Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.6048
Peer-review started: May 28, 2020
First decision: September 13, 2020
Revised: September 26, 2020
Accepted: October 19, 2020
Article in press: October 19, 2020
Published online: December 6, 2020
Processing time: 189 Days and 19.2 Hours
Hyporesponsiveness to erythropoiesis-stimulating agents (ESAs) is a prevalent problem in patients with chronic kidney disease. It is associated with increased morbidity and mortality in patients who undergo dialysis. A significant proportion of patients do not respond to iron supplementation and conventional ESAs. We report a case of severe ESA hyporesponsiveness-related anemia that was successfully treated with oral roxadustat.
A 59-year-old Chinese woman had high blood glucose for 25 years, maintenance hemodialysis for 7 years, and recurrent dizziness and fatigue for more than 2 years. Laboratory tests showed severe anemia (hemoglobin level of 54 g/L), though bone marrow biopsy, fluorescence in situ hybridization, and hemolysis tests were within normal ranges. We initially administered first-line therapies and other adjuvant treatments, such as blood transfusions, ESAs, and adequate dialysis, but the patient did not respond as anticipated. Her erythropoietin-resistant anemia was probably not only due to chronic renal insufficiency. The patient received the hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (100 mg, three times weekly). After 12 wk of treatment, the patient’s hemoglobin increased significantly, and her symptoms were alleviated. During the follow-up period, adverse drug reactions were controllable and tolerable.
Oral roxadustat is effective and tolerable for the treatment of ESA hypores-ponsiveness-related anemia in patients undergoing hemodialysis.
Core Tip: We present a case of refractory erythropoiesis-stimulating agent hyporesponsiveness-related anemia in which oral roxadustat increased and maintained hemoglobin. Roxadustat may be a useful optional treatment for anemia in patients with chronic kidney disease. Future clinical trials are needed to assess potential problems with hypoxia-inducible factor prolyl hydroxylase inhibitors.
- Citation: Yu WH, Li XJ, Yuan F. Roxadustat for treatment of erythropoietin-hyporesponsive anemia in a hemodialysis patient: A case report. World J Clin Cases 2020; 8(23): 6048-6055
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/6048.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.6048
Hyporesponsiveness to erythropoiesis-stimulating agents (ESAs) is a common condition in maintenance hemodialysis patients with chronic kidney disease, and it is associated with increased hospitalizations and mortality[1] and frequent blood transfusions. The most common causes are iron deficiency, inflammation, inadequate dialysis, and hyperparathyroidism[2]. ESAs and injection of veinc iron treatments do not work well in some cases. Hepcidin is a key regulator of iron metabolism[3]. The cytokine interleukin 6 plays an important role in inducing hepcidin synthesis during inflammation[4], which leads to hypoferremia, ineffective erythropoiesis, and anemia. Causes should be treated appropriately, and roxadustat may promote the production of erythropoietin (EPO) and upregulate iron utilization to ameliorate renal anemia. We report a case of refractory ESA hyporesponsiveness-related anemia in which first-line therapies were ineffective but oral roxadustat effectively relieved symptoms.
A 59-year-old woman was admitted because of high blood glucose for 25 years, maintenance hemodialysis for 7 years, and recurrent dizziness and fatigue for more than 2 years.
Approximately 2 years ago, the patient suffered from recurrent dizziness, chest discomfort, and weakness of both lower extremities, but had no hematemesis, black stool, bone pain, or gingival bleeding. She was in poor spirits, had a poor appetite, and was sleeping badly, which deeply influenced her daily life. She was hospitalized several times because the dizziness was unresolved, and the weakness in both lower extremities worsened.
She had been diagnosed as having diabetes mellitus for 25 years and grade 3 hypertension for more than 10 years, and she received subcutaneous injections of insulin before meals and bedtime. She also took levamlodipine benzenesulfonate (5 mg), metoprolol tartrate tablets (25 mg), and prazosin hydrochloride (1 mg) daily, and calcitriol and materials for hematopoiesis (iron, folic acid, and vitamin B 12) were administered. Her blood pressure and blood glucose were well-controlled.
Approximately 7 years ago, she underwent an arteriovenous fistula surgery and began hemodialysis (3 times per week). In the past 2 years, she had frequent blood transfusions (once every 1 to 2 mo) because of severe anemia.
The patient’s personal and family history was unremarkable.
The patient presented with the following vital signs: Body temperature, 36.3 °C; blood pressure, 135/80 mmHg; pulse rate, 75 beats/min; and respiratory rate, 20 breaths/min. She was chronic and ill-looking with an anemic appearance. She had clear breathing sounds in both lungs. Her heart rate was 75 bpm, with a normal rhythm, and no other abnormalities were noted.
Hematological examination revealed an erythrocyte count of 1.58 × 1012/L, hemoglobin (Hb) level of 54 g/L, platelet count of 80 × 109/L, and ferritin of 4218.40 ng/mL (Table 1). The weekly dose ESA-to-Hb ratio was calculated as an index of ESA responsiveness[1], and basal state was 35.6 UI weekly/kg/g Hb.
| Parameter | Recorded value | Reference range |
| White blood cell count (× 109/L) | 3.92 | 3.50-9.50 |
| Red blood cell count (× 1012/L) | 1.58 | 3.80-5.10 |
| Hemoglobin (g/L) | 54 | 115-150 |
| Platelet count (× 109/L) | 80 | 125-350 |
| Procalcitonin (ng/mL) | 1.7 | < 0.1 |
| Erythrocyte sedimentation rate (mm/h) | 90 | 1-20 |
| Albumin (g/L) | 44.8 | 40.0-55.0 |
| Calcium (mmol/L) | 2.25 | 2.11-2.52 |
| Phosphonium (mmol/L) | 2.99 | 0.85-1.51 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 7.37 | - |
| Blood urea nitrogen (mmol/L) | 11.99 | 2.90-7.14 |
| Serum creatinine (µmol/L) | 555.7 | 44.0-133.0 |
| Ferritin (ng/mL) | 4218.40 | 21.80-274.66 |
| Serum iron (µmol/L) | 23.1 | 7.8-32.2 |
| High-density lipoprotein (mmol/L) | 0.70 | > 1.04 |
| Triglyceride (mmol/L) | 4.00 | < 1.71 |
| Cholesterol (mmol/L) | 4.03 | 2.90-5.20 |
| Low-density lipoprotein (mmol/L) | 2.65 | < 3.12 |
| 25 (nmol/L) | 22 | 75-250 |
| Glucose (mmol/L) | 10.85 | 3.90-6.10 |
| Hemoglobin A1c (%) | 7.0 | 3.90-6.10 |
| EPO concentration (mIU/mL) | 53.9 | 4.3-29 |
| Sucrose hemolysis test | Negative | Negative |
| Direct Coombs (IgG, C3) tests | Negative | Negative |
| Anti-EPO antibodies | Negative | Negative |
The results of the first bone marrow biopsy showed that she had bone marrow hyperplasia. The proportion of erythroids, primarily including polychromatic normoblasts and orthochromolatic normoblasts, was increased. The morphological characteristics of the granulocytes and erythroids were normal.
Reticulocyte tests had the following results: Total reticulocyte count, 0.019 × 109/L; Immature reticulocyte fraction, 10.7%; low fluorescent reticulocytes, 89.3%; and middle fluorescent reticulocytes, 10.3%.
A cluster of differentiation (CD) tests was performed on CD55 and CD59. In peripheral red blood cells, the levels of CD55 and CD59 were both 97%, while in blood granulocytes, the levels of CD55 and CD59 were both 96%. Flow cytometry was used to diagnose paroxysmal nocturnal hemoglobinuria. Flow cytometry assays were mainly based on the detection of CD59 (membrane inhibitor of reactive lysis) and CD55 (decay accelerating factor) on red blood cells, which provided an estimation of the haemolysis severity. By detecting the lack of CD55 and CD59 on the cell surface, it can be used as the most direct evidence for the diagnosis of paroxysmal nocturnal hemoglobinuria.
Bone puncture was performed again, and no obvious abnormalities were found in the bone marrow smear and by fluorescence in situ hybridization
Genetic tests showed that the patient carried a splice site mutation c.2408 + 1 G > A (low frequency hybrid, 5.21% of mutation frequency, sequencing depth, 2455 X) in the DNMT3A gene, which was deemed associated with myeloid hematological system diseases and a bad prognosis.
The antigen results related to autoimmune thrombocytopenia, coagulation test, cardiac biomarkers, thyroid function tests, and other blood biochemistry were within normal ranges.
Abdominal ultrasonography revealed diffuse lesions in renal parenchyma on both sides. Cardiac ultrasound images showed heart changes caused by hypertension. Chest-computed tomography scans detected recurrent pneumonia, bilateral diffuse exudation, and pleural effusion. Magnetic resonance imaging revealed that foreign objects were deposited in the liver and spleen, and the patient was suspected to have iron overload.
Type 2 diabetes, chronic kidney disease stage 5 (diabetic nephropathy), and renal anemia (iron overload); maintenance hemodialysis; grade 3 hypertension in a very high-risk group.
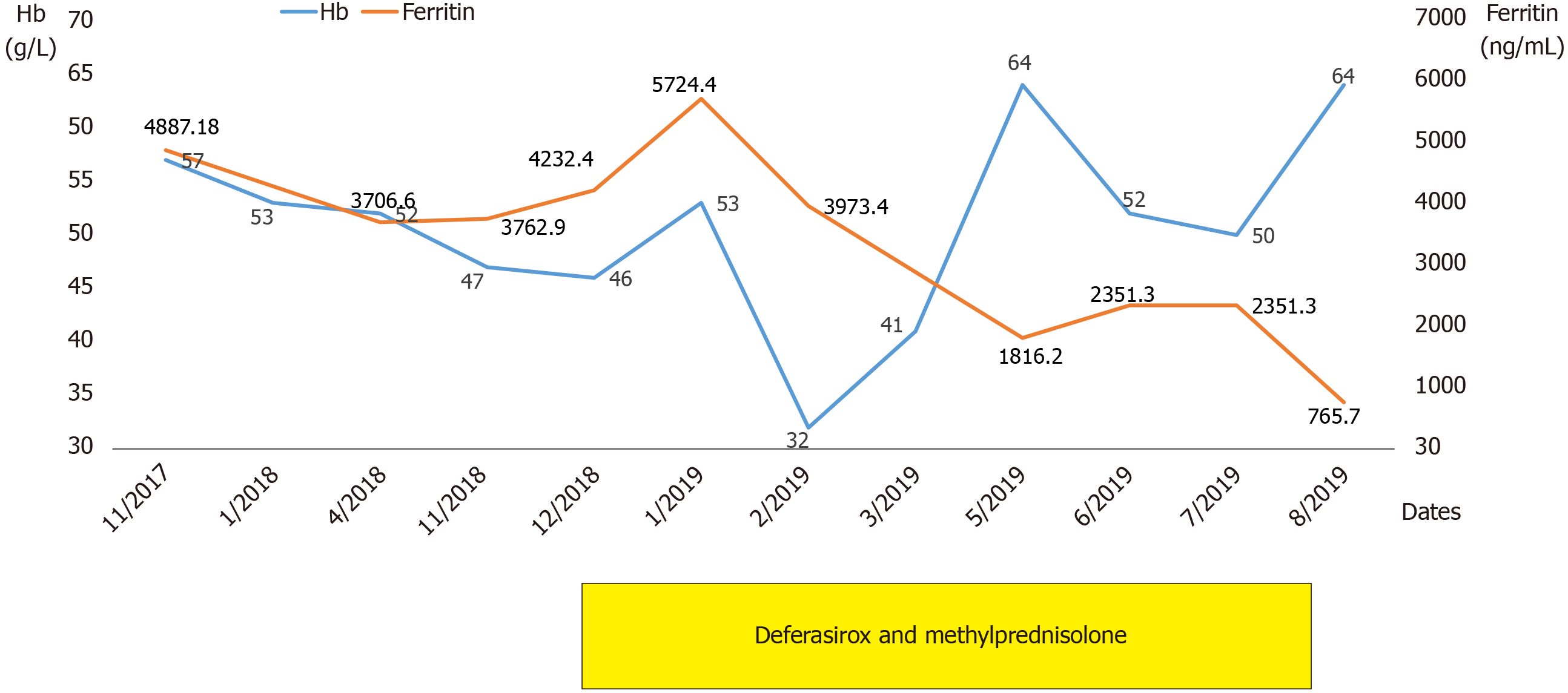
After admission, blood transfusion, recombinant human erythropoietin (rhEPO, 16000 U/W), adequate dialysis, and other symptomatic treatments were given. However, her symptoms did not improve despite these treatments. Serum ferritin values were far above the upper limit of the normal range (Figure 1), which provides a sensitive indicator for patients who are at risk for clinical manifestations of hemochromatosis[5]. Based on the genetic examination, the patient was suspected of suffering from immuno-related pancytopenia, iron overload due to the intermittent blood transfusions, and myelodysplastic syndromes. Therefore, treatments of oral deferasirox (875 mg) and methylprednisolone tablets (24 mg) were administered daily. However, the patient’s anemia did not improve (Figure 1).
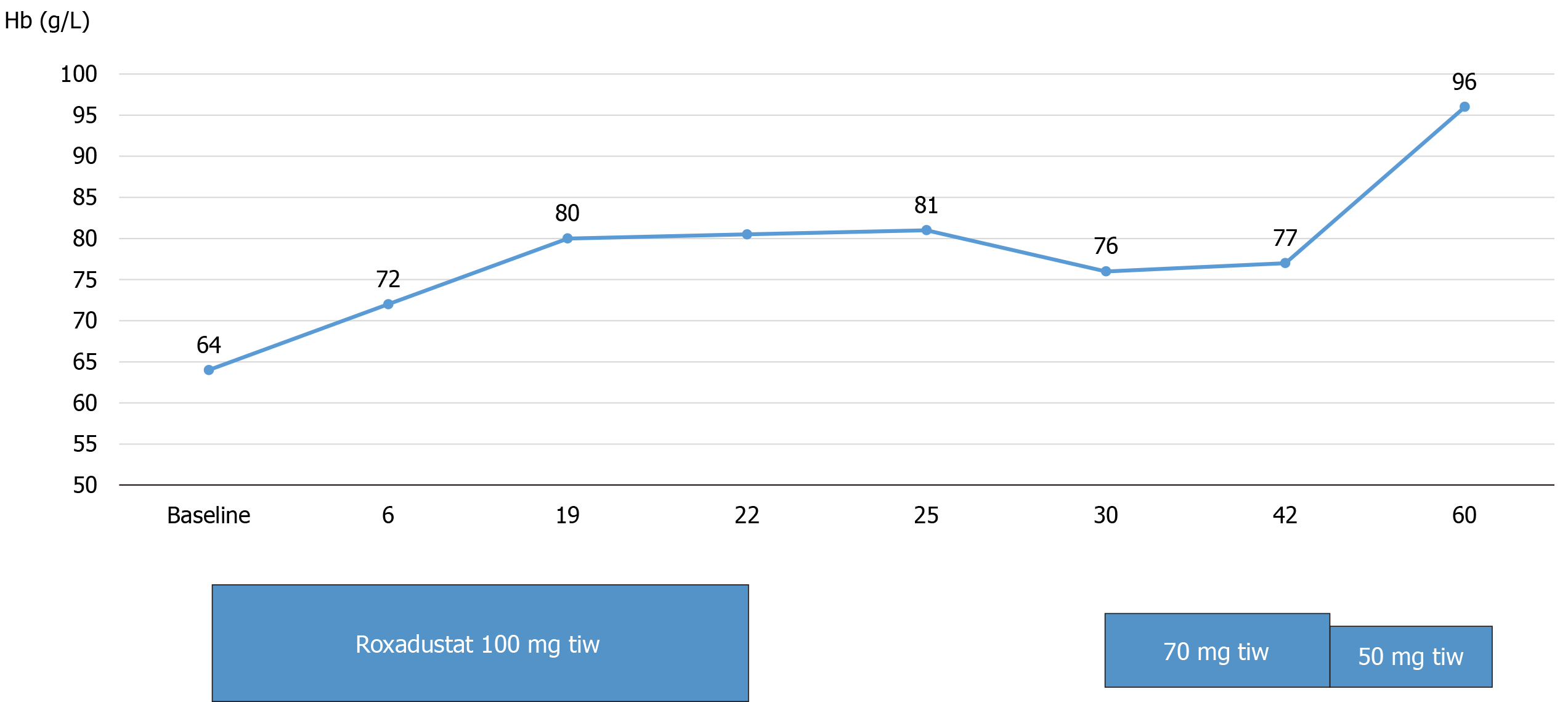
On August 18, 2019, the patient arrived at the hospital with the same complaint of recurrent dizziness. Her hemoglobin level was 64 g/L. Due to the iron overload and low hemoglobin level, we recommended oral roxadustat to improve her anemia and immediate cessation of methylprednisolone and deferasirox. The patient (57 kg) received roxadustat [100 mg, three times weekly (tiw)] beginning the next day and was required to visit our hospital weekly for evaluation of the therapeutic effects.
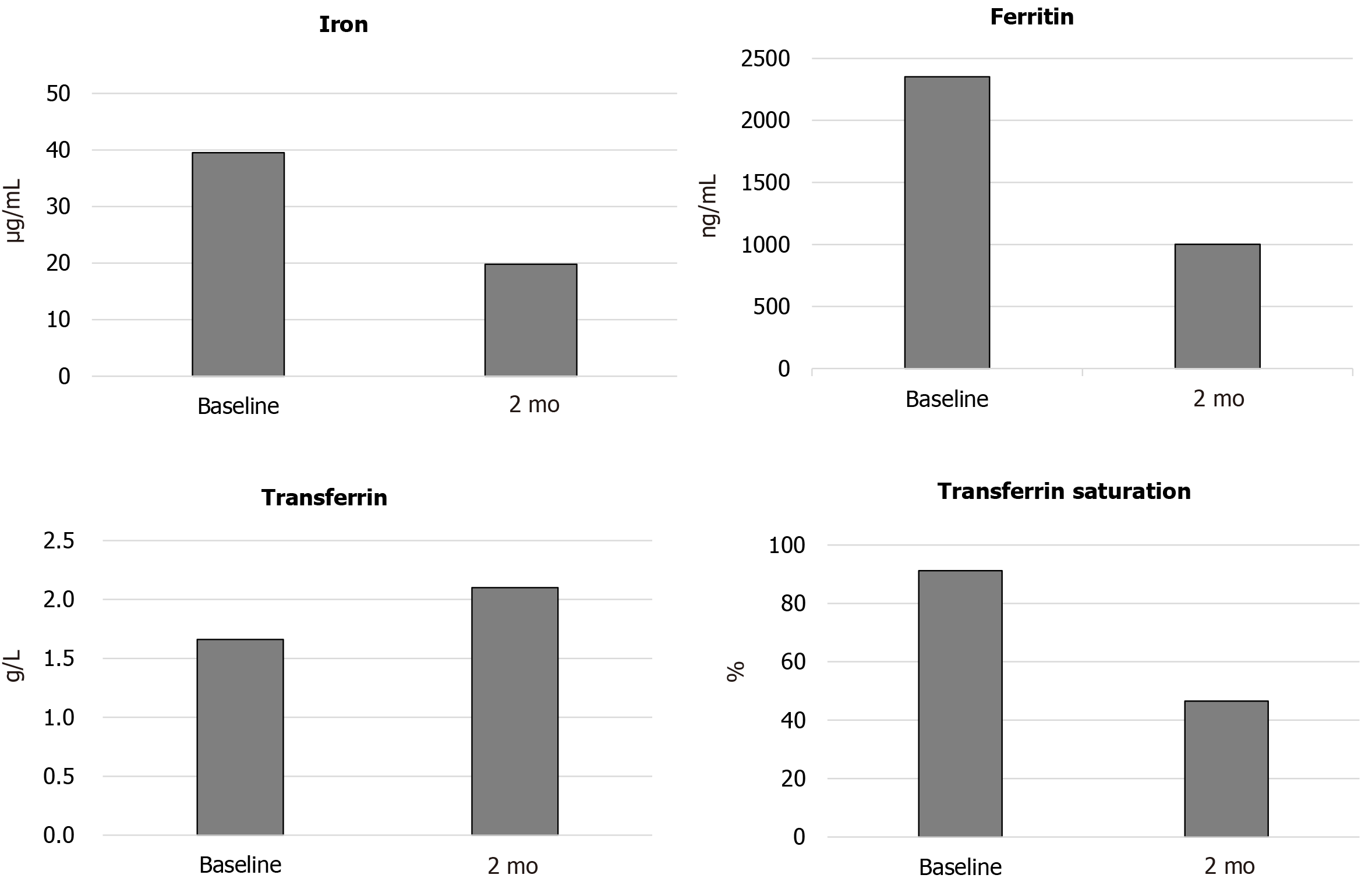
We adjusted the roxadustat dose based on the findings of previous studies. At the follow-up visit, the patient had adhered to the above medications. On the 6th day, blood tests showed that hemoglobin levels increased from the baseline of 64 g/L to 72 g/L. Roxadustat was discontinued on September 10, 2019 because of high systolic blood pressure (200 mmHg), and this side effect was well resolved with valsartan amlodipine (85 mg, quaque nocte). The patient restarted roxadustat (70 mg, tiw) treatment when her blood pressure stabilized. The patient’s hemoglobin level increased by a mean rate of 6.2 g/L/W (Figure 2), and reached 96 g/L after 60 d. The levels of serum ferritin, transferrin saturation, and iron decreased and that of transferrin increased (Figure 3). There was a decrease in triglyceride and cholesterol (Table 2). We did not observe side effects other than hypertension from the onset of treatment through the follow-up. Roxadustat decreased her hospitalization days and greatly improved her quality of life. The patient is currently on a continuous regimen of oral roxadustat, and her hemoglobin stabilized at approximately 100 g/L.
| Laboratory test | TG (mmol/L) | CHO (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) | WBC (× 109/L) | PLT (× 1012/L) |
| Baseline | 2.2 | 4.34 | 3.03 | 0.78 | 4.5 | 45 |
| 2 mo | 1.4 | 2.62 | 1.64 | 0.7 | 4.06 | 81 |
To the best of our knowledge, this is the first case report of roxadustat treatment for a maintenance hemodialysis patient with ESA hyporesponsiveness-related anemia. ESA hyporesponsiveness, which is the inability to achieve or maintain target hemoglobin levels despite higher than usual doses of ESA[6], is associated with greater mortality, greater iron and ESA use, and lower hemoglobin levels compared to non–ESA hyporesponsiveness patients[7]. However, the underlying mechanism of ESA responsiveness is unclear. Studies showed that the primary mediating factors included iron deficiency (absolute or functional) and vitamin deficiency, inflammation[8], poor nutritional status, inadequate dialysis, oxidative stress, and hyper-parathyroidism. Hypoferremia results from hepcidin-mediated inhibition of iron release[9]. Hepcidin is the main hormone responsible for maintaining systemic iron[3]. Interleukin 6 is a major inducer of hepcidin expression by the liver during inflammatory processes[10]. Hepcidin binds to ferroportin, which blocks iron transport into the plasma from macrophages involved in iron recycling, from iron stores in hepatocytes, and from enterocytes that absorb dietary iron[3,11]. Hepcidin is also homeostatically regulated via iron loading. Dietary iron or transfusions increase hepcidin synthesis. Excess hepcidin is the main cause of functional iron deficiency and iron-restricted erythropoiesis. Hypoxia-inducible factors (HIFs) are heterodimers consisting of an oxygensensitive α subunit and a constitutively expressed β subunit. HIF2-α is the key regulator of EPO synthesis and iron metabolism. Under normal oxygen conditions, HIF-α subunits are prolyl hydroxylated by PHD1, PHD2, and PHD3. Under hypoxia prolyl-4-hydroxylation of HIF-α is inhibited, resulting in its translocation to the nucleus, where it heterodimerizes with the HIF-β subunit and transactivates a large number of oxygen-regulated genes, leading to an increase in EPO production, improved uptake and use of iron, and changes to the bone marrow microenvironment. Roxadustat is an HIF prolyl hydroxylase inhibitor that may be used to reduce hepcidin, increase iron mobilization and utilization, and increase erythropoietin production[12]. A trial of roxadustat indicated that roxadustat–induced hemoglobin increases were independent of baseline C–reactive protein levels and iron repletion status[13]. Several studies reported that roxadustat was effective in correcting anemia in dialysis patients[14-16]. However, limited cases of roxadustat treatment for ESA hyporesponsiveness-related anemia have been reported.
Laboratory tests showed that this patient was in a chronic inflammatory state with serious ESA responsiveness and iron overload. Therefore, correcting the anemia might have been extremely difficult. During treatment with roxadustat, the patient’s hemoglobin level increased by a mean rate of 6.2 g/L/W, and it was stably maintained at 90-100 g/L without blood transfusion or injection of vein iron. In a large series of patients on dialysis, it was shown that those with more severe anemia received higher doses of ESA, suggesting that these patients were more resistant to treatment. The therapy increased transferrin and decreased the levels of serum iron, ferritin, and transferrin saturation (Figure 3). These results indicate that roxadustat improved the iron metabolism disorder of the patient. There is evidence to support lowering low-density lipoprotein cholesterol (LDL-C) to reduce cardiovascular morbidity and mortality. Cumulative LDL arterial burden is a central determinant for the initiation and progression of atherosclerotic cardiovascular disease. The lower the LDL-C attained, the greater the benefit accrued. The blood biochemistry results showed that triglyceride, cholesterol, and LDL-C levels declined, which may reduce the occurrence of atherosclerotic cardiovascular disease and its clinical manifestations, such as myocardial infarction and ischaemic stroke. The patient did not experience severe side effects except hypertension, which was well controlled with medications. Roxadustat effectively increased hemoglobin levels and was well-tolerated in our case, and it may be beneficial for ESA hyporesponsiveness-related anemia patients undergoing hemodialysis.
However, this report is a case study and has confused and complex background. Thus, the patient’s EPO-resistant anemia was probably not only due to chronic renal insufficiency, but also some possibility of the interaction of these drugs. Future clinical trials are required to confirm its effectiveness and assess safety concerns.
Oral roxadustat for the treatment of ESA hyporesponsiveness-related anemia in a patient undergoing hemodialysis is effective and tolerable. Roxadustat may be an additional treatment option for anemia in patients undergoing maintenance hemodialysis. Treatment of anemia in patients should be individualized. It is necessary to closely examine the risks and carefully monitor adverse events.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Khattab MMA, Tanaka H S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Li JH
| 1. | Panichi V, Rosati A, Bigazzi R, Paoletti S, Mantuano E, Beati S, Marchetti V, Bernabini G, Grazi G, Rizza GM, Migliori M, Giusti R, Lippi A, Casani A, Barsotti G, Tetta C; RISCAVID Study Group. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: results from the RISCAVID study. Nephrol Dial Transplant. 2011;26:2641-2648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Alves MT, Vilaça SS, Carvalho Md, Fernandes AP, Dusse LM, Gomes KB. Resistance of dialyzed patients to erythropoietin. Rev Bras Hematol Hemoter. 2015;37:190-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Rishi G, Wallace DF, Subramaniam VN. Hepcidin: regulation of the master iron regulator. Biosci Rep. 2015;35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 4. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3533] [Article Influence: 168.2] [Reference Citation Analysis (0)] |
| 5. | Waalen J, Felitti VJ, Gelbart T, Beutler E. Screening for hemochromatosis by measuring ferritin levels: a more effective approach. Blood. 2008;111:3373-3376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Fukuma S, Yamaguchi T, Hashimoto S, Nakai S, Iseki K, Tsubakihara Y, Fukuhara S. Erythropoiesis-stimulating agent responsiveness and mortality in hemodialysis patients: results from a cohort study from the dialysis registry in Japan. Am J Kidney Dis. 2012;59:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Luo J, Jensen DE, Maroni BJ, Brunelli SM. Spectrum and Burden of Erythropoiesis-Stimulating Agent Hyporesponsiveness among Contemporary Hemodialysis Patients. Am J Kidney Dis. 2016;68:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | López-Gómez JM, Portolés JM, Aljama P. Factors that condition the response to erythropoietin in patients on hemodialysis and their relation to mortality. Kidney Int Suppl. 2008: S75-S81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Ganz T. Hepcidin--a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 268] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 711] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 11. | Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest. 2004;113:1251-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Sonkar SK, Kumar A, Singh NK, Sonkar GK, Pandey S, Bhosale V. Role of Hepcidin on Response of Erythropoietin Stimulating Agents in Anaemic Advanced Chronic Kidney Disease Patients. JCDR. 2018;12:OC14-OC16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Provenzano R, Besarab A, Sun CH, Diamond SA, Durham JH, Cangiano JL, Aiello JR, Novak JE, Lee T, Leong R, Roberts BK, Saikali KG, Hemmerich S, Szczech LA, Yu KH, Neff TB. Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for the Treatment of Anemia in Patients with CKD. Clin J Am Soc Nephrol. 2016;11:982-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 228] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 14. | Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, Jiang G, Lin H, Zhang X, Zuo L, He Q, Fu P, Li X, Ni D, Hemmerich S, Liu C, Szczech L, Besarab A, Neff TB, Peony Yu KH, Valone FH. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32:1373-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Besarab A, Chernyavskaya E, Motylev I, Shutov E, Kumbar LM, Gurevich K, Chan DT, Leong R, Poole L, Zhong M, Saikali KG, Franco M, Hemmerich S, Yu KH, Neff TB. Roxadustat (FG-4592): Correction of Anemia in Incident Dialysis Patients. J Am Soc Nephrol. 2016;27:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 16. | Akizawa T, Otsuka T, Reusch M, Ueno M. Intermittent Oral Dosing of Roxadustat in Peritoneal Dialysis Chronic Kidney Disease Patients with Anemia: A Randomized, Phase 3, Multicenter, Open-Label Study. Ther Apher Dial. 2020;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |