Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.6086
Peer-review started: June 1, 2020
First decision: September 13, 2020
Revised: September 28, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 6, 2020
Processing time: 185 Days and 20 Hours
Abscess formation is one of the complications after radical resection of rectal cancer; cases with delayed postoperative anastomotic abscess are rare. Here, we report a rare case of postoperative anastomotic abscess with a submucosal neoplasm appearing after rectal surgery. Ultimately, the patient was diagnosed and treated by endoscopic fenestration. In addition, we review the literature on the appearance of an abscess as a complication after rectal cancer surgery.
A 57-year-old man with a history of rectal malignancy resection complained of a smooth protuberance near the anastomotic stoma. Endoscopic ultrasonography revealed a hypoechoic structure originating from the muscularis propria, and a submucosal tumor was suspected. The patient was subsequently referred to our hospital and underwent pelvic contrast-enhanced computed tomography, which revealed no thickening or strengthening of the anastomotic wall. In order to clarify the origin of the lesion and obtain the pathology, endoscopic fenestration was performed. After endoscopic procedure, a definitive diagnosis of delayed anastomotic submucosal abscess was established. The patient achieved good recovery and prognosis after the complete clearance of abscess.
Endoscopic fenestration may be safe and effective for the diagnosis/treatment of delayed intestinal smooth protuberance after rectal cancer surgery.
Core Tip: Delayed postoperative abscess is a rare complication after radical resection of rectal cancer, especially those presenting several years after surgery. Here, we report a rare case of postoperative anastomotic abscess with a submucosal neoplasm appearing who was treated by endoscopic fenestration. In addition, we review the literature on abscess after rectal cancer surgery. Although extremely rare, delayed submucosal abscess should be considered in the differential diagnosis in cases with suspected submucosal tumors in patients after rectal cancer resection with intestinal smooth swelling. Meanwhile, endoscopic fenestration may be safe and effective for the diagnosis/treatment of delayed intestinal postoperative smooth protuberance.
- Citation: Zhang BZ, Wang YD, Liao Y, Zhang JJ, Wu YF, Sun XL, Sun SY, Guo JT. Endoscopic fenestration in the diagnosis and treatment of delayed anastomotic submucosal abscess: A case report and review of literature. World J Clin Cases 2020; 8(23): 6086-6094
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/6086.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.6086
Currently, laparoscopic radical resection is the standard and mainstay surgical treatment for rectal cancer[1-3]. Abscess formation is one of the complications after rectal cancer resection and is usually found within a few weeks post-surgery[4-6]. However, delayed postoperative anastomotic abscess is extremely rare, especially that presenting several years after surgery. Here, we report a rare case of delayed anastomotic submucosal abscess in a patient after rectal surgery who was diagnosed and treated by endoscopic fenestration. Furthermore, we performed a literature review on abscess complication following rectal cancer surgery.
A 57-year-old male patient was referred to our hospital for definite diagnosis and treatment of an intestinal smooth protuberance that appeared more than 3 years after rectal cancer surgery.
Initially, a smooth protuberance was found following a colonoscopy examination during a regular medical examination at a local hospital 3.5 years after rectal cancer surgery. The patient did not complain of any symptoms.
The patient presented with irregular stool and bloody stool since September 2015. He was admitted to a local hospital complaining of lower abdominal pain in February 2016. Colonoscopy revealed a large protuberant lesion located 15-18 cm from the anus, and a biopsy was taken. Endoscopic diagnosis was advanced rectal cancer, and pathological diagnosis was rectal adenocarcinoma (moderately differentiated). Laparoscopic radical resection of rectal cancer was performed in March 2016. Intra- and post-operative pathology confirmed rectal adenocarcinoma (moderately differentiated). Colonoscopy performed during postoperative follow-up at 6 mo, 1 year, 2 years, and 3 years after the operation showed good anastomotic healing (Figure 1A).
The patient had no specific personal and family history.
The patient did not have positive signs on physical examination.
Laboratory testing including C-reactive protein level and leukocyte count, showed no abnormalities.
In November 2019 (3.5 years after operation), a smooth protuberance measuring approximately 15 mm × 15 mm (Figure 1B) was found near the anastomotic site by colonoscopy at the local hospital. The diagnosis was a protuberant lesion near the anastomotic stoma. Subsequent endoscopic ultrasonography (EUS) at the local hospital revealed a hypoechoic structure (Figure 1C) of approximately 1.12 cm × 0.91 cm in the rectal wall. A submucosal tumor (SMT) originating from the muscularis propria was suspected. The patient was referred to our hospital for definitive diagnosis and further treatment. We analyzed the patient's past history and examination and suspected that the protuberance was local recurrence of rectal cancer, postoperative abscess, or SMT. Subsequently, pelvic contrast-enhanced computed tomography (CT) was performed, which revealed no thickening or strengthening of the anastomotic wall (Figure 1D).
