Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.5976
Peer-review started: June 27, 2020
First decision: September 23, 2020
Revised: September 26, 2020
Accepted: October 13, 2020
Article in press: October 13, 2020
Published online: December 6, 2020
Processing time: 155 Days and 21.9 Hours
Abdominal ventral rectopexy (AVR) with colectomy is controversial in the treatment of obstructed defecation syndrome (ODS). Literature data on this technique for ODS are very limited.
To evaluate the safety and efficacy of AVR with colectomy for selected patients with ODS.
Consecutive patients who underwent AVR with colectomy for ODS were identified prospectively from 2016 to 2017 in our department. Patient demographics, perioperative surgical results, and postoperative follow-up outcomes were collected and analyzed. Long-term follow-up was evaluated with standardized questionnaires. The severity of symptoms was assessed by the objective Wexner Constipation Score (WCS) and ODS Score. The quality of life was assessed by the Patients Assessment of Constipation Quality of Life score. Functional outcome was compared pre- and post-operatively for each patient. The primary outcomes were determined by the improvement in symptoms and quality of life. Secondary outcome measures were operating time, postoperative length of stay, morbidity and mortality, improvement of pelvic floor structure, and patient satisfaction.
Four patients underwent robotic-assisted surgery, and two patients underwent a laparoscopic-assisted procedure. The mean operating time for the robotic approach was 243 min (range 160–300 min), and the mean operating time for the laparoscopic approach was 230 min (range 220-240 min). The mean postoperative length of stay was 8.2 d (range 6-12 d). There was no conversion to open procedure and no postoperative mortality. No urinary retention, wound infection, prolonged ileus, pelvic infection and anastomosis leakage occurred. Six patients were followed up for 36 mo. The WCS, ODS, and Patients Assessment of Constipation Quality of Life score improved significantly postoperatively (P < 0.05). The WCS and ODS scores showed the best remission and stabilization at 6 to 12 mo after surgery. There was no recurrence or novel constipation after surgery. None of the patients used laxative medication.
Robotic and laparoscopic-assisted ventral rectopexy with colectomy is a safe and effective procedure for selected patients with ODS. However, comprehensive preoperative evaluation and careful patient selection are essential.
Core Tip: Many different surgical procedures have been developed to treat patients with obstructed defecation syndrome (ODS), which mainly includes transperineal and transabdominal approaches, and no optimal procedure has been identified for all patients to date. Abdominal rectopexy with colectomy is controversial. It has not been reported whether abdominal ventral rectopexy combined with an additional colon resection provide better functional results in the treatment of ODS. Therefore, we report our preliminary experience of abdominal ventral rectopexy with additional colectomy to evaluate its safety and efficacy in selected patients with ODS.
- Citation: Wang L, Li CX, Tian Y, Ye JW, Li F, Tong WD. Abdominal ventral rectopexy with colectomy for obstructed defecation syndrome: An alternative option for selected patients. World J Clin Cases 2020; 8(23): 5976-5987
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/5976.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.5976
Obstructed defecation syndrome (ODS) is the most common type of chronic constipation. It comprises a wide spectrum of defecation disorders, mainly including incomplete evacuation, defecation straining, digital assistance, and repetitive toilet visits[1]. ODS is mainly caused by pelvic floor structural abnormalities, such as rectal prolapse, rectal intussusception, and rectocele[2]. The common clinical symptom of internal rectal prolapse (IRP) is ODS, while the patient with external rectal prolapse (ERP) usually complains of prolapse of an anal mass, obstructed defecation (OD), and fecal incontinence (FI)[3,4]. Patients with ODS often depend on a laxative and/or enema, and they have accompanying psychological disorders. Their quality of life is severely impaired when medication becomes less effective over time. Unlike ERP, which always requires surgery, the indication for surgery in IRP arises from severe symptoms of chronic constipation with failure of conservative treatment and a subjective reduction in quality of life, and does not depend on the finding of abnormal morphology alone. In these patients who may benefit from surgical therapy, this not only depends on imaging findings but more importantly relies on the patient’s feeling.
Many different surgical procedures have been developed to treat patients with ODS, which mainly include transperineal and transabdominal approaches, and no optimal procedure has been identified for all patients to date[2,3,5-7]. Perineal surgery is usually used in elderly frail patients who are unfit for a general anesthetic, but it has a higher risk of recurrence[8]. In recent years, abdominal surgery has become a common practice in the treatment of ODS with the development of mini-invasive techniques, especially for patients with accompanying pelvic floor dysfunction[6,7,9-14]. Laparoscopic surgery is routinely performed, and the use of robotic assistance is progressively increasing in pelvic floor surgery due to its technical advantage in a narrow confined space[15-17]. However, technically, there are ongoing debates on the transabdominal approach that can be categorized into with or without resection of the colon, and with or without implantation of mesh. After the technique of laparoscopic ventral rectopexy (LVR) was first described by D’Hoore et al[18], the procedure became a novel method adopted rapidly all over the world, which is a safe solution for relieving intussusception- and prolapse-related symptoms, including OD and FI, with a lower morbidity rate[19]. It is currently considered to be the standard procedure for full-thickness ERP in Europe[6,20].
Laparoscopic resection rectopexy (LRR) is also a safe and effective procedure achieving good long-term results with an improvement in ODS symptoms and an acceptable morbidity[21]. Abdominal posterior rectopexy with sigmoid resection has shown satisfactory functional improvements in patients with prolapse and preoperative constipation[22]. A systematic review by Emile et al[13] showed that resection rectopexy for IRP had a lower recurrence rate, whereas ventral rectopexy achieved better symptomatic improvement, a shorter operative time and a lower complication rate. Therefore, rectopexy with or without resection is controversial, and there have been few reports on resection rectopexy in recent years[20,23,24].
It has not been reported whether abdominal ventral rectopexy (AVR) combined with an extended colon resection provides better functional results in the treatment of ODS, and this is worthy of exploration. Therefore, we report our preliminary experience of AVR with an extended colectomy to evaluate its safety and efficacy in selected patients with ODS.
Consecutive patients who received AVR with colectomy for ODS between January 2016 and December 2017 in our department were identified prospectively. Data regarding patient demographics, perioperative surgical results and postoperative follow-up outcomes were obtained prospectively. The study was performed according to the approval of the Medical Ethics Committee of Daping Hospital. Written informed consent was obtained from all participants.
Patients with ODS who conformed to the Rome III criteria of functional constipation and who failed maximal conservative treatments including biofeedback and medical therapy were eligible for the study. A precise patient history, diagnostic workup, including physical examination, colonoscopy, anorectal manometry, defecography, barium enema, pelvic dynamic magnetic resonance imaging, and colonic transit test, was examined preoperatively. Patients with severe constipation symptoms [Wexner Constipation Score (WCS) > 15 and ODS score > 10], grade III-IV full-thickness rectal intussusception, along with pelvic floor anatomic abnormalities, such as abnormally deep cul-de-sac, descending perineum syndrome, rectocele, enterocele, retroversion of the uterus, and redundant colon, were recruited into the study. The grade of rectal prolapse was according to the Oxford radiological prolapse grading system[25,26].
Exclusion criteria were previous anal surgery or colorectal resection, malignancy, inflammatory bowel disease, megacolon, ERP, pregnancy, as well as contraindications for general anesthesia. Patient characteristics are summarized in Table 1.
| Patient No. | Sex | Age (yr) | BMI (kg/ | Duration of ODS (mo) | Career | History of surgery | Defecography | Barium enema | Pelvic dynamic magnetic resonance imaging | Colonic transit time (h) |
| 1 | Female | 43 | 18.7 | 120 | Middle school teacher | Laparoscopic ovariocystectomy | IRP (grade IV) DPS | Redundant sigmoid and transverse colon | POP, deep cul-de-sac, sigmoidocele | 54 |
| 2 | Female | 77 | 16 | 13 | Farmer | None | IRP (grade IV) DPS | Redundant sigmoid colon | POP, deep cul-de-sac, retroversion of uterus | 52 |
| 3 | Female | 58 | 26.8 | 96 | Self-employed person | None | IRP (grade III) rectocele | Redundant sigmoid colon | POP, deep cul-de-sac | Normal |
| 4 | Female | 63 | 24 | 360 | Retired worker | None | IRP (grade IV) | Redundant sigmoid and transverse colon | POP, deep cul-de-sac, sigmoidocele | 64 |
| 5 | Female | 39 | 23.2 | 84 | Factory worker | PPH | IRP (grade IV) | Redundant sigmoid colon | POP, deep cul-de-sac | Normal |
| 6 | Female | 37 | 26.8 | 120 | Factory worker | None | IRP (grade IV) rectocele | Redundant sigmoid colon | POP, deep cul-de-sac | 54 |
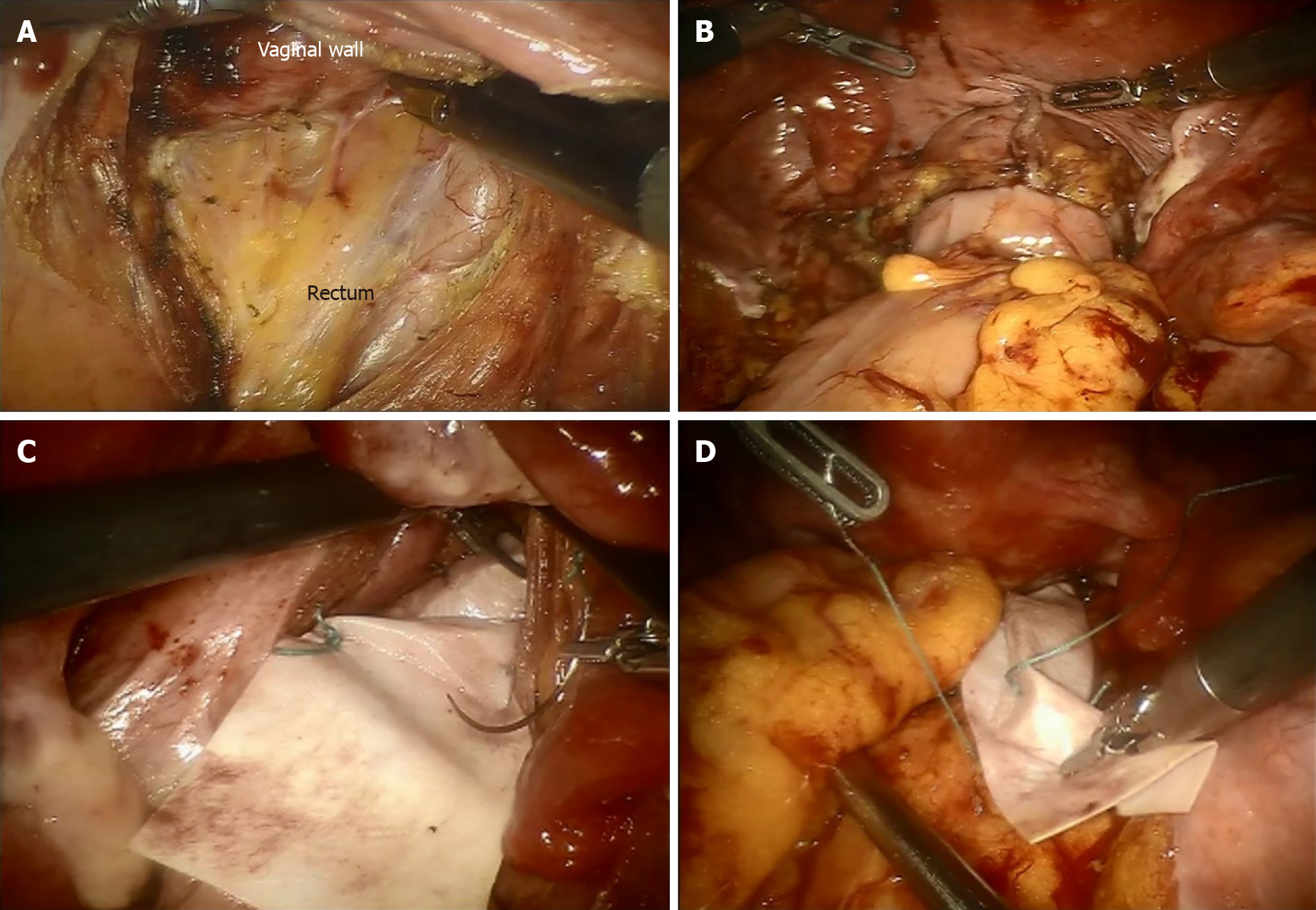
The operation was performed via robotic or laparoscopic-assisted surgery. The left colon was mobilized completely with release of the splenic flexure, and protection of the superior rectal artery and the hypogastric nerves at the same time. The anterior plane of the rectum was dissected from the vagina downwards to the levator ani muscle. The colonic mesentery was dissociated close to the colon wall of the rectal sigmoid junction, and the colon was transected using an Endoscopic Cutting stapler. The proximal colon was pulled out via an enlarged trocar incision in the left lower quadrant, and the sigmoid colon and descending colon (50-60 cm) were resected. The anastomosis was achieved with a laparoscopic double-staple technique. Then, pelvic and surgical field irrigation was performed to avoid potential contamination. The ventral mesh rectopexy (VMR) technique was primarily performed by following the details described by D’Hoore et al[18]. A strip of 3 cm × 17 cm tailored rectangular mesh was positioned at the lowest point of the rectovaginal space. Fixation between the anterior wall of the distal rectum and mesh was sutured with 2-0 PDS. The proximal end of the mesh was sutured on the sacral promontory after the rectum was pulled upwards. Finally, the right lateral peritoneal incision was closed to cover the mesh and the pelvic floor peritoneum was reconstructed with a running suture with 2-0 Prolene to obliterate the cul-de-sac. The uterus was fixed to the anterior position by a suture of round ligaments. The operative technique was similar in the robotic and laparoscopic surgeries. The excised specimens were sent for pathological examination. All operations were performed by the same surgical team to ensure quality control (Figure 1).
Postoperative evaluation, including improvement in symptoms, quality of life, patient satisfaction, intake of laxative medication, and recurrence was performed. Patients were followed up at 3, 6, 12, 24, and 36 mo after surgery. Long-term follow-up was evaluated with standardized questionnaires in the outpatient clinic. The severity of symptoms was assessed by the objective WCS and ODS score. Quality of life was assessed by the Patients Assessment of Constipation Quality of Life score. The functional result was compared pre- and post-operatively for each patient. Recurrence was defined as symptoms of persistent obstructed defecation or new symptoms with an ODS score > 10 or WCS > 15, or an anatomic rectal prolapse (Oxford grade ≥ 2) diagnosed by defecography or dynamic magnetic resonance imaging.
The primary outcomes were determined by the improvement in symptoms and quality of life. Secondary outcome measures were operating time, postoperative length of stay, complications and mortality, improvement of pelvic floor structure, and patient satisfaction.
Statistical analyses were performed using SPSS 24.0 (SPSS, Chicago, IL, United States). The Mann–Whitney U test was used to compare the preoperative scores with the follow-up scores. A P value < 0.05 was considered statistically significant.
Six patients underwent robotic-assisted and laparoscopic-assisted VMR with colectomy. All patients were female with a mean age of 53 years (range 37-77 years). Mean body mass index was 22.6 kg/m2 (range 16-26.8 kg/m2). Mean duration of constipation symptoms before surgery was 132.2 mo (range 13-360 mo). Patient career included teacher, farmer, self-employed person, and factory worker. Two patients had a previous history of surgery. One patient underwent laparoscopic ovariocystectomy, and another patient underwent a procedure for prolapse and hemorrhoids. All patients were diagnosed as having grade III and IV IRP along with pelvic organ prolapse, deep cul-de-sac, and redundant sigmoid colon, two patients had accompanying rectocele, and two patients had a sigmoidocele. Four patients had abnormally prolonged colonic transit times measured by radiopaque markers, while two patients had normal colonic transit before surgery (Table 1).
Four patients underwent robotic-assisted surgery, while the other two patients received laparoscopic-assisted procedures. The mean operating time for the robotic approach was 243 min (range 160-300 min), while the mean operating time for the laparoscopic approach was 230 min (range 220-240 min). In three patients, a synthetic polypropylene mesh was used, and a biologic mesh was used in the other patients. Mean postoperative length of stay was 8.2 d (range 6-12 d). There was no conversion to open procedure and no postoperative mortality. No urinary retention, wound infection, prolonged ileus, pelvic infection, and anastomosis leakage occurred. One patient who developed mesh erosion into the rectum complained of pelvic pain and was examined by pelvic magnetic resonance imaging and proctoscopy at 15 mo postoperatively. The patient achieved complete symptomatic remission after the mesh was removed transanally by colonoscopy (Table 2).
| Clinical outcomes | |
| Robotic | 4 |
| Laparoscopic | 2 |
| synthetic mesh | 3 |
| biologic mesh | 3 |
| Mean operating time (min) | |
| Robotic | 243 (160-300) |
| Laparoscopic | 230 (220-240) |
| Conversion | 0 |
| Mean postoperative length of stay (d) | 8.2 (6-12) |
| Urinary retention | 0 |
| Wound infection | 0 |
| Prolonged ileus | 0 |
| Anastomotic leakage | 0 |
| Pelvic infection | 0 |
| Mesh complication | 1 |
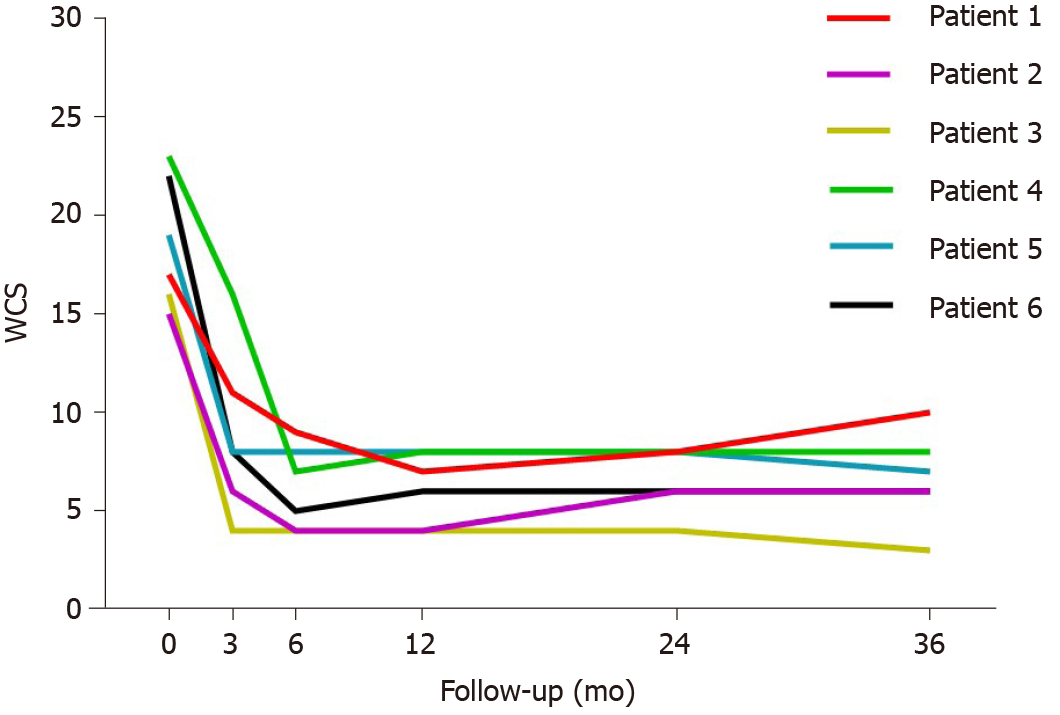
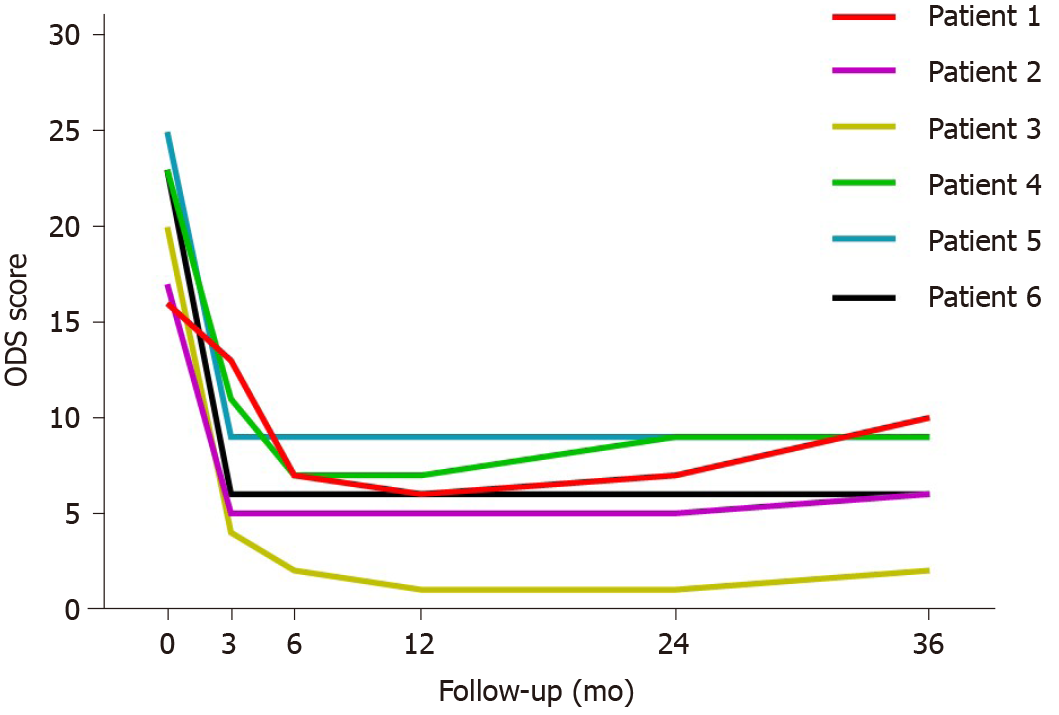
All patients completed 36 mo follow-up after surgery. Six patients had severe constipation preoperatively with a WCS ≥ 15. The mean WCS before surgery was 18.6 (range 15-23). At 3 mo, the mean WCS improved to 8.3 (range 4-16). At 6 mo, the mean WCS improved to 6.2 (range 4–9). The mean WCS was 6.2 (range 4-8), 6.7 (range 4-8), and 6.7 (range 3-10) at 12, 24, and 36 mo, respectively. The scores obviously decreased in the first 3 to 6 mo, while the decline slightly improved subsequently. Six patients showed significant improvement in constipation after surgery compared with the preoperative condition (P < 0.05). The WCS scores showed the best remission and stabilization at 6 to 12 mo after surgery, and four of the six patients had a slight increase after 12 mo (Figure 2). The mean ODS score decreased from 20.7 (range 16-25) preoperatively to 7 (range 2-10) at the end of follow-up (P < 0.05, respectively). Similarly, the scores of four patients showed a slight increase after 12 to 24 mo (Figure 3).
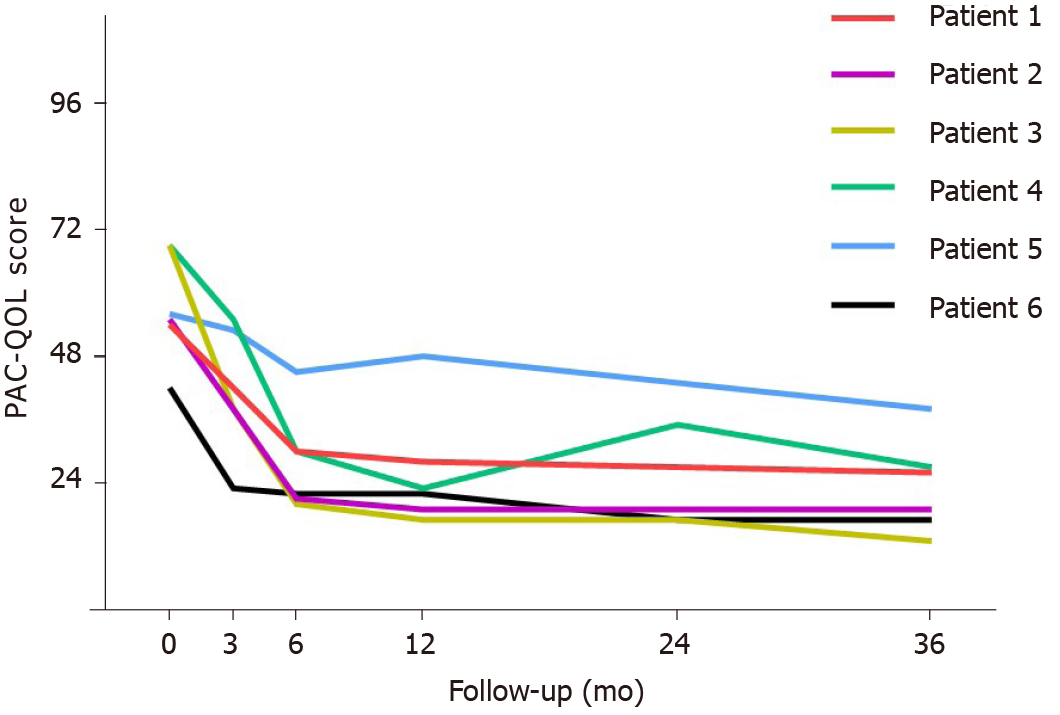
The mean Patients Assessment of Constipation Quality of Life score improved from 57.5 (range 42-69) to 23.3 (range 13-38) (P < 0.05, respectively). The score was then stable after 6 mo (Figure 4). An improvement in patient’s quality of life was observed in all four subsets (physical discomfort, psychosocial discomfort, worries and concerns, and satisfaction). All six patients were satisfied with the surgery and had no regrets regarding the surgical treatment. There was no recurrence or novel constipation after surgery. None of the patients used laxative medication.
ODS is a benign condition characterized by a multifactorial etiology that predo-minantly affects females and the elderly with symptoms of obstructed defecation[27,28]. When treatment with medication becomes less effective, the patient has to consider surgery as quality of life is severely impaired. The goal of surgical treatment is not only to repair anatomic abnormalities, but also to restore defecation function and avoid novel symptoms. Many operative procedures have been described in the literature, but there is no one procedure that suits all patients. The decision on surgical procedure depends on the comorbidities of the patient, the patient’s age and bowel function, and the surgeon’s preference and experience[22].
The present study is the first on AVR with colectomy in selected patients with ODS, which achieved satisfactory functional results during a 3-year follow-up period, despite the small number of cases. Our results showed that all six patients showed significant improvement in constipation after surgery. Their quality of life also improved significantly, and all six patients were satisfied with the surgical treatment at each follow-up postoperatively. The scores showed the best remission and stabilization at 6 to 12 mo during long-term follow-up, which is similar to that in the other studies[29]. It is worth noting that the scores in four patients showed a slight increase after 12 mo, which implied that treatment of constipation requires a multidisciplinary approach for a long period after surgery. Although surgery corrected the abnormal anatomical structures, bowel motility function, hormone level, psychological effect, and other comprehensive factors can also affect the patient's defecation function[1]. The clinical outcomes were acceptable, although one patient developed mesh erosion. There was no pelvic infection, anastomosis leakage, or other complications in these six patients.
LVR was initially designed for ERP, and it was later applied to the treatment of high-grade IRP accompanied by ODS[30]. The advantages of purely anterior rectal mobilization include the prevention of anterior recto-rectal intussusception and reinforcement of the rectovaginal septum, as well as avoidance of autonomic nerve injury and the risk of new-onset constipation. Many studies have shown that LVR had a lower recurrence rate and better functional improvement of OD, FI, and gastrointestinal quality of life[15,17,31-33]. Postoperative constipation after LVR may improve up to 80% in patients with mesh use over a long-term follow-up[34]. Nevertheless, the functional results are still not satisfactory for patients with ODS, which is different to ERP. Due to the multifactorial etiology and a large spectrum of symptoms, patients with IRP usually have accompanying different morphological alterations of the pelvic floor, the colon and rectum. Abdominal surgery is performed to deal with the whole spectrum of underlying alterations as much as possible.
According to the clinical practice guidelines of rectal prolapse, sigmoid resection may be added to posterior rectopexy in patients with prolapse and preoperative constipation[22]. LRR is a safe and effective procedure achieving greater improvement of constipation, especially in patients with a symptomatic sigmoidocele[21]. However, some studies showed that the functional results of LRR were similar to those of LVR, but postoperative complications may be increased with an additional resection[20,23,24].
The generic term ‘‘resection rectopexy’’ includes resection and rectopexy, but the extent of colonic resection, method of rectal mobilization and fixation vary considerably in historical literature. The procedure of LRR was performed by sigmoidectomy and posterior rectal suture fixation, including mobilization of the left colon with the splenic flexure unmobilized and retrorectal mobilization to the pelvic floor[35]. Therefore, different methods of rectopexy and resection might result in different functional outcomes.
In this study, we implemented VMR combined with extended resection of the sigmoid colon and descending colon based on the fact that all six patients suffered from severe constipation accompanied by a lengthy colon and pelvic floor structural abnormalities. Two patients had accompanying rectocele, and two patients had sigmoidocele. Four patients had abnormally prolonged colonic transit time, and residual markers existed in the descending colon and sigmoid colon. Interestingly, El Muhtaseb et al[36] reported that the colonic transit time was not changed after resection rectopexy. This might be attributed to the potential damage of autonomic nerves due to mobilization of the posterior and lateral rectum. However, the changes in colonic transit time after AVR with colectomy are not clear, and further study is needed in these patients.
A recent review by Albayati et al[17] showed that robotic ventral rectopexy had fewer conversions to open surgery and fewer complications, but it required a longer operative time with no added benefit over LVR. Robotic ventral rectopexy and LVR are good options for ODS associated with IRP. In the current study, the operating time between robotic- and laparoscopic-assisted procedures was slightly different. Although robotic-assisted surgery took more time in the preparation stage, it had an obvious advantage in pelvic structure dissection, especially in lower mesh suture fixation.
It was considered that synthetic mesh had a lower recurrence rate but a higher complication rate compared with biological mesh[37]. The postoperative improvement in constipation was approximately 80% in patients with biologic mesh used for a long-term follow-up, which is similar to synthetic mesh[34]. Unfortunately, one patient developed mesh erosion at 15 mo after surgery in our study. The rectum was corroded by the biologic mesh and the patient achieved complete symptomatic remission after the mesh was removed. The reason for this might be related to the mesh material or the suture technique. Although the rate of biologic mesh erosion is obviously lower than that of synthetic mesh[38,39], technical errors in dissection of the rectovaginal septum and different sized meshes may be possible causes of erosion[40]. Attention should be paid to the risk factors involved, including poor physical condition and tissue healing, uncontrolled diabetes mellitus, smoking, and previous pelvic irradiation.
Colectomy is usually not advocated in combination with repairs involving mesh. However, a strict aseptic operation, pelvic irrigation, complete coverage of the pelvic peritoneum during surgery, and the use of biological mesh can reduce the risk of potential pelvic infection and complications.
The present study has some limitations. First, this was only an observational preliminary study of several selected patients, due to the small number of cases and different heterogeneities of the patients. The safety and effectiveness need further exploration in a large number of patients. Second, there may have been some differences between the robotic and laparoscopic surgeries in the study. For example, robotic-assisted surgery requires a longer operative time with no significant benefit over laparoscopic surgery. More studies are required to clarify the potential technical advantage of robotic surgery in achieving an improvement in the clinical outcome. Third, although there are no studies comparing biological mesh with synthetic mesh in VMR, two different types of mesh were used in this study, which could have affected the clinical outcomes. Finally, a control group of AVR without colectomy is lacking in this study, which could be more powerful in proving whether extended colectomy is necessary.
The postoperative results and long-term functional outcomes are satisfactory in selected patients with ODS. Robotic and laparoscopic-assisted ventral rectopexy with colectomy is a safe and effective procedure. However, comprehensive preoperative evaluation and careful patient selection are essential.
Abdominal ventral rectopexy (AVR) with colectomy is controversial in the treatment of obstructed defecation syndrome (ODS). Literature data on this technique are very limited.
ODS is the most common type of chronic constipation. In patients with severe symptoms of constipation, reduced quality of life, and conservative treatment have failed, surgery should be considered. Many different surgical procedures have been reported, but no optimal procedure has been identified for all patients to date. It is controversial whether an additional colon resection in rectopexy is required. Laparoscopic ventral rectopexy has become a salutatory procedure with better functional outcomes in recent years, and it achieved an 80% improvement in constipation. Therefore, whether AVR combined with an additional colon resection can provide better functional results in the treatment of ODS has not been reported and is worthy of exploration.
The research objectives were to evaluate the efficacy and safety of AVR with colectomy in selected patients with ODS. The primary objectives were improvement in functional outcomes determined by constipation symptoms and quality of life assessed by the Wexner Constipation Score, ODS score and the Patients Assessment of Constipation Quality of Life score through patient-self comparison pre- and post-operatively. Secondary objectives were operating time, postoperative length of stay, complications and mortality, improvement of pelvic floor structure, and patient satisfaction.
Consecutive patients who received AVR with colectomy for ODS between January 2016 and December 2017 in our department were identified prospectively. Data regarding patient demographics, perioperative surgical results and postoperative follow-up outcomes were obtained prospectively. Patients with ODS who conformed to the Rome III criteria of functional constipation and who failed maximal conservative treatments including biofeedback and medical therapy were eligible. These patients had severe constipation symptoms [Wexner Constipation Score (WCS) > 15 and ODS score > 10], and high grade (III/IV) full-thickness rectal intussusception, along with pelvic floor anatomic abnormalities. All operations were performed by the same surgical team to ensure quality control (Figure 1). Statistical analyses were performed using SPSS 24.0 (SPSS, Chicago, IL, United States). The Mann–Whitney U test was used to compare the preoperative scores with the follow-up scores.
Perioperative outcomes: Six patients underwent robotic-assisted and laparoscopic-assisted ventral mesh rectopexy with colectomy. All patients were diagnosed as having grade III and IV internal rectal prolapse along with pelvic organ prolapse, deep cul-de-sac, and redundant sigmoid colon. There was no conversion to open procedure and no postoperative mortality. No urinary retention, wound infection, prolonged ileus, pelvic infection, and anastomosis leakage occurred. One patient who developed mesh erosion into the rectum complained of pelvic pain and was examined by pelvic magnetic resonance imaging and proctoscopy at 15 mo postoperatively. The patient achieved complete symptomatic remission after the mesh was removed transanally by colonoscopy. Functional outcomes: All patients completed 36 mo follow-up after surgery. The mean WCS before surgery was 18.6 (range 15-23). The mean WCS improved to 6.7 (range 3-10) at 36 mo. Six patients showed significant improvement in constipation after surgery compared with the preoperative condition (P < 0.05). The mean ODS score decreased from 20.7 (range 16-25) preoperatively to 7 (range 2-10) at the end of follow-up (P < 0.05, respectively). The mean Patients Assessment of Constipation Quality of Life score improved from 57.5 (range 42-69) to 23.3 (range 13-38) (P < 0.05, respectively). Six patients were satisfied with the surgery and had no regrets regarding surgical treatment. There was no recurrence or novel constipation after surgery. None of the patients used laxative medication.
The postoperative results and long-term functional outcomes are satisfactory for selected patients with ODS. Robotic and laparoscopic-assisted ventral rectopexy with colectomy is a safe and effective procedure. However, comprehensive preoperative evaluation and careful patient selection are essential.
This was the first study to explore AVR with colectomy for the treatment of ODS. The results showed that these patients achieved satisfactory functional results during a 3-year follow-up, despite the small number of cases. Although the present study has some limitations, we will increase the number of patients and include a control group of AVR without colectomy in a subsequent study, which could be more powerful in proving whether colectomy is necessary.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nacif L S-Editor: Zhang L L-Editor: Webster JR P-Editor: Liu JH
| 1. | Podzemny V, Pescatori LC, Pescatori M. Management of obstructed defecation. World J Gastroenterol. 2015;21:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 2. | Murad-Regadas SM, Regadas FS, Rodrigues LV, Fernandes GO, Buchen G, Kenmoti VT. Management of patients with rectocele, multiple pelvic floor dysfunctions and obstructed defecation syndrome. Arq Gastroenterol. 2012;49:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Cariou de Vergie L, Venara A, Duchalais E, Frampas E, Lehur PA. Internal rectal prolapse: Definition, assessment and management in 2016. J Visc Surg. 2017;154:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Cannon JA. Evaluation, Diagnosis, and Medical Management of Rectal Prolapse. Clin Colon Rectal Surg. 2017;30:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Collinson R, Cunningham C, Lindsey I. Surgery for internal rectal prolapse. Colorectal Dis. 2009;11:11-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Kim M, Meurette G, Ragu R, Lehur PA. Current surgical treatment of obstructed defecation among selected European opinion leaders in pelvic floor surgery. Tech Coloproctol. 2016;20:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Murphy PB, Wanis K, Schlachta CM, Alkhamesi NA. Systematic review on recent advances in the surgical management of rectal prolapse. Minerva Chir. 2017;72:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Riansuwan W, Hull TL, Bast J, Hammel JP, Church JM. Comparison of perineal operations with abdominal operations for full-thickness rectal prolapse. World J Surg. 2010;34:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Giannini A, Caretto M, Russo E, Mannella P, Simoncini T. Advances in surgical strategies for prolapse. Climacteric. 2019;22:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Hori T, Yasukawa D, Machimoto T, Kadokawa Y, Hata T, Ito T, Kato S, Aisu Y, Kimura Y, Takamatsu Y, Kitano T, Yoshimura T. Surgical options for full-thickness rectal prolapse: current status and institutional choice. Ann Gastroenterol. 2018;31:188-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Bjerke T, Mynster T. One decade of rectal prolapse surgery: a national study. Int J Colorectal Dis. 2018;33:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Joubert K, Laryea JA. Abdominal Approaches to Rectal Prolapse. Clin Colon Rectal Surg. 2017;30:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Emile SH, Elfeki HA, Youssef M, Farid M, Wexner SD. Abdominal rectopexy for the treatment of internal rectal prolapse: a systematic review and meta-analysis. Colorectal Dis. 2017;19:O13-O24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Rogers AC, McCawley N, Hanly AM, Deasy J, McNamara DA, Burke JP. Trends in the treatment of rectal prolapse: a population analysis. Int J Colorectal Dis. 2018;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Mäkelä-Kaikkonen J, Rautio T, Pääkkö E, Biancari F, Ohtonen P, Mäkelä J. Robot-assisted vs laparoscopic ventral rectopexy for external or internal rectal prolapse and enterocele: a randomized controlled trial. Colorectal Dis. 2016;18:1010-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | van Iersel JJ, Paulides TJ, Verheijen PM, Lumley JW, Broeders IA, Consten EC. Current status of laparoscopic and robotic ventral mesh rectopexy for external and internal rectal prolapse. World J Gastroenterol. 2016;22:4977-4987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Albayati S, Chen P, Morgan MJ, Toh JWT. Robotic vs laparoscopic ventral mesh rectopexy for external rectal prolapse and rectal intussusception: a systematic review. Tech Coloproctol. 2019;23:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | D'Hoore A, Cadoni R, Penninckx F. Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg. 2004;91:1500-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 317] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 19. | Samaranayake CB, Luo C, Plank AW, Merrie AE, Plank LD, Bissett IP. Systematic review on ventral rectopexy for rectal prolapse and intussusception. Colorectal Dis. 2010;12:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 20. | Panis Y. Laparoscopic ventral rectopexy: resection or no resection? Tech Coloproctol. 2014;18:611-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Laubert T, Kleemann M, Roblick UJ, Bürk C, Hildebrand P, Lewejohann J, Schlöricke E, Bruch HP. Obstructive defecation syndrome: 19 years of experience with laparoscopic resection rectopexy. Tech Coloproctol. 2013;17:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Bordeianou L, Paquette I, Johnson E, Holubar SD, Gaertner W, Feingold DL, Steele SR. Clinical Practice Guidelines for the Treatment of Rectal Prolapse. Dis Colon Rectum. 2017;60:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 23. | Formijne Jonkers HA, Maya A, Draaisma WA, Bemelman WA, Broeders IA, Consten EC, Wexner SD. Laparoscopic resection rectopexy vs laparoscopic ventral rectopexy for complete rectal prolapse. Tech Coloproctol. 2014;18:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | von Papen M, Ashari LH, Lumley JW, Stevenson AR, Stitz RW. Functional results of laparoscopic resection rectopexy for symptomatic rectal intussusception. Dis Colon Rectum. 2007;50:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Wijffels NA, Collinson R, Cunningham C, Lindsey I. What is the natural history of internal rectal prolapse? Colorectal Dis. 2010;12:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Wijffels NA, Jones OM, Cunningham C, Bemelman WA, Lindsey I. What are the symptoms of internal rectal prolapse? Colorectal Dis. 2013;15:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 28. | Kairaluoma MV, Kellokumpu IH. Epidemiologic aspects of complete rectal prolapse. Scand J Surg. 2005;94:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Tsiaoussis J, Chrysos E, Athanasakis E, Pechlivanides G, Tzortzinis A, Zoras O, Xynos E. Rectoanal intussusception: presentation of the disorder and late results of resection rectopexy. Dis Colon Rectum. 2005;48:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Mercer-Jones MA, D'Hoore A, Dixon AR, Lehur P, Lindsey I, Mellgren A, Stevenson AR. Consensus on ventral rectopexy: report of a panel of experts. Colorectal Dis. 2014;16:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | van Iersel JJ, Consten EC. Ventral mesh rectopexy for rectal prolapse: level-I evidence. Lancet Gastroenterol Hepatol. 2016;1:264-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Inaba CS, Sujatha-Bhaskar S, Koh CY, Jafari MD, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A. Robotic ventral mesh rectopexy for rectal prolapse: a single-institution experience. Tech Coloproctol. 2017;21:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | van Iersel JJ, Formijne Jonkers HA, Paulides TJC, Verheijen PM, Draaisma WA, Consten ECJ, Broeders IAMJ. Robot-Assisted Ventral Mesh Rectopexy for Rectal Prolapse: A 5-Year Experience at a Tertiary Referral Center. Dis Colon Rectum. 2017;60:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Franceschilli L, Varvaras D, Capuano I, Ciangola CI, Giorgi F, Boehm G, Gaspari AL, Sileri P. Laparoscopic ventral rectopexy using biologic mesh for the treatment of obstructed defaecation syndrome and/or faecal incontinence in patients with internal rectal prolapse: a critical appraisal of the first 100 cases. Tech Coloproctol. 2015;19:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Roblick UJ, Bader FG, Jungbluth T, Laubert T, Bruch HP. How to do it--laparoscopic resection rectopexy. Langenbecks Arch Surg. 2011;396:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | El Muhtaseb MS, Bartolo DC, Zayiae D, Salem T. Colonic transit before and after resection rectopexy for full-thickness rectal prolapse. Tech Coloproctol. 2014;18:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Mercer-Jones MA, Brown SR, Knowles CH, Williams AB. Position Statement by The Pelvic Floor Society on behalf of The Association of Coloproctology of Great Britain and Ireland on the use of mesh in ventral mesh rectopexy (VMR). Colorectal Dis. 2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Borie F, Coste T, Bigourdan JM, Guillon F. Incidence and surgical treatment of synthetic mesh-related infectious complications after laparoscopic ventral rectopexy. Tech Coloproctol. 2016;20:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Albayati S, Morgan MJ, Turner CE. Laparoscopic ventral rectopexy for rectal prolapse and rectal intussusception using a biological mesh. Colorectal Dis. 2017;19:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Madbouly KM, Youssef M. Laparoscopic Ventral Rectopexy versus Laparoscopic Wells Rectopexy for Complete Rectal Prolapse: Long-Term Results. J Laparoendosc Adv Surg Tech A. 2018;28:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |