Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.6036
Peer-review started: May 15, 2020
First decision: August 8, 2020
Revised: August 18, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 6, 2020
Processing time: 170 Days and 18.9 Hours
Apocrine carcinoma of the breast is a special type of invasive ductal carcinoma of the breast that is rare in clinical practice. Neoadjuvant therapy, especially neoadjuvant targeted therapy, has rarely been reported for apocrine carcinoma of the breast.
A 63-year-old woman presented with apocrine carcinoma of the left breast underwent core needle biopsy. The patient was diagnosed with apocrine carcinoma by immunohistochemical staining and negative hormone status (estrogen receptor and progesterone receptor) but showed overexpression of human epidermal factor receptor 2 (HER-2). Moreover, positive expression of androgen receptor (approximately 60%) and gross cystic disease fluid protein 15 was observed. The patient was treated with neoadjuvant targeted therapy consisting of the TCH regimen (docetaxel, carboplatin area under curve 6 and trastuzumab) every 21 d. The mass in the left breast was significantly reduced, and pain in the breast and left upper arm also improved.
HER-2 positive apocrine carcinoma of the breast can be improved by neoadjuvant chemotherapy combined with targeted therapy.
Core Tip: A woman with apocrine carcinoma of the left breast underwent core needle biopsy. The patient was diagnosed with apocrine carcinoma by immunohistochemical staining and negative hormone status (estrogen receptor and progesterone receptor) but showed overexpression of human epidermal factor receptor 2. Moreover, positive expression of androgen receptor (approximately 60%) and gross cystic disease fluid protein 15 was observed. The patient was treated with neoadjuvant targeted therapy consisting of the TCH regimen (docetaxel, carboplatin area under curve 6 and trastuzumab) every 21 d. The mass in the left breast was significantly reduced, and pain in the breast and left upper arm also improved.
- Citation: Yang P, Peng SJ, Dong YM, Yang L, Yang ZY, Hu XE, Bao GQ. Neoadjuvant targeted therapy for apocrine carcinoma of the breast: A case report. World J Clin Cases 2020; 8(23): 6036-6042
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/6036.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.6036
Breast cancer is the most common malignant tumor in women worldwide and has become a major threat to women's health[1]. Invasive ductal carcinoma of no-special-type is the most common type of breast cancer and constitutes up to 75% of all breast carcinomas. The remaining 25% are special histologic types including invasive apocrine carcinoma of the breast, which accounts for 0.4%-4.0% of all breast cancers in women[2-4]. Apocrine carcinoma of the breast is a rare invasive malignant tumor that is mainly composed of apocrine cells, which is defined by the World Health Organization as an invasive carcinoma with cytological features of apocrine cells[5]. More strictly, it is characterized by abundant eosinophilic and granular cytoplasm, centrally/eccentrically located nuclei with prominent nucleoli and distinct cell borders, and a distinct steroid receptor profile: Hormone receptor (HR)-negative and androgen receptor (AR)-positive cells[6]. “pure” apocrine carcinoma is defined by apocrine morphology in > 90% of tumor cells and immunohistochemical criteria, and it appears to have a worse disease-free survival rate[3]. So far, the precise significance of AR expression is not completely clear. Ni et al[7] showed that androgen stimulated the human epidermal factor receptor 2 (HER2) signaling pathway in estrogen receptor (ER)-negative and HER2-positive breast cancer, and AR blockade in these tumors impaired cell growth. Naderi et al[8] reported similar observations and demonstrated a functional “cross-talk” between androgen and HER2. Unfortunately, it is rarely used clinically at present. Anti-HER2 therapy is still the cornerstone for HR-negative and HER2-positive apocrine carcinoma of the breast. Neoadjuvant therapy is preferred for locally advanced breast cancer, especially HR-negative and HER2-amplified breast cancer. However, there are few reports on the efficacy of neoadjuvant targeted therapy for apocrine carcinoma. Informed content was obtained from the patient.
A 63-year-old woman found a mass in the superior lateral quadrant of the left breast, with no obvious discomfort, and she paid no attention to it.
During the next 2 years, the left breast mass gradually increased in size and was painful. She developed discomfort in the left upper limb and visited a local hospital many times (without an examination report). Surgical treatment was suggested, but the patient refused.
The patient had a free previous medical history.
The patient has a history of good health, and there is no similar patient in the family members.
Physical examination showed that the skin in the outer upper quadrant of the left mammary gland was obviously sunken, and a hard mass about 5 cm × 6 cm, with an unclear boundary, irregular shape, and poor activity was found on palpation. No obvious enlarged lymph nodes were found in the axilla.
No abnormalities were found in the patient's laboratory examinations.
Ultrasound showed a solid hypoechoic mass approximately 3.5 cm × 5.3 cm × 4.5 cm in size at the margin of the gland at 12-3 o'clock in the superior lateral quadrant of the left breast, and several hypoechoic lymph nodes of different sizes were detected in the left axilla. The largest lymph node was about 0.6 cm × 0.6 cm, and the lymphatic hilum structure had disappeared. No abnormalities were found in the right breast. Therefore, chest computed tomography (CT) scanning was performed, and the left breast mass was diagnosed as breast cancer, with no abnormalities in the lungs. Other results, including abdominal ultrasound, bone scan, and laboratory examinations, showed no abnormalities. There was no family history in genetic disorders.
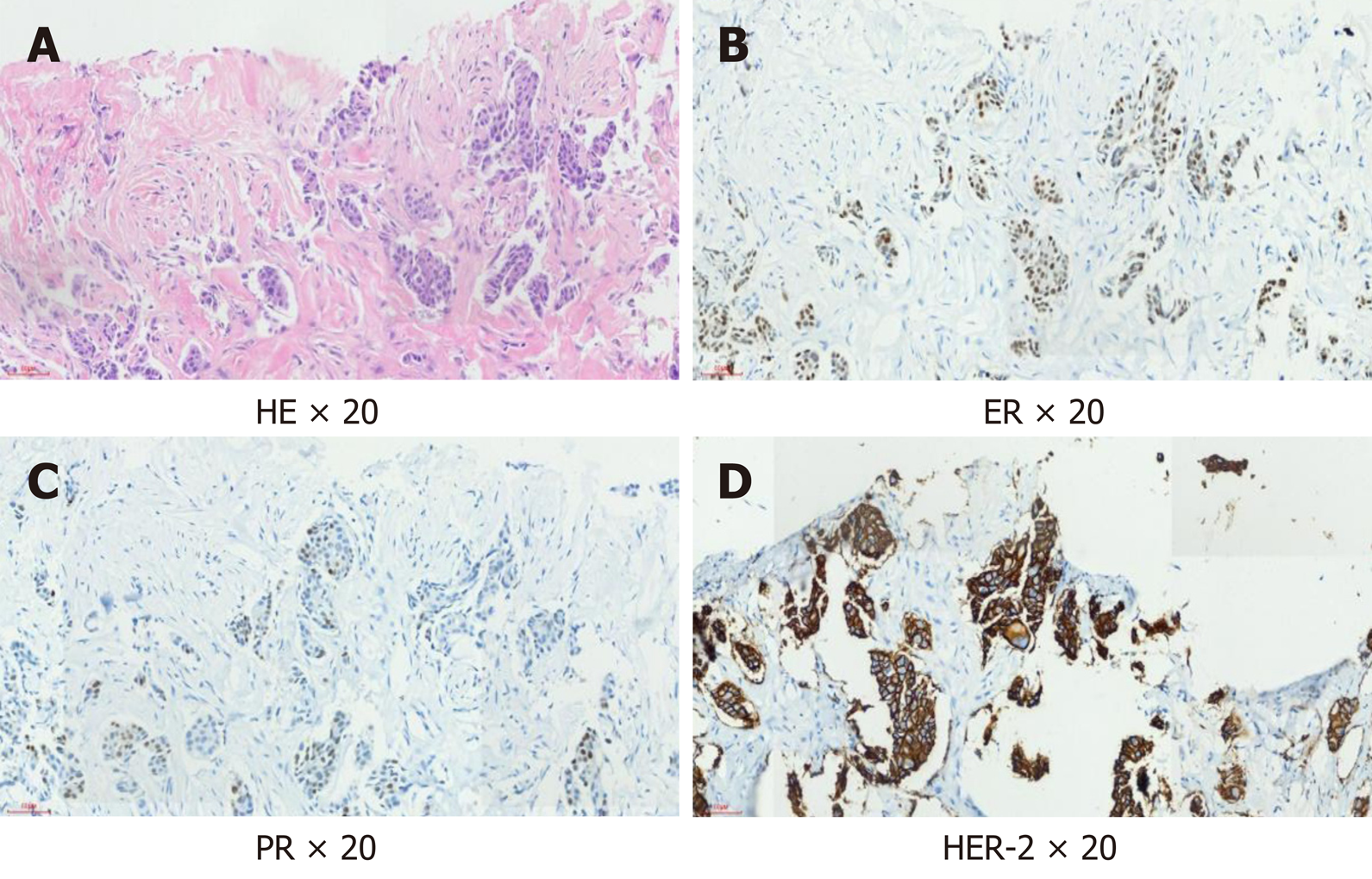
The patient underwent core needle biopsy of the left breast mass under ultrasound guidance, and the histopathological diagnosis was apocrine carcinoma. Immunohistochemical analysis showed negative expression of ER and progesterone receptor (PR), but positive expression of HER2 and AR (approximately 60%); gross cystic disease fluid protein 15 (GCDFP-15) was also positive (Figure 1).
The final diagnosis of the presented case is apocrine carcinoma of the breast. The clinical stage was T4N1M0 (IIIB).
Considering that the patient had small breast volume, large breast lesion, late clinical stage, and local advanced breast cancer and her molecular type, neoadjuvant therapy seemed an effective choice. Therefore, after informed consent was obtained from her family members and the patient, neoadjuvant chemotherapy using the TCH regimen [docetaxel, carboplatin area under curve 6 and trastuzumab 8 mg/kg followed by 6 mg/kg every 21 d] and targeted therapy were administered. No significant side effects were observed during the treatment.
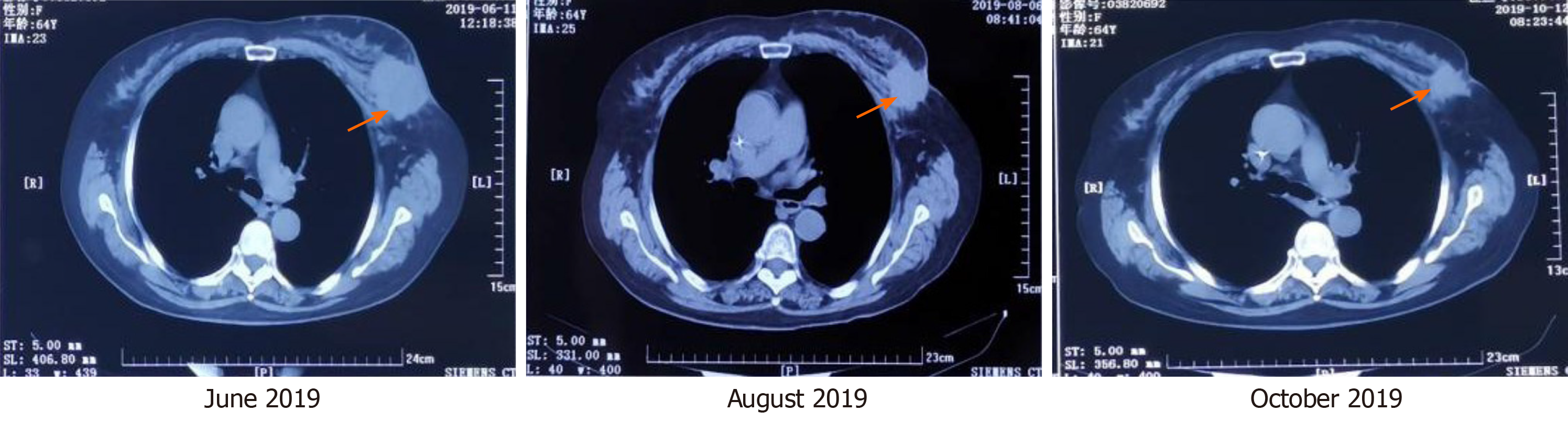
After 5 cycles of treatment, the left breast lesion was significantly reduced on CT examination (Figure 2). In November 2019, the patient underwent modified radical mastectomy. During the operation, mild edema and thickening of the left breast skin, unclear boundary of the breast lesions, and irregular morphology were observed, and no capsule or obvious calcified lesions were found in the specimen. The specimen was soaked in formalin solution and sent to the pathology department. Apocrine carcinoma of the breast was confirmed by pathology. The immunohistochemical results were as previously determined, but Ki-67 was reduced to 50%.
Apocrine carcinoma of the breast remains largely unknown as studies have recruited a small number of patients and did not use well-defined criteria for apocrine carcinoma. “Molecular apocrine” carcinomas exhibit a prognostically poor gene signature with a high-risk recurrence score and a poor 70-gene prognosis signature[9]. Zhang et al[10] analyzed data from the SEER Program and suggested that compared with other invasive carcinomas, overall survival and disease-specific survival (DSS) were both worse in invasive apocrine adenocarcinoma patients than in invasive ductal carcinoma patients. Based on the Kaplan-Meier analysis, the prognosis of apocrine carcinoma is poor. Further study on the risk factors influencing the overall survival and disease-specific survival by multifactor analysis confirmed that histological grade II/III, tumor size > 2 cm, and positive lymph nodes were associated with poor prognosis and that ER/PR positive breast conserving surgery, and radiotherapy were protective factors in DSS[10]. Nevertheless, Japaze et al[11] suggested that “pure” invasive apocrine carcinoma may be a distinct clinicopathological entity with less aggressive behavior than high-grade no-special-type cancer. In addition, Wu et al[12] identified 366 patients with triple-negative apocrine carcinoma of the breast and 30996 patients with triple-negative breast cancer and invasive ductal carcinoma from the SEER database. Patients with triple-negative apocrine carcinoma had a better prognosis than patients with triple-negative breast cancer, and chemotherapy was associated with survival advantages in patients with triple-negative apocrine carcinoma.
There are some contradictions in the prognostic study of apocrine carcinoma of the breast, which may be related to its unclear definition. In addition, the latest cla-ssification of breast tumors by the World Health Organization is equally inaccurate[13]. At present, most experts agree that the definition of pure apocrine carcinoma should be based on typical cell morphology and unique immunohistochemical char-acteristics[14,15], as this profile matches closely the normal apocrine epithelium. Our patient was characterized by typical morphological manifestations and immune combinations.
Mammary apocrine cells have abundant eosinophilic and granular cytoplasm, centrally to eccentrically located nuclei with prominent nucleoli and distinctive cell borders, or less common apocrine cells with a foamy and vacuolated cytoplasm[16]. AR activation and signaling is a hallmark of apocrine differentiation in breast pathology; moreover, AR has been associated with HER2 signaling in apocrine carcinoma[17]. HER2 protein is overexpressed in approximately 15% of all breast carcinomas[18], but the rate of HER2 overexpression is approximately 50% in apocrine carcinoma[19]. GCDFP-15, identified by Haagensen et al[20] in 1977, is a 15-kDa protein mapped to chromosome 7, and its expression has been associated with apocrine differentiation, including apocrine carcinoma, although its positivity has been confirmed in other breast carcinoma subtypes[20-22]. GCDFP-15 positive patients are associated with local recurrence and distant metastases[23].
At present, clinical and radiological presentations of apocrine carcinoma do not differ from those seen in invasive ductal carcinomas[24]. Clinically, apocrine carcinoma usually presents with a palpable tumor mass, rarely with a bloody discharge from the nipple or as a cyst[25,26]. However, some studies have indicated a higher rate of apocrine carcinoma (and AR expression) in the elderly[26].
With regard to treatment, some studies have indicated a poor response to chemotherapy in patients with apocrine carcinoma[27], although HER2 enriched breast carcinomas tend to have the highest rate of complete response to neoadjuvant chemotherapy. Iizuka et al[28] described a case of triple-negative apocrine carcinoma treated with docetaxel/cyclophosphamide (four courses) and epirubicin/cyclo-phosphamide (four courses), with a complete pathologic response to neo-adjuvant chemotherapy. However, neoadjuvant targeted therapy for apocrine carcinoma of breast has rarely been reported. The breast mass in our patient was initially up to 5.3 cm in diameter and was locally advanced; thus, she received the neoadjuvant TCH regimen and then underwent surgery after five cycles of treatment, which resulted in partial remission. Therefore, we believe that HER2 positive apocrine carcinoma of the breast can be treated with neoadjuvant targeted therapy.
As a rare invasive carcinoma, there is no special treatment at present for apocrine carcinoma of the breast. Appropriate treatment can be given based on imm-unohistochemical results. For HER2 positive locally advanced apocrine carcinomas of the breast, according to our experience, neoadjuvant targeted therapy is appropriate and can yield desirable results. The predictive role of AR expression and the efficacy of anti-AR therapy in the treatment of breast cancer have not been determined. More clinical evidence is needed.
The authors thank the patient for agreeing to publish this report.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rim CH S-Editor: Liu M L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Lee H, Lee DE, Park S, Kim TS, Jung SY, Lee S, Kang HS, Lee ES, Sim SH, Park IH, Lee KS, Kwon YM, Kong SY, Joo J, Jeong HJ, Kim SK. Predicting Response to Neoadjuvant Chemotherapy in Patients With Breast Cancer: Combined Statistical Modeling Using Clinicopathological Factors and FDG PET/CT Texture Parameters. Clin Nucl Med. 2019;44:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Tan PH, Ellis IO. Myoepithelial and epithelial-myoepithelial, mesenchymal and fibroepithelial breast lesions: updates from the WHO Classification of Tumours of the Breast 2012. J Clin Pathol. 2013;66:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Dellapasqua S, Maisonneuve P, Viale G, Pruneri G, Mazzarol G, Ghisini R, Mazza M, Iorfida M, Rotmensz N, Veronesi P, Luini A, Goldhirsch A, Colleoni M. Immunohistochemically defined subtypes and outcome of apocrine breast cancer. Clin Breast Cancer. 2013;13:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Choi J, Jung WH, Koo JS. Clinicopathologic features of molecular subtypes of triple negative breast cancer based on immunohistochemical markers. Histol Histopathol. 2012;27:1481-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 5. | Cserni G. Histological type and typing of breast carcinomas and the WHO classification changes over time. Pathologica. 2020;112:25-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Vranic S, Schmitt F, Sapino A, Costa JL, Reddy S, Castro M, Gatalica Z. Apocrine carcinoma of the breast: a comprehensive review. Histol Histopathol. 2013;28:1393-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 7. | Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, Rimm DL, Liu XS, Brown M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20:119-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 297] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 8. | Naderi A, Hughes-Davies L. A functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia. 2008;10:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Weigelt B, Horlings HM, Kreike B, Hayes MM, Hauptmann M, Wessels LF, de Jong D, Van de Vijver MJ, Van't Veer LJ, Peterse JL. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 380] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 10. | Zhang N, Zhang H, Chen T, Yang Q. Dose invasive apocrine adenocarcinoma has worse prognosis than invasive ductal carcinoma of breast: evidence from SEER database. Oncotarget. 2017;8:24579-24592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Japaze H, Emina J, Diaz C, Schwam RJ, Gercovich N, Demonty G, Morgenfeld E, Rivarola E, Gil Deza E, Gercovich FG. 'Pure' invasive apocrine carcinoma of the breast: a new clinicopathological entity? Breast. 2005;14:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Wu W, Wu M, Peng G, Shi D, Zhang J. Prognosis in triple-negative apocrine carcinomas of the breast: A population-based study. Cancer Med. 2019;8:7523-7531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Yang WT, Zhu XZ. [The introduction of 2012 WHO classification of tumours of the breast]. Zhonghua Bing Li Xue Za Zhi. 2013;42:78-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 14. | Kolečková M, Kolář Z, Ehrmann J, Kořínková G, Zlámalová N, Melichar B, Trojanec R. Tumor-Infiltrating Lymphocytes/Plasmocytes in Chemotherapeutically Non-Influenced Triple-Negative Breast Cancers - Correlation with Morphological and Clinico-Pathological Parameters. Klin Onkol. 2019;32:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Provenzano E, Gatalica Z, Vranic S. Carcinoma with apocrine differentiation. 5th ed. In: WHO lassification of Tumours 5th Edition-Tumours of the Breast Lyon, France: IARC. 2019. Available from: https://qspace.qu.edu.qa/handle/10576/12569. |
| 16. | Page DL. Apocrine carcinomas of the breast. Breast. 2005;14:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 18. | Baehner FL, Achacoso N, Maddala T, Shak S, Quesenberry CP Jr, Goldstein LC, Gown AM, Habel LA. Human epidermal growth factor receptor 2 assessment in a case-control study: comparison of fluorescence in situ hybridization and quantitative reverse transcription polymerase chain reaction performed by central laboratories. J Clin Oncol. 2010;28:4300-4306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Vranic S, Gatalica Z, Deng H, Frkovic-Grazio S, Lee LM, Gurjeva O, Wang ZY. ER-α36, a novel isoform of ER-α66, is commonly over-expressed in apocrine and adenoid cystic carcinomas of the breast. J Clin Pathol. 2011;64:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Haagensen DE Jr, Dilley WG, Mazoujian G, Wells SA Jr. Review of GCDFP-15. An apocrine marker protein. Ann N Y Acad Sci. 1990;586:161-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Naderi A, Meyer M. Prolactin-induced protein mediates cell invasion and regulates integrin signaling in estrogen receptor-negative breast cancer. Breast Cancer Res. 2012;14:R111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Lewis GH, Subhawong AP, Nassar H, Vang R, Illei PB, Park BH, Argani P. Relationship between molecular subtype of invasive breast carcinoma and expression of gross cystic disease fluid protein 15 and mammaglobin. Am J Clin Pathol. 2011;135:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Ilhan B, Emiroğlu S, Türkay R, Ilhan R. The role of histopathologic testing on apocrine carcinoma of the breast. Curr Probl Cancer. 2020;44:100501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | O'Malley FP, Bane AL. The spectrum of apocrine lesions of the breast. Adv Anat Pathol. 2004;11:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Mardi K, Sharma J, Sharma N. Apocrine carcinoma of the breast presenting as a solitary cyst: cytological and histopathological study of a case. Indian J Pathol Microbiol. 2004;47:268-270. [PubMed] |
| 26. | Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003;120:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Nagao T, Kinoshita T, Hojo T, Tsuda H, Tamura K, Fujiwara Y. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: the relationship between the outcome and the clinicopathological characteristics. Breast. 2012;21:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Iizuka M, Enomoto K, Sakurai K. [A case of breast cancer treated with neoadjuvant chemotherapy and segmentectomy]. Gan To Kagaku Ryoho. 2012;39:2027-2029. [PubMed] |