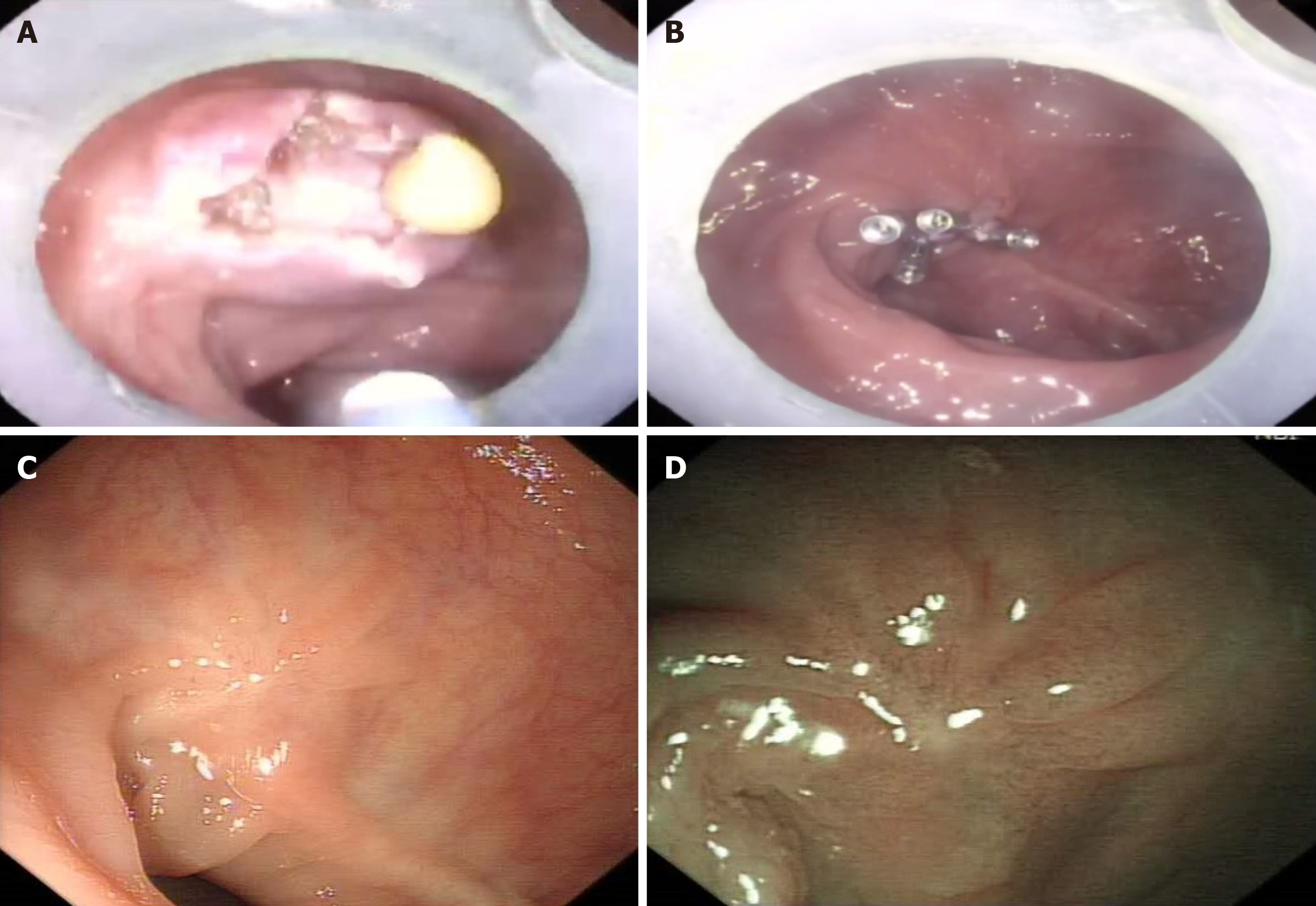
To clarify the origin of the lesion and obtain the pathology, endoscopic resection was performed. After dissecting the mucosal layer by using a dual knife, a soft cystic structure was observed. After opening the sac wall, a yellow viscous liquid can be seen flowing out (Figure 2A). Generally speaking, pus needs bacterial culture for further diagnosis; however, the lesion was at the rectum and highly susceptible to contamination by intestinal faeces and flora. Therefore, we did not culture the pus. We subsequently repeated the suction and irrigation procedures during endoscopic procedure to clean the area of the purulent exudate. The wound was closed using five metal clips finally (Figure 2B).
Based on the clinical, imaging, and endoscopic findings, we finally made a definitive diagnosis of delayed anastomotic submucosal abscess following rectal surgery.
Diagnostic endoscopic fenestration that the patient underwent was also performed as a treatment for the intestinal protrusion lesion. In the process, the purulent exudate was cleaned completely, and the wound was entirely closed using clips.
After the endoscopic fenestration, the patient had no adverse effects and was treated with cephalosporin and glucose for anti-infection and nutrition therapy, respectively. The patient was discharged without complications 2 d after the procedure. Postoperative follow-up examination was performed, showing no evidence of recurrence in both white light endoscopy and narrow-band imaging (Figure 2C and D).
We report a case of delayed anastomotic submucosal abscess in a patient after rectal cancer surgery with a review of the literature. To the best of our knowledge, only 15 cases of abscess formation following rectal cancer surgery have been reported in the literature, including our case (Table 1), and this is the first report of delayed intestinal anastomotic abscess diagnosed and treated by endoscopic fenestration.
| Ref. | Year | Study design | No. of patient | Age, yr | Operation method | Time from surgery to abscess | Chemoradiotherapy before or after surgery | Fistula or leakage formation | Abscess position | Reoperation | Success | Follow-up | Result |
| Aras et al[12] | 2016 | Case report | 1 | 34 | TME; coloanal anastomosis; diverting ileostomy | Postoperation | Before | Leakage | Pelvic | Drainage; intraluminal vacuum associated closure | Yes | 45 d | Development of granulation tissue at the pelvic sinus |
| Honma et al[13] | 2007 | Case report | 1 | 68 | LAR | 10 d | Before | Leakage | Pelvic | Colostomy | Yes | ND | ND |
| Martins et al[14] | 2012 | Case report | 1 | 37 | Hartmann procedure | 14 d | Before | ND | Pelvic | Transrectal endoscopic drainage facilitated by TEM access | Yes | 60 d | Reduction in the pelvic fluid |
| Kollmorgen et al[15] | 1994 | Case report | 1 | 32 | LAR; abdominal perineal resection for recurrent rectal cancer | 8 d | After | Fistula | Pelvic | Drainage | Yes | 90 d | A smaller pelvic abscess cavity recurrence and resolved by ciprofloxacin and proscar |
| Brehant et al[16] | 2009 | Case report | 1 | 62 | Restorative proctectomy with TME, circular stapled low colorectal side-toend anastomosis, and loop ileostomy | Postoperation; 90 d; 225 d | ND | Leakage | Pelvic | Drainage | Yes | 300 d | No abscess recurrence |
| Rahimi et al[17] | 2018 | Case report | 1 | 61 | LAR with a diverting loop ileostomy | 14 d | Before; after | Fistula; leakage | Presacral | Drainage | Yes | ND | ND |
| Scabini et al[18] | 2009 | Case report | 2 | ND | AR; transanal anastomosis; temporary colostomy | 30 d; 60 d | Before | Leakage | Presacral | No; drainage | Yes | ND | ND |
| D'Hondt et al[19] | 2009 | Case report | 1 | 76 | AR; hartmann procedure; completion proctectomy | 6 yr | After | ND | Presacral | ENDO-sponge treatment | Yes | 150 d | No abscess recurrence |
| Mandai et al[20] | 2015 | Case report | 1 | 60 | LAR | 17 and 64 d | ND | ND | Around the anastomotic intestine; in the subdiaphragmatic area | EUS-guided transgastric drainage; naso-cystic drainage | Yes | 3 yr | No abscess recurrence |
| Sadatomo et al[21] | 2013 | Case report | 1 | 64 | ND | 28 chemotherapy courses | After | Leakage | Intra-abdominal | Drainage | Yes | 19 d | No abscess recurrence |
| Kimura et al[22] | 2012 | Case report | 1 | 50 | ISR | Postoperation | Before; after | ND | Dissection area | Drainage | Yes | ND | ND |
| Ikeda et al[23] | 2009 | Case report | 1 | 60 | LAR | 6 d | ND | Leakage | In the left inguinal hernial sac | Hernioplasty and resection of the inflamed sac | Yes | ND | ND |
| Goldman et al[24] | 1989 | Case report | 1 | 76 | LAR | 30 d | After | Fistula; leakage | Anastomotic; right seminal vesicle | Cutaneous; vasostomy | Yes | 2 yr | No abscess recurrence until death due to stroke associated with cerebral metastases |
| Present case | 2020 | Case report | 1 | 57 | ND | 3 and a half years | No | No | Anastomotic | Endoscopic; fenestration | Yes | 90 d | No abscess recurrence and well anastomotic healing |
Currently, laparoscopic radical resection of rectal cancer has the advantages of less trauma, less bleeding, and rapid recovery of the intestinal function, and remains the most significant treatment for rectal cancer[2,3]. However, in any invasive procedures, complications cannot be completely avoided. These complications have an important impact on the postoperative recovery of patients. Postoperative complications of rectal cancer resection include postoperative hemorrhage, infection-related complications, and anastomosis-related complications (anastomotic fistula and stricture)[4-6]. Postoperative abscess is one of the severe complications after rectal cancer surgery. Patients with postoperative abscess are usually symptomatic. Indicators of infection in laboratory tests will also increase accordingly, including C-reactive protein level and white blood cell count[7-9]. Postoperative abscess is related to the following factors: (1) After anti-infective therapy, viable bacteria are stored in the deep rectal wall, stimulating the rectal wall to form an abscess for a long time; (2) Local hematomas resulting from intra-incisional bleeding are not fully absorbed and develop into abscesses in the event of infection; and (3) Anastomotic fistulas are caused by poor blood supply and excessive tension, which lead to infection and abscess formation around the anastomotic site[10,11].
In the 15 cases reviewed herein, the patient ages ranged from 32-76 years, with an average age of 56.7 years[12-24]. The retrieved literature indicated that when patients were diagnosed with postoperative abscess after rectal cancer surgery, they usually presented with abdominal pain, obvious mass, and fever, accompanied by fistula or leakage formation (66.67%). Among the 15 patients (including this case), nine achieved the ideal treatment effect by drainage (60%), and the remaining patients underwent colostomy and cutaneous vasostomy for excretion of the purulent secretions to relieve symptoms in a timely manner. Five (33.33%) of the 15 patients had pelvic abscess after rectal cancer surgery; in four (80%) of these patients, drainage was performed, whereas one (20%) was treated by colostomy. All five patients underwent successful abscess treatment (100%); four of these patients were followed (> 45 d), and the results showed improvement in three patients. In the fourth patient, Kollmorgen et al[15] reported an abscess recurrence after drainage of a small pelvic abscess, which improved after anti-infective treatment with ciprofloxacin. This suggests that conservative anti-infective treatment is a feasible option for limited abscess without increased risk of spreading. Presacral abscess was found in four (26.67%) cases, and three (75%) of these cases were treated by drainage. D'Hondt et al[19] reported intermittent fever, massive mucopurulent discharge from a perineal wound, and severe pain during radiotherapy after rectal cancer resection. After admission, CT showed a presacral abscess. Endo-sponge therapy was performed on the presacral abscess after biopsy confirmed no recurrent tumor. The prognosis was good after 5 mo of follow-up. A case reported by Mandai et al[20] showed abscess formation in the para-anastomotic and subphrenic areas after low anterior resection of rectal cancer. The patient was treated by EUS-guided transgastric drainage and naso-cystic drainage innovatively and had no abscess recurrence at the 3-year follow-up. EUS-guided drainage is suggested as a safe and effective method for the treatment of postoperative abdominal abscess.
Patients with postoperative abscess in the abdominal and pelvic cavity are usually symptomatic, whereas those with SMT are asymptomatic[25-27]. In the present case, the patient had no discomfort until colonoscopic examination detected the abnormality near the anastomotic site. Combined with the history of past illness, the patient was easily misdiagnosed with rectal cancer recurrence or rectal submucosal lesions. At this point, differential diagnosis was difficult, especially with SMT. The clinical features and EUS imaging were strongly suggestive of SMT after local tumor recurrence was disregarded based on pelvic CT findings. Endoscopic fenestration, however, ultimately indicated a rare delayed submucosal abscess rather than SMT.
To date, there is little information on delayed postoperative anastomotic abscess, especially that appearing several years after an operation. The later an abscess develops, the more complicated the causal relationship between the abscess and previous surgery is, making differential diagnosis more difficult. Patients with postoperative abscess usually present with fervescence, abdominal pain, and abdominal mass. Inflammatory indicator levels on blood tests usually increase and imaging examination may also suggest inflammatory exudation. However, these conditions may not occur when the abscess is wrapped around the cyst wall and does not spread. Therefore, abscess can mistakenly and easily be ruled out as a diagnosis, thereby delaying the patient's treatment. Currently, there are no guidelines for the treatment of postoperative abscess of rectal cancer. In general and based on the reviewed literature, large abscesses with complex anatomical locations are more commonly treated by drainage. If an abscess is associated with peritonitis, emergency surgical treatment can be performed. However, endoscopic fenestration is a better minimally invasive procedure for enveloping an abscess in the intestinal tract.
Endoscopic fenestration is an intuitive, safe, and reliable diagnostic method when clinical features and imaging findings are uncertain. Endoscopic fenestration has been widely used for intracranial cysts[28,29]; in recent years, it has also emerged as an effective method for the diagnosis and treatment of gastrointestinal protuberance[30,31]. Endoscopic fenestration can safely and effectively diagnose abscess, reduce severe complications such as peritonitis, and reduce the use of invasive procedures such as abdominal drainage.
Although extremely rare, delayed submucosal abscess should be considered in the differential diagnosis in cases of suspected SMTs based on imaging during the late postoperative period in rectal cancer patients presenting with intestinal smooth swelling. When the protuberance is wrapped around the cyst wall without definite evidence of tumor recurrence or metastasis, endoscopic fenestration can be considered as a safe, effective, and feasible strategy for the definitive diagnosis and treatment of delayed intestinal smooth protuberance in patients after rectal surgery.
We thank Professor Sun SY, the co-corresponding author of this article, for his support and guidance to this study, as well as all other doctors who participated in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chawla S, Fogli L, Rawat K S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55765] [Article Influence: 7966.4] [Reference Citation Analysis (132)] |
| 2. | Lirici MM, Hüscher CG. Techniques and technology evolution of rectal cancer surgery: a history of more than a hundred years. Minim Invasive Ther Allied Technol. 2016;25:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 3. | Małczak P, Mizera M, Torbicz G, Witowski J, Major P, Pisarska M, Wysocki M, Strzałka M, Budzyński A, Pędziwiatr M. Is the laparoscopic approach for rectal cancer superior to open surgery? Wideochir Inne Tech Maloinwazyjne. 2018;13:129-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010;251:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 5. | Shearer R, Gale M, Aly OE, Aly EH. Have early postoperative complications from laparoscopic rectal cancer surgery improved over the past 20 years? Colorectal Dis. 2013;15:1211-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Lyall A, Mc Adam TK, Townend J, Loudon MA. Factors affecting anastomotic complications following anterior resection in rectal cancer. Colorectal Dis. 2007;9:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Medina-Fernández FJ, Garcilazo-Arismendi DJ, García-Martín R, Rodríguez-Ortiz L, Gómez-Barbadillo J, Gallardo-Valverde JM, Martínez-Dueñas JL, Navarro-Rodríguez E, Torres-Tordera E, Díaz-López CA, Briceño J. Validation in colorectal procedures of a useful novel approach for the use of C-reactive protein in postoperative infectious complications. Colorectal Dis. 2016;18:O111-O118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Pedersen T, Roikjær O, Jess P. Increased levels of C-reactive protein and leukocyte count are poor predictors of anastomotic leakage following laparoscopic colorectal resection. Dan Med J. 2012;59:A4552. [PubMed] |
| 9. | Ortega-Deballon P, Radais F, Facy O, d'Athis P, Masson D, Charles PE, Cheynel N, Favre JP, Rat P. C-reactive protein is an early predictor of septic complications after elective colorectal surgery. World J Surg. 2010;34:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Chambers WM, Mortensen NJ. Postoperative leakage and abscess formation after colorectal surgery. Best Pract Res Clin Gastroenterol. 2004;18:865-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Caulfield H, Hyman NH. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg. 2013;148:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Aras A, Celik S, Kiziltan R, Yilmaz Ö, Kotan Ç. Successful Treatment of a Large Pelvic Abscess Using Intraluminal VAC: A Case Report. J Clin Diagn Res. 2016;10:PD19-PD20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Honma K, Tango Y, Honma K, Isomoto H. Perioperative management of severe interstitial pneumonia for rectal surgery: a case report. Kurume Med J. 2007;54:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Martins BC, Marques CF, Nahas CS, Hondo FY, Pollara W, Nahas SC, Ribeiro Junior U, Cecconello I, Maluf-Filho F. A novel approach for the treatment of pelvic abscess: transrectal endoscopic drainage facilitated by transanal endoscopic microsurgery access. Surg Endosc. 2012;26:2667-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Kollmorgen TA, Kollmorgen CF, Lieber MM, Wolff BG. Seminal vesicle fistula following abdominoperineal resection for recurrent adenocarcinoma of the rectum. Report of a case. Dis Colon Rectum. 1994;37:1325-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Brehant O, Hanes A, Fuks D, Sabbagh C, Blanpain S, Brazier F, Regimbeau JM. Stapled marsupialisation of chronic low rectal anastomotic sinuses. Int J Colorectal Dis. 2009;24:1233-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Rahimi H, Venbrux AC, Obias V. Successful embolization of a enterocutaneous fistula tract with Onyx 34 following low anterior resection for rectal cancer. Radiol Case Rep. 2018;13:728-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Scabini S, Pertile D, Boaretto R, Rimini E, Romairone E, Scordamaglia R, Ferrando V. [Leakage of colorectal anastomosis after neoadjuvant therapy with bevacizumab. Case report]. G Chir. 2009;30:413-416. [PubMed] |
| 19. | D'Hondt M, De Hondt G, Malisse P, Vanden Boer J, Knol J. Chronic pelvic abscedation after completion proctectomy in an irradiated pelvis: another indication for ENDO-sponge treatment? Tech Coloproctol. 2009;13:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Mandai K, Uno K, Yasuda K. Endoscopic ultrasound-guided drainage of postoperative intra-abdominal abscesses. World J Gastroenterol. 2015;21:3402-3408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Sadatomo A, Koinuma K, Miki A, Horie H, Yasuda Y. [A case of metachronous gastrointestinal perforation of a patient with metastatic rectal cancer during treatment with bevacizumab-based chemotherapy]. Gan To Kagaku Ryoho. 2013;40:943-945. [PubMed] |
| 22. | Kimura A, Nishikawa S, Yachi T, Ito Y, Kudo Y, Kubo N, Tokura T, Umehara Y, Kurushima M, Takahashi K, Morita T. [A case of advanced rectal cancer treated effectively with intersphincteric resection and preoperative chemotherapy]. Gan To Kagaku Ryoho. 2012;39:2201-2203. [PubMed] |
| 23. | Ikeda S, Takeda H, Yoshimitsu M, Hinoi T, Yoshida M, Sumitani D, Takakura Y, Kawaguchi Y, Shimomura M, Tokunaga M, Kawahori K, Ohdan H, Okajima M. Abscess in the inguinal hernial sac after peritonitis surgery: a case report. World J Gastroenterol. 2009;15:1007-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Goldman HS, Sapkin SL, Foote RF, Taylor JB. Seminal vesicle-rectal fistula. Report of a case. Dis Colon Rectum. 1989;32:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Kovacevic B, Kalaitzakis E, Klausen P, Brink L, Hassan H, Karstensen JG, Vilmann P. EUS-guided through-the-needle microbiopsy of pancreatic cysts: Technical aspects (with video). Endosc Ultrasound. 2020;9:220-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Oh D, Ligresti D, Seo DW. Novel swine biliary dilatation model with temperature-controlled endobiliary radiofrequency ablation: An effective tool for training in EUS-guided biliary drainage. Endosc Ultrasound. 2020;9:245-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Rana SS, Sharma R, Gupta R. EUS-guided transmural pancreatic duct interventions for relief of pain in patients with chronic pancreatitis and failed ERCP. Endosc Ultrasound. 2020;9:274-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Guerson A, Ho S. The use of EUS-microforceps biopsies to evaluate patients with pancreatic cystic lesions. Endosc Ultrasound. 2020;9:209-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 30. | Pesenti C, Bories E, Caillol F, Ratone JP, Godat S, Monges G, Poizat F, Raoul JL, Ries P, Giovannini M. Characterization of subepithelial lesions of the stomach and esophagus by contrast-enhanced EUS: A retrospective study. Endosc Ultrasound. 2019;8:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Antonini F, Delconte G, Fuccio L, De Nucci G, Fabbri C, Armellini E, Frazzoni L, Fornelli A, Magarotto A, Mandelli E, Occhipinti P, Masci E, Manes G, Macarri G. EUS-guided tissue sampling with a 20-gauge core biopsy needle for the characterization of gastrointestinal subepithelial lesions: A multicenter study. Endosc Ultrasound. 2019;8:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |