lesions were removed in the first stage of surgery, and the pathologic evaluation was shown to be a meningioma (WHO grade I). In the second stage, the right transverse sinus, sigmoid sinus, jugular bulb and jugular vein were removed, and the stump of the jugular vein was ligated. Due to the high recurrence rate, no venous reconstruction was performed[6].
A 68-year-old man developed back pain for 4 mo with sudden left lower limb weakness. MRI revealed a tumor in the spinal cord (C7–T2) that had invaded the mediastinal lymph nodes. After surgical resection, the post-operative pathologic evaluation showed a highly malignant anaplastic meningioma. The patient declined radiotherapy after surgery, and had dysfunction of the left fingers, hypoesthesia of the forearm, groin, and bladder, and grade 5 muscle strength in the lower limbs[7].
A 45-year-old woman had persistent headaches. MRI and CT revealed that a large tumor eroded the occipital bone, resulting in complete occlusion of multiple sinuses, including the bilateral transverse sinus, straight sinus, and posterior one-third of the superior sagittal sinus. The tumor activated compensatory reflux pathways and reshaped an extensive venous network within the brain. The tumor was removed palliatively via surgery and the residual part was removed using a gamma knife. The post-operative pathologic evaluation was shown to be a meningioma (WHO grade I)[8].
All of the above cases suggest that meningiomas, whether malignant or benign, can invade the intracranial venous system and can directly infiltrate or penetrate the arterial and venous walls, causing extracranial dissemination via the circulation[5,9,10]. In the current case, it was considered that after the spontaneous rupture of the primary intracranial meningioma, the tumor metastasized to the jugular vein via the superior sagittal sinus, transverse sinus, and sigmoid sinus, gradually blocked the vein, and infiltrated into the surrounding anatomic content. Finally, the tumor invaded the common carotid artery and the brachiocephalic trunk, leading to occlusion of the common carotid artery. The hematogenous metastasis in this case was invasive and destructive, which was also indicated by the post-operative imaging findings of suspicious metastatic tumors in the lungs.
The histologic morphology of malignant meningiomas resembles melanomas, carcinomas, and sarcomas[11]; however, meningiomas can be identified based on different immune phenotypes. The cell proliferation marker, Ki-67, is positively correlated with meningioma classification and the Ki-67 marker index is 11.3% ± 4.08% in malignant meningiomas, ranging from 7%-46%. When the proliferation index is > 20%, the mortality rate is extremely high[12]. Because of the rapid growth, malignant meningiomas can display cystic changes, necrosis, and bleeding. It has been reported that cystic changes are a characteristic of atypical and anaplastic meningiomas[13]. Malignant meningioma cyst walls are mostly uneven in thickness and mural nodules may appear inside. Necrosis is a pathologic phenomenon that occurs when a tumor is rapidly growing and the blood supply in the tumor is insufficient, which is an indicator of malignancy. The larger the meningioma area of necrosis, the more irregular the shape, the greater the possibility of malignancy. The tumor in this case showed significant cystic changes, including a large amount of calcifications, necrosis, hemorrhage, and uneven thickness of the capsule wall with rough nodules. Moreover, the results of immunohistochemistry were not consistent with a carcinoma or melanoma, and Ki-67 was positive in 10%–30% of cells. Each of the above histologic findings were consistent with the characteristics of a malignant meningioma.
There are three types of surgical approaches for tumors that invade the common carotid arterial wall (tumor dissection with the artery intact, carotid artery resection plus ligation, and carotid artery resection plus reconstruction). Regarding the first method, simply stripping the malignant tumor violates the surgical principle of radical tumor resection. Huvos et al[14] performed a histopathologic examination on 64 cases of carotid artery invasion after surgical resection and reported that 37 cases (57.8%) had microscopic invasion of the artery wall. McCready et al[15] performed a histopathologic examination on 16 carotid artery specimens and found that 37.5% had tumor invasion. If the tumor and the invaded artery cannot be completely removed, the patient will most likely die in the short term due to tumor recurrence or arterial rupture. Kennedy et al[16] reported that the local recurrence rate of tumor dissection alone is as high as 46% in patients with malignant tumors invading the common carotid artery, the distant metastasis rate is 67%, and the 5-year survival rate is 7%. Therefore, active surgical treatment with complete tumor resection can yield a greater probability of survival for these patients[17]. Due to extensive local spread and potential distant metastasis, most malignant tumors that invade the carotid artery have exceeded the field of radical surgical resection and may not be able to retain effective safety limits. The surgery for such patients is actually a palliative surgical resection. This palliative procedure can relieve pain symptoms, improve the quality of life, and extend the survival time. Some patients can even achieve long-term survival.
Whether reconstruction of the carotid artery should be performed after resection of an invaded carotid artery is controversial. Studies supporting carotid artery reconstruction have revealed that the internal carotid artery passes 350 mL of blood per minute, and bilateral internal carotid arteries supply 85% of all blood in the brain. Reconstruction of the carotid artery after resection is significant because reconstruction helps maintain brain blood circulation and reduce post-operative complications. Meleca et al[18] reported that in 12 cases of resection and ligation of the common carotid artery, 7 had neurologic complications (5 cases had cerebrovascular accidents and two had transient ischemic attack), while in 8 cases of reconstruction of the common carotid artery, only one case had a cerebrovascular accident 3 mo after surgery. Katsuno et al[19] reviewed a total of 148 cases in 11 studies and found that the mortality associated with invaded carotid artery resection and reconstruction was only 6.8%, and the incidence of neurologic dysfunction was only about 10%, suggesting that head and neck surgeons should actively perform carotid artery resection and reconstruction. Ozer et al[20] reviewed 18 cases of carotid artery resection and reconstruction and reported a stroke rate of only 5.5%. Zheng et al[21] published a comparison between 17 cases of carotid artery resection and ligation and 11 cases of carotid artery resection and reconstruction. The incidence of neurologic complications of ligation was 50%, while only 9.1% for the vascular reconstruction group. Ricco et al[22] reported 42 cases of carotid artery resection and reconstruction. No strokes or death occurred within 30 d after surgery; 19% had transient dysphagia, 14.3% had vocal cord paralysis, 4.8% had delayed wound healing and 2.4% had local flap necrosis. The above studies suggest that although carotid artery resection and ligation provide a possibility for complete tumor resection, carotid artery resection and ligation increases the risk of post-operative mortality and neurologic complications, and carotid artery reconstruction has a lower incidence of complications and is thus worth consideration.
In contrast, a series of studies have shown that resection and reconstruction of a carotid artery invaded by tumor does not improve the occurrence of complications and prolong the survival of patients. Verhaeghe et al[23] analyzed 59 cases of carotid artery resection and ligation and 213 cases of carotid artery resection and reconstruction, and found that the peri-operative mortality of carotid resection and ligation was 0%-30%, and the stroke rate was 0%-25%. Peri-operative mortality in patients undergoing resection plus reconstruction is 0%–33% and the stroke rate is 0%-22%. Aslan et al[24] summarized 28 cases of resection of invaded carotid arteries and compared the neurologic complications of patients undergoing arterial reconstruction and non-reconstruction, and found that there was no difference in neurologic complications between the two groups of patients. Snyderman et al[25] compared the incidence of central nervous system complications in patients with and without carotid artery reconstruction after carotid artery resection, and concluded that there was no statistical difference between the two groups. Németh et al[26] was of the opinion that resection of the tumor and the invaded carotid artery and simultaneous carotid artery reconstruction did not improve the long-term survival of patients. In addition, after reviewing 58 patients, Freeman et al[27] found that among 41 patients who underwent carotid resection and reconstruction, eight had a stroke (20%); however, no stroke occurred in six cases after carotid artery resection and ligation. A list of the complications reported[28-34] for carotid artery ligation or reconstruction is shown in Table 1.
| Type of operation | Number of cases | Complications | Year of publication |
| Carotid artery resection + ligation | 88 | 22 cases of common carotid artery ligation: 41% neurologic complications, 36% mortality; 66 cases of internal carotid artery ligation: 47% neurologic complications, 29% mortality[27] | 1955 |
| 156 | Death or coma, 15.4%; Death, coma, or hemiplegia, 30.1%; Transient cerebral dysfunction, 10.3%[28] | 1981 | |
| 12 | Cerebrovascular accident, 42%; TIA, 17%; Mortality, 8%[17] | 1994 | |
| 7 | Cerebrovascular accident, 29%; Mortality, 29%[29] | 1994 | |
| 18 | Cerebrovascular accident, 33%; Mortality, 17%[30] | 1995 | |
| 20 | Peri-operative mortality, 10%; Neurologic deficits, 30%[23] | 2002 | |
| 59 | Peri-operative mortality, 0-30%; Stroke incidence, 0-25%[22] | 2003 | |
| 17 | Stroke incidence, 20% (graft occlusion, 12.2%)[26] | 2004 | |
| 17 | Neurologic complications, 50%[20] | 2007 | |
| Carotid artery resection + reconstruction | 8 | Cerebrovascular accident, 13%; Mortality, 0%[17] | 1994 |
| 148 | Neurologic complications, 4.7%; Mortality, 6.8%[18] | 2001 | |
| 8 | Peri-operative mortality, 12.5%; Neurologic dysfunction, 25%[23] | 2002 | |
| 213 | Peri-operative mortality, 0%-33%; Stroke incidence, 0-22%; Local infection, 11%-33%; Anastomotic rupture, 0%-33%; Pharyngeal fistula, 0%-33%[22] | 2003 | |
| 41 | Stroke, 17.6%[26] | 2004 | |
| 11 | Neurologic complications, 9.1%[20] | 2007 | |
| 18 | Carotid artery rupture, 5.5%; Stroke, 5.5%[19] | 2008 | |
| 13 | Graft rupture, 7.7%; Stroke, 7.7%[31] | 2008 | |
| 10 | Mild hemiplegia, 10%; Pharyngeal fistula, 10%[32] | 2011 | |
| 19 | Stroke, 5.3%; Pharyngeal fistula, 5.3%; Local infection, 10.5%; Local flap necrosis, 10.5%; Local hematoma, 10.5%[16] | 2014 | |
| 31 | Transient dysphagia, 19.4%; Vocal cord paralysis 9.7%; Local wound dehiscence 6.5%; Local flap necrosis 3.2%; Stroke and thrombosis, 0%[33] | 2016 | |
| 42 | Transient dysphagia 19%; Vocal cord paralysis, 14.3%; Wound healing delayed, 4.8%; Local flap necrosis, 2.4%; Stroke, 0%[21] | 2017 |
These studies suggest that the occurrence of post-operative neurologic complications may not depend on whether the carotid artery is reconstructed, rather there are more important factors, such as the collateral circulation compensation of the circle of Willis in the brain. If the arterial reconstruction is performed indiscriminately, reconstruction will increase the financial burden on patients, waste medical resources, and the risks of post-operative cerebral ischemia, thrombosis, and carotid anastomotic rupture are not reduced. Therefore, under the premise that the technology, materials, and equipment meet the requirements, the patient is a candidate for surgery, the financial status and psychological preparation of the family are adequate, the pre-operative BOT is negative, and the carotid return pressure (carotid artery residual end pressure) exceeds 50–70 mmHg, which reflects good compensation of cerebral circulation after carotid artery occlusion, then carotid artery resection and ligation can be selected. In the current case involving a recurrent metastatic malignant meningioma, the pre-operative examination confirmed that the right common carotid artery was completely occluded and the BOT was negative. Additionally, intra-operatively it was shown that the tumor completely enveloped the common carotid artery and caused an arterial embolism, the arterial wall was violated and the structure was incomplete. The carotid return pressure was monitored and exceeded 70 cmH2O (approximately 52.6 mmHg). To remove the tumor as completely as possible, better local control was obtained without increasing the risk of post-operative neurologic deficits, and the quality of life was improved, and the right common carotid artery was removed. Considering that the posterior brain circulation was well-compensated, the pathology showed extracranial metastasis of a malignant meningioma with invasion of the subclavian artery, which is difficult to repair, and post-operative radiotherapy may be required, thus a carotid artery reconstruction was not selected.
Although the subclavian artery has more collateral circulation around the shoulder, ligation of the subclavian artery generally does not cause limb necrosis, but often results in symptoms of limb ischemia, so the subclavian artery should be repaired as much as possible. The principles and methods are the same as other arterial injury repairs, and can be directly sutured. For minor arterial defects, autologous vein or deep fascia can be repaired in sheets and wrapped around the arterial opening for sealing and suturing. Obtaining materials is easy for this method and there is no bleeding after suturing. The method is especially suitable for repair of the first segment of the subclavian artery. If the arterial defect is large (> 2 cm in diameter) or most of the defect has detached, the arterial defect should be cut off and pruned, and an end-to-end anastomosis should be performed without tension. If there is tension, an autologous great saphenous vein or artificial vessel transplantation should be applied. A balloon can be used to occlude the artery pre-operatively when necessary[35,36]. In this case, the defect involving the right subclavian artery was approximately 1 cm after tumor resection, thus a 1 × 1 cm jugular vein wall was directly sutured to repair the defect. No thrombosis, infection, or limb ischemia occurred post-operatively and the repair was satisfactory.
Equally important is post-operative management, the key to which lies in the prevention and treatment of complications. Within 48 h after resection of the carotid artery, the patient may present with mild hemiplegia and aphasia. Cerebral thrombosis may occur within 48 h to several weeks after surgery. Local infection, a pharyngeal fistula, and a carotid artery stump ligation rupture can occur approximately 1 wk post-operatively. Therefore, in view of the possible complications, adequate pre-operative evaluation of the cerebral circulation compensation is required. In this case, controlled hypertension and systemic heparinization were applied, and a myocutaneous flap was obtained to cover the operative area. Post-operatively, low-molecular-weight heparin and low-molecular dextran were administered systemically to prevent thrombosis, and mannitol, furosemide, and albumin were used to prevent cerebral edema. Papaverine and alprostadil improve the local circulation. Strengthening anti-infection, strong systemic nutritional support, and careful observation of the nervous system is necessary, which includes consciousness, the pupils, phonation, muscle tension, and pathologic reflexes.
Here we report a rare case of a malignant meningioma with hematogenous metastasis to the cervical-thoracic junction and invaded the jugular vein and common carotid artery causing complete occlusion of the two vessels. The diagnosis needs to be combined with the medical history, imaging, surgical observation, and post-operative pathologic evaluation. The key to successful surgical resection lies in mastering the vessels and nerves in this area and grasping the indications for vascular reconstruction and proper management of the peri-operative complications. Even though neck metastatic meningiomas are rare and reported in individual cases worldwide, there are still rules to follow. As the literature accumulates, our knowledge of cervical metastatic meningiomas will continue to grow.
Manuscript source: Unsolicited manuscript
Specialty type: Otorhinolaryngology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mehdipour P S-Editor: Zhang H L-Editor: Webster JR P-Editor: Zhang YL
| 1. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10993] [Cited by in RCA: 10846] [Article Influence: 1205.1] [Reference Citation Analysis (0)] |
| 2. | Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, Sun MZ, Barani IJ, James CD, Parsa AT. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol. 2014;16:628-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Dziuk TW, Woo S, Butler EB, Thornby J, Grossman R, Dennis WS, Lu H, Carpenter LS, Chiu JK. Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 1998;37:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 197] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Chamberlain MC, Glantz MJ. Cerebrospinal fluid-disseminated meningioma. Cancer. 2005;103:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Yamashiro K, Hasegawa M, Higashiguchi S, Kato H, Hirose Y. Intravenous sinus meningioma with intraluminal extension to the internal jugular vein: case report and review of the literature. Br J Neurosurg. 2020;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Vachhrajani S, Jea A, Rutka JA, Blaser S, Cusimano M, Rutka JT. Meningioma with dural venous sinus invasion and jugular vein extension. J Neurosurg Pediatr. 2008;2:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Nishida N, Kanchiku T, Imajo Y, Suzuki H, Yoshida Y, Kato Y, Hoshii Y, Taguchi T. A case of an anaplastic meningioma metastasizing to the mediastinal lymph nodes. J Spinal Cord Med. 2016;39:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Debernardi A, Quilici L, La Camera A, Boccardi E, Cenzato M. Torcular Meningioma with Multi-Venous Sinus Invasion: Compensatory Drainage Veins and Surgical Strategy. World Neurosurg. 2018;109:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Dincer A, Chow W, Shah R, Graham RS. Infiltration of Benign Meningioma into Sagittal Sinus and Subsequent Metastasis to Lung: Case Report and Literature Review. World Neurosurg. 2020;136:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Guclu B, Sindou M. Reconstruction of vein of Labbé in temporo-occipital meningioma invading transverse sinus: technical report. Acta Neurochir (Wien). 2010;152:941-945; discussion 945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Cimino PJ. Malignant progression to anaplastic meningioma: Neuropathology, molecular pathology, and experimental models. Exp Mol Pathol. 2015;99:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Cornelius JF, Slotty PJ, Steiger HJ, Hänggi D, Polivka M, George B. Malignant potential of skull base versus non-skull base meningiomas: clinical series of 1,663 cases. Acta Neurochir (Wien). 2013;155:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Hsu CC, Pai CY, Kao HW, Hsueh CJ, Hsu WL, Lo CP. Do aggressive imaging features correlate with advanced histopathological grade in meningiomas? J Clin Neurosci. 2010;17:584-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Huvos AG, Leaming RH, Moore OS. Clinicopathologic study of the resected carotid artery. Analysis of sixty-four cases. Am J Surg. 1973;126:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | McCready RA, Miller SK, Hamaker RC, Singer MI, Herod GT. What is the role of carotid arterial resection in the management of advanced cervical cancer? J Vasc Surg. 1989;10:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Kennedy JT, Krause CJ, Loevy S. The importance of tumor attachment to the carotid artery. Arch Otolaryngol. 1977;103:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Nishinari K, Krutman M, Valentim LA, Chulam TC, Yazbek G, Kowalski LP, Wolosker N. Late surgical outcomes of carotid resection and saphenous vein graft revascularization in patients with advanced head and neck squamous cell carcinoma. Ann Vasc Surg. 2014;28:1878-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Meleca RJ, Marks SC. Carotid artery resection for cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1994;120:974-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Katsuno S, Takemae T, Ishiyama T, Usami SI. Is carotid reconstruction for advanced cancer in the neck a safe procedure? Otolaryngol Head Neck Surg. 2001;124:222-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Ozer E, Agrawal A, Ozer HG, Schuller DE. The impact of surgery in the management of the head and neck carcinoma involving the carotid artery. Laryngoscope. 2008;118:1771-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Zheng JW, Zhong LP, Zhang ZY, Zhang CP, Zhu HG, Sun J, Fan XD, Hu YJ, Ye WM, Li J, Suen J. Carotid artery resection and reconstruction: clinical experience of 28 consecutive cases. Int J Oral Maxillofac Surg. 2007;36:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Ricco JB, Illuminati G, Belmonte R. [Resection of recurrent neck cancers with replacement of the carotid artery]. J Med Vasc. 2017;42:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Verhaeghe JL, Montagne S, Belotzerkovski I, Bracard S, Henneton C, Lapeyre M, Meistelman C, Dolivet G. [Is carotid resection a valuable option in advanced head and neck squamous cell carcinomas]. Bull Cancer. 2003;90:607-613. [PubMed] |
| 24. | Aslan I, Hafiz G, Baserer N, Yazicioglu E, Kiyak E, Tinaz M, Biliciler N. Management of carotid artery invasion in advanced malignancies of head and neck comparison of techniques. Ann Otol Rhinol Laryngol. 2002;111:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Snyderman CH, D'Amico F. Outcome of carotid artery resection for neoplastic disease: a meta-analysis. Am J Otolaryngol. 1992;13:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Németh Z, Dömötör G, Tálos M, Barabás J, Ujpál M, Szabó G. Resection and replacement of the carotid artery in metastatic head and neck cancer: literature review and case report. Int J Oral Maxillofac Surg. 2003;32:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Freeman SB, Hamaker RC, Borrowdale RB, Huntley TC. Management of neck metastasis with carotid artery involvement. Laryngoscope. 2004;114:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Illuminati G, Schneider F, Minni A, Calio FG, Pizzardi G, Ricco JB. Resection of recurrent neck cancer with carotid artery replacement. J Vasc Surg. 2016;63:1272-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | He XB, Li JJ, Chen YH, Shu C, Tang QL, Yang XM. Treatment of recurrent head and neck carcinoma involving the carotid artery: carotid reconstruction with ePTFE graft. Eur Arch Otorhinolaryngol. 2011;268:1817-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Miao B, Lu Y, Pan X, Liu D. Carotid artery resection and reconstruction with expanded polytetrafluoroethylene for head and neck cancer. Laryngoscope. 2008;118:2135-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Nayak UK, Donald PJ, Stevens D. Internal carotid artery resection for invasion of malignant tumors. Arch Otolaryngol Head Neck Surg. 1995;121:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Brennan JA, Jafek BW. Elective carotid artery resection for advanced squamous cell carcinoma of the neck. Laryngoscope. 1994;104:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Konno A, Togawa K, Iizuka K. Analysis of factors affecting complications of carotid ligation. Ann Otol Rhinol Laryngol. 1981;90:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | MOORE O, BAKER HW. Carotid-artery ligation in surgery of the head and neck. Cancer. 1955;8:712-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Aksoy M, Tunca F, Yanar H, Guloglu R, Ertekin C, Kurtoglu M. Traumatic injuries to the subclavian and axillary arteries: a 13-year review. Surg Today. 2005;35:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Hyre CE, Cikrit DF, Lalka SG, Sawchuk AP, Dalsing MC. Aggressive management of vascular injuries of the thoracic outlet. J Vasc Surg. 1998;27:880-884; discussion 884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